Abstract
Purpose
Scopolamine is a nonselective muscarinic cholinergic receptor antagonist, which induces impairment of learning ability and memory function. Exercise is known to ameliorate brain disturbance induced by brain injuries. In the present study, we investigated the effect of treadmill exercise on short-term memory in relation to acetylcholinesterase (AChE) expression in the hippocampus, using a scopolamine-induced amnesia model in mice.
Methods
To induce amnesia, 1 mg/kg scopolamine hydrobromide was administered intraperitoneally once per day for 14 days. A step-down avoidance test for short-term memory was conducted. AChE histochemistry, immunohistochemistry for collagen IV, and doublecortin were performed.
Results
Short-term memory deteriorated in the mice with scopolamine-induced amnesia, concomitant with enhanced AChE expression and suppression of angiogenesis in the hippocampus. Critically, treadmill exercise ameliorated short-term memory impairment, suppressed AChE expression, and enhanced angiogenesis in the mice with scopolamine-induced amnesia.
Conclusions
Overexpression of AChE is implicated in both brain and renal disease. The findings of our study indicate that treadmill exercise may be of therapeutic value in neurodegenerative and renal diseases by suppressing the effects of AChE expression.
Keywords: Amnesia, Exercise test, Short-term memory, Acetylcholinesterase
INTRODUCTION
Dementia is one of the defining symptoms of neurodegenerative disorders, including Alzheimer disease (AD). Memory loss is induced by a cholinergic deficit, and the degree of this deficit is correlated with the severity of dementia [1]. Critically, memory loss is due to a reduction of acetylcholine (ACh) production, which leads to a decrease of ACh available at the neuronal synapse [2].
The activity of nicotinic and muscarinic ACh receptors is reduced in patients with AD [3,4]. Muscarinic acetylcholinergic receptor antagonists are known to impair learning ability and memory function, and this impairment can be observed with the nonselective antagonist, scopolamine. Scopolamine can therefore be effective for use in animal models of amnesia [5], and it also inhibits central cholinergic neuronal activity [6].
The degeneration of basal forebrain cholinergic neurons is associated with impaired cortical cholinergic neurotransmission, induced by hyperactivity of choline acetyltransferase and acetylcholinesterase (AChE) [7]. As ACh is a major neurotransmitter involved in the process of learning and memory, enhanced AChE activity decreases the brain's ACh concentration, which can lead to memory impairment [8].
The hippocampus is a brain structure critical for learning and memory function. It is vulnerable to neuronal injury produced by scopolamine-induced cholinergic activity dysregulation, which can result in impairment of synaptic plasticity and loss of spatial learning memory [9]. Newborn neurons in the hippocampus play an important role in learning ability and memory function. The developmental stages of neurogenesis are characterized by specific markers, including nestin, NeuroD, doublecortin (DCX), polysialylated neural cell adhesion molecule, stathmin, and calretinin [10]. DCX is a marker of neuroblasts, neuronal precursor cells, and immature neurons. It is associated with structural plasticity in the adult mammalian brain, and has been used as a marker of newly formed neurons in the dentate gyrus of the adult hippocampus [11]. DCX is involved in neuronal migration and development, and it is continuously expressed during adult neurogenesis [12].
Cerebral blood vessels play a critical role in the regulation of neural stem cell proliferation and migration [13]. Several microenvironmental elements, such as blood vessels, mediate cell proliferation, differentiation, and maturation [14].
The beneficial effects of physical exercise on cognitive functions such as spatial learning ability and memory, have been reported previously [15,16,17]. In the present study, we investigated the effect of treadmill exercise on short-term memory in relation to AChE expression using a mouse model of scopolamine-induced amnesia. AChE histochemistry and immunohistochemistry for collagen IV and DCX were performed.
MATERIALS AND METHODS
Experimental Animals
Male ICR mice weighing 35±0.5 g were used in this experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The mice were housed under controlled temperature (20℃±2℃) and lighting (7 AM to 7 PM) conditions with food and water available ad libitum. The mice were randomly divided into four groups (n=10 in each group), as follows: the control group, the control and exercise group, the scopolamine-induced amnesia group, and the scopolamine-induced amnesia and exercise group. All mice received 50 mg/kg BrdU (Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally once a day 30 minutes before performing treadmill exercises for five consecutive days from the start of the experiment.
Administration of Scopolamine
To induce amnesia, the mice were subcutaneously injected with 1 mg/kg scopolamine hydrobromide (Sigma Chemical Co.) dissolved in physiological saline, once a day for 14 consecutive days. The mice in the control group received saline instead of scopolamine. A step-down avoidance test was carried out 30 minutes after the last injection of scopolamine.
Treadmill Exercise Protocol
The mice in the two exercise groups were forced to run on a motorized treadmill for 30 minutes, once a day, for 14 consecutive days. Exercise load consisted of running at a speed of 5 meters/min with a 0° incline. The mice in the nonexercise groups were left on the treadmill without running, for the same duration as the exercise groups.
Step-Down Avoidance Test
Short-term memory was evaluated in the step-down avoidance test according to a previously described method [17,18]. Mice were positioned on a 7-cm×25-cm platform, at a height of 2.5 cm, and allowed to rest on the platform for 2 minutes. The platform faced a 42-cm×25-cm grid of parallel 0.1-cm caliber stainless steel bars, which were spaced 1 cm apart. In the training session, the animals received a 0.5-mA scramble foot shock for 3 seconds immediately upon stepping down. Retention time was assessed 24 hours after the training session. The time between being placed on the platform and stepping down and placing all four paws on the grid, was defined as the latency in the step-down avoidance task. If this latency was over 300 seconds, it was recorded as 300 seconds.
Tissue Preparation
The mice were sacrificed immediately after determining the latency in the step-down test. The mice were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were dissected and postfixed in the same fixative overnight, and transferred to a 30% sucrose solution for cryoprotection. Forty-micrometer-thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany). On average 10-slice sections in the hippocampus were collected from each mouse.
AChE Histochemistry
AChE histochemistry was performed according to a previously described method [19]. The sections were washed in PBS and incubated in a solution with 25 mg of acetylthiocholine iodine for 1 hour. This solution was composed of 32.5 mL of 0.1 M sodium hydrogen PB (NaH2PO4·H2O, pH 6.0), 2.5 mL of 0.1 M sodium citrate, 5 mL of 30 mM copper sulfate, 5 mL of 5 mM potassium ferricyanide, and 5 mL of distilled water. The color of the mixing solution was green. The density of the stained nuclei of the hippocampal CA1 region was measured using the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
Collagen IV Immunohistochemistry
Pepsin digestion was carried out on free-floating sections using a previously described method [20]. Prior to pepsin treatment, sections were incubated in distilled water for 5 minutes at 37℃ and then transferred to 1 mg/mL pepsin (Dako, Carpinteria, CA, USA) in 0.2 N HCl. Sections were incubated in the pepsin solution at 37℃ for 10 minutes. They were subsequently washed in PBS for 15 minutes at 27℃, followed by three 10 minutes washes at room temperature. The sections were then incubated overnight with rabbit anticollagen IV antibody (1:500; Abcam, Cambridge, Cambridgeshire, UK), and then for another 1 hour with biotinylated rabbit secondary antibody (1:100; Vector Laboratories, Burlingame, CA, USA). Bound secondary antibody was then amplified with Vector Elite ABC kit (Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% diaminobenzidine tetrahydro-chloride (DAB) and the sections were mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and cover slips were mounted using Permount (Fisher Scientific, Pittsburgh, PA, USA).
DCX Immunohistochemistry
Immunohistochemistry was performed for visualization of the expression of DCX, using a previously described method [21,22]. The brain sections were incubated overnight with a mouse anti-DCX antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and then for another 1 hour with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified with a Vector Elite ABC kit (Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% DAB. The sections were mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and the cover slips were mounted using Permount (Fisher Scientific).
Data Analysis
The number of DCX-positive cells in the dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and they were expressed as the numbers of cells per mm2 in the dentate gyrus. The data are expressed as the number of cells per mm2 of the hippocampus. The measurement of expression of AChE was defined as the optical density. The perimeter of blood vessels within the medial boundaries of the dentate gyrus was expressed as µm. Statistical analyses were performed using one-way analysis of variance followed by Duncan's post-hoc test. The results are presented as the mean±standard error of the mean (SEM). Significance was set as P<0.05.
RESULTS
Effect of Treadmill Exercise on Short-term Memory in Step-Down Avoidance Test
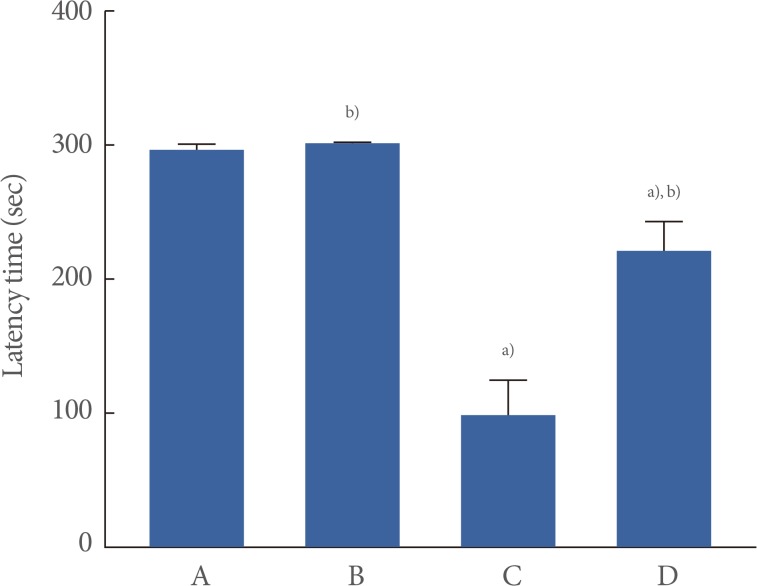
Short-term memory was measured using the step-down avoidance test (Fig. 1). The mean latency was 297.10±2.90 seconds in the control group, 300±0.00 seconds in the control and exercise group, 97.40±25.65 seconds in the scopolamine-induced amnesia group, and 219.11±21.74 seconds in the scopolamine-induced amnesia and exercise group.
Fig. 1.
The effect of treadmill exercise on short-term memory. (A) Control group, (B) control and exercise group, (C) scopolamine-induced amnesia group, and (D) scopolamine-induced amnesia and exercise group. The data are presented as the mean±standard error of the mean. a)P<0.05 compared to the control group. b)P<0.05 compared to the scopolamine-induced amnesia group.
These results revealed that short-term memory was significantly impaired due to induced amnesia (P<0.05). In contrast, treadmill exercise significantly improved short-term memory in the scopolamine-induced amnesia mice (P<0.05). In the normal mice, treadmill exercise exerted no significant effect on short-term memory.
Effect of Treadmill Exercise on Perimeter of Blood Vessels
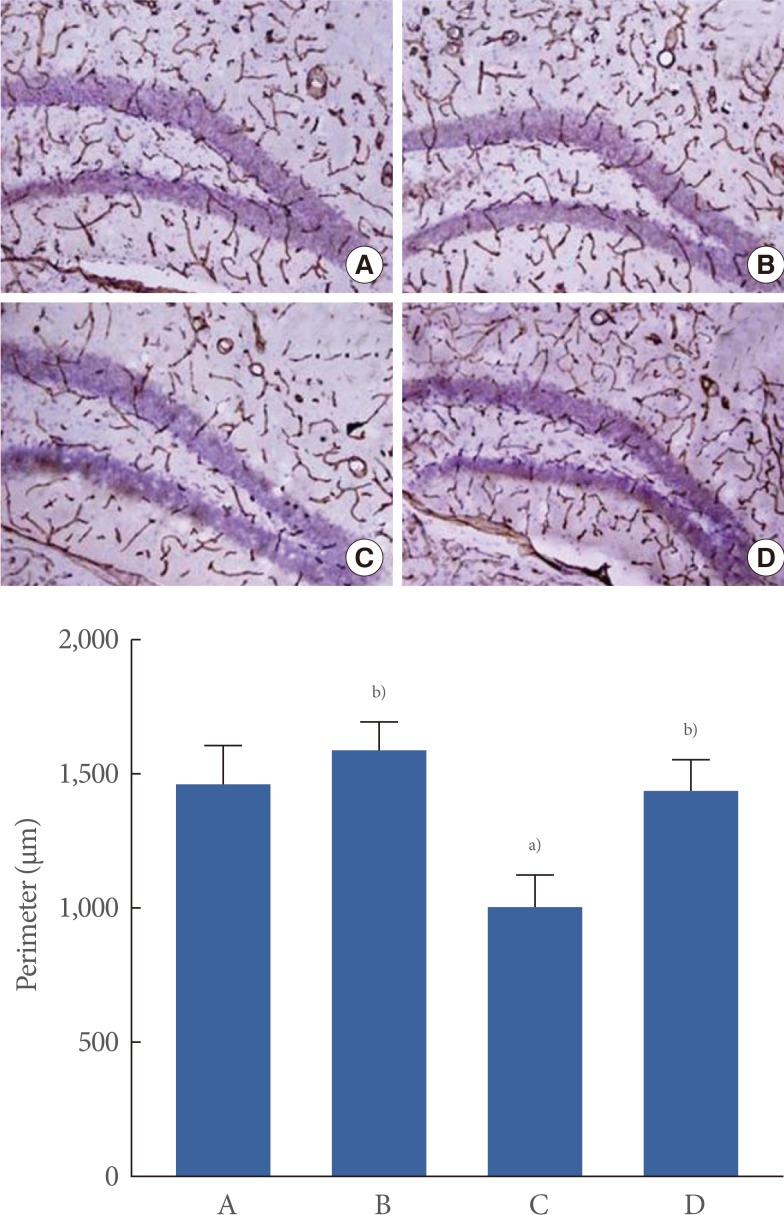
The mean perimeter of the blood vessels in the hippocampal dentate gyrus was 1,460±138.75 µm in the control group, 1,588.51±105.60 µm in control and exercise group, 998.87±122.94 µm in the scopolamine-induced amnesia group, and 1,429.97±114.11 µm in the scopolamine-induced amnesia and exercise group (Fig. 2).
Fig. 2.
The effect of treadmill exercise on the perimeters of cerebral blood vessels. (A) Control group, (B) control and exercise group, (C) scopolamine-induced amnesia group, and (D) scopolamine-induced amnesia and exercise group. (A-D) Acetylcholinesterase histochemistry was performed. The scale bar represents 100 µm. The data are presented as the mean±standard error of the mean. a)P<0.05 compared to the control group. b)P<0.05 compared to the scopolamine-induced amnesia group.
These results showed that the perimeter of blood vessels in the hippocampal dentate gyrus was decreased in the mice with scopolamine-induced amnesia (P<0.05). In contrast, treadmill exercise enhanced the perimeter of blood vessels in the mice with scopolamine-induced amnesia (P<0.05). In the control mice, treadmill exercise exerted no significant effect on the perimeter of blood vessels in the hippocampal dentate gyrus.
Effect of Treadmill Exercise on AChE Expression
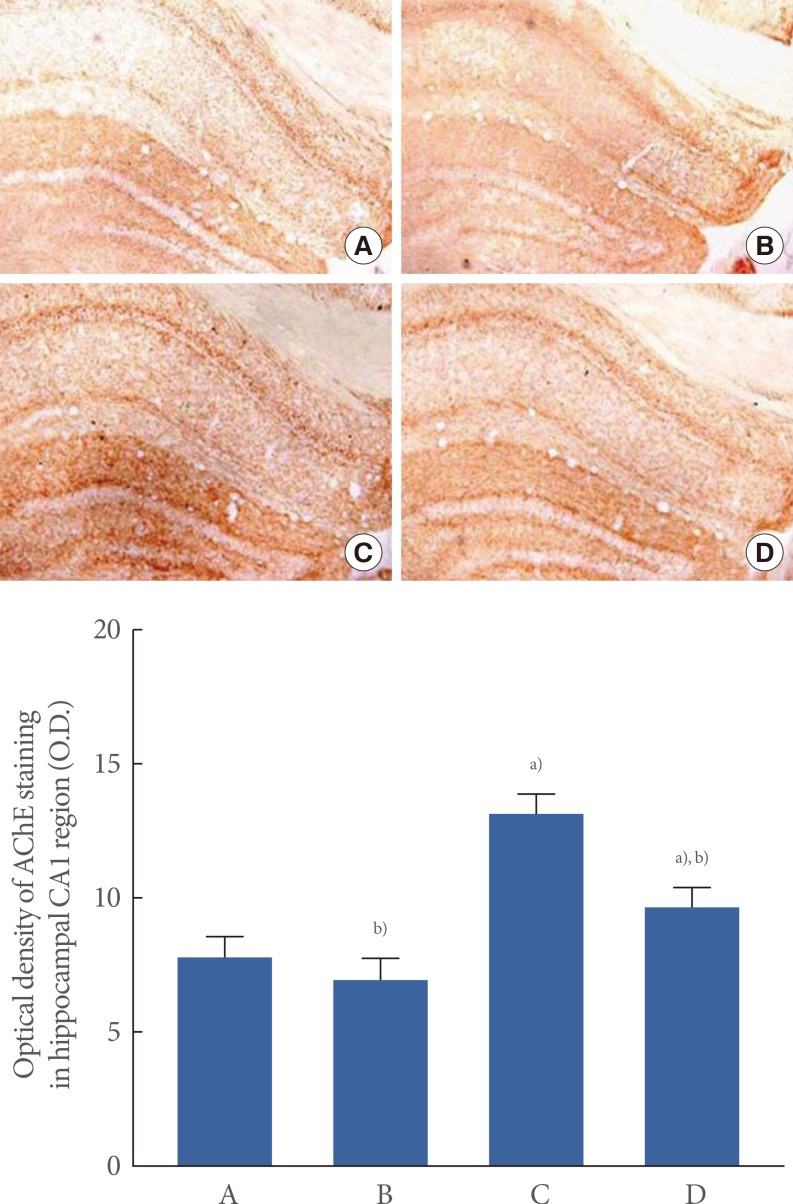
The optical density of AChE in the hippocampal CA1 region was 7.79±0.81 in the control group, 6.94±0.75, in the control and exercise group, 13.02±0.70 in the scopolamine-induced amnesia group, and 9.54±0.63 in the scopolamine-induced amnesia and exercise group (Fig. 3).
Fig. 3.
The effect of treadmill exercise on acetylcholinesterase (AChE) expression. (A) Control group, (B) control and exercise group, (C) scopolamine-induced amnesia group, and (D) scopolamine-induced amnesia and exercise group. (A-D) Collagen IV histochemistry was performed. The scale bar represents 200 µm. The data are presented as the mean±standard error of the mean. a)P<0.05 compared to the control group. b)P<0.05 compared to the scopolamine-induced amnesia group.
These results demonstrated that AChE expression in the hippocampal CA1 region was increased in the mice with scopolamine-induced amnesia (P<0.05). In contrast, treadmill exercise suppressed AChE expression in the mice with scopolamine-induced amnesia (P<0.05). In the control mice, treadmill exercise exerted no significant effect on AChE expression in the hippocampal CA1 region.
Effect of Treadmill Exercise on DCX Expression
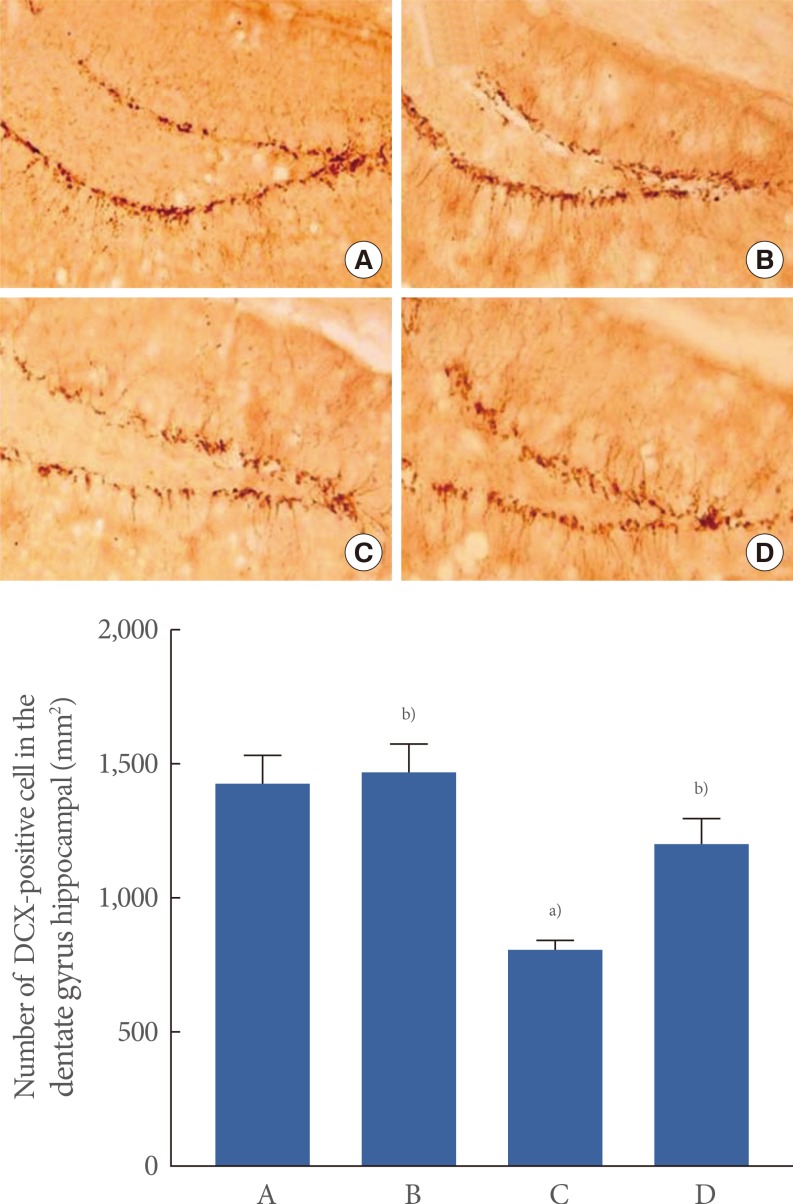
The number of DCX cells in the hippocampal dentate gyrus was 1,431.90±104.36/mm2 in the control group, 1,470.68±99.11/mm2 in the control and exercise group, 807.87±35.83/mm2 in the scopolamine-induced amnesia group, and 1,197.13±97.09/mm2 in the scopolamine-induced amnesia and exercise group. The expression of DCX is shown in Fig. 4.
Fig. 4.
The effect of treadmill exercise on doublecortin (DCX) expression in the hippocampal dentate gyrus. (A) Control group, (B) control exercise group, (C) scopolamine-induced amnesia group, and (D) scopolamine-induced amnesia and exercise group. (A-D) Doublecortin histochemistry was performed. The scale bar represents 200 µm. The data are presented as the mean±standard error of the mean. a)P<0.05 compared to the control group. b)P<0.05 compared to the scopolamine-induced amnesia group.
These data demonstrated that DCX expression in the hippocampal dentate gyrus was significantly decreased in the mice with scopolamine-induced amnesia (P<0.05). In contrast, treadmill exercise significantly increased DCX expression in the mice with scopolamine-induced amnesia (P<0.05). In the control mice, treadmill exercise exerted no significant effect on DCX expression in the hippocampal dentate gyrus.
DISCUSSION
Dementia is the most striking feature of patients with AD, and degeneration of cholinergic neurons in the central nervous system is correlated with the severity of dementia in patients with AD [23]. The central cholinergic system is closely associated with learning ability and memory function via the muscarinic and nicotinic receptors [24]. Scopolamine is known to inhibit ACh transmission, resulting in a deterioration of memory [25]. Here, we found that latency in the step-down avoidance test was shortened in mice due to the scopolamine-induced amnesia. In contrast, treadmill exercise extended the latency in mice with scopolamine-induced amnesia. Together, these results demonstrate that treadmill exercise ameliorates scopolamine-induced amnesia in mice.
It has been reported that scopolamine dysregulates cholinergic activity, which results in performance deficits on learning and memory tasks [26]. Scopolamine-induced memory impairments are associated with cholinergic dysfunction [27]. In the results of the present study, AChE expression was increased in the hippocampal CA1 region following scopolamine-injection, suggesting that destruction of ACh was enhanced by scopolamine. In contrast, treadmill exercise suppressed scopolamine-induced AChE expression in the hippocampal CA1 region. These results suggest that treadmill exercise suppresses the anti-amnestic effect of AchE, by inhibiting the destruction of ACh.
Expression of DCX can be used as a marker for immature neurons, therefore the level of DCX expression in the adult brain can be used to measure neurogenesis [28]. For example, Brown et al. [29] demonstrated that DCX expression decreased during aging in proportion to a decrease in neurogenesis. In the results reported here, the number of DCX-positive cells in the hippocampal dentate gyrus was decreased in the mice with scopolamine-induced amnesia, while treadmill exercise increased the number of DCX-positive cells in mice with induced amnesia. These findings suggest that cell proliferation was increased by treadmill exercise in the scopolamine-induced amnesia model in mice.
In the hippocampal dentate gyrus, new cells cluster close to blood vessels [30], and blood vessels are important for neurogenesis [13]. Physical exercise increases cerebral blood volume [31], cerebral blood flow [32], blood vessel perimeter, and angiogenesis [31,33]. For example, Lopez-Lopez et al. [34] reported that exercise improved cerebral blood volume and vessel remodeling in various brain regions, including the hippocampus. In the present results, the density of microvessels in the hippocampal dentate gyrus was decreased in the mice with scopolamine-induced amnesia, while treadmill exercise increased the density of microvessels in mice with scopolamine-induced amnesia. These results show that vascularization in the hippocampus was up-regulated by treadmill exercise.
Antimuscarinics are widely used for the treatment of voiding dysfunction in urology. Therefore, cognitive impairment related with the antimuscarinic medication is also considered as an important side effect in urology. AChE inhibitors are current standard for the care of AD, however they have side effects from indiscriminate activation of muscarinic and nicotinic receptors [35]. As shown in the present study, treadmill exercise suppressed AChE expression and enhanced vascularization without alteration of muscarinic and nicotinic receptors. Overexpression of AChE is implicated in both brain and renal disease, the findings of our study suggest that the suppression of AChE due to treadmill exercise may be of therapeutic value for these diseases.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-327-G00124).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Howes MJ, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer's disease and other cognitive disorders. Phytother Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- 2.Kim MJ, Choi SJ, Lim ST, Kim HK, Kim YJ, Yoon HG, et al. Zeatin supplement improves scopolamine-induced memory impairment in mice. Biosci Biotechnol Biochem. 2008;72:577–581. doi: 10.1271/bbb.70480. [DOI] [PubMed] [Google Scholar]
- 3.Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer's disease. Biol Psychiatry. 2001;49:175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- 4.Mulugeta E, Karlsson E, Islam A, Kalaria R, Mangat H, Winblad B, et al. Loss of muscarinic M4 receptors in hippocampus of Alzheimer patients. Brain Res. 2003;960:259–262. doi: 10.1016/s0006-8993(02)03542-4. [DOI] [PubMed] [Google Scholar]
- 5.Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6:e27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amenta F, Parnetti L, Gallai V, Wallin A. Treatment of cognitive dysfunction associated with Alzheimer's disease with cholinergic precursors: ineffective treatments or inappropriate approaches? Mech Ageing Dev. 2001;122:2025–2040. doi: 10.1016/s0047-6374(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 8.Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012;232:66–76. doi: 10.1016/j.bbr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- 10.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 11.Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci. 2008;11:429–439. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki T. Microenvironmental elements supporting adult hippocampal neurogenesis. Anat Sci Int. 2003;78:69–78. doi: 10.1046/j.0022-7722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, et al. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Baek SS, Jun TW, Kim KJ, Shin MS, Kang SY, Kim CJ. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain Dev. 2012;34:45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Kim SE, Ko IG, Park CY, Shin MS, Kim CJ, Jee YS. Treadmill and wheel exercise alleviate lipopolysaccharide-induced short-term memory impairment by enhancing neuronal maturation in rats. Mol Med Rep. 2013;7:31–36. doi: 10.3892/mmr.2012.1160. [DOI] [PubMed] [Google Scholar]
- 18.Cho HS, Shin MS, Song W, Jun TW, Lim BV, Kim YP, et al. Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson's rats. J Exerc Rehabil. 2013;9:354–361. doi: 10.12965/jer.130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooi EJ, Prins M, Bajic N, Belien JA, Gerritsen WH, van Horssen J, et al. Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol. 2011;122:313–322. doi: 10.1007/s00401-011-0849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franciosi S, De Gasperi R, Dickstein DL, English DF, Rocher AB, Janssen WG, et al. Pepsin pretreatment allows collagen IV immunostaining of blood vessels in adult mouse brain. J Neurosci Methods. 2007;163:76–82. doi: 10.1016/j.jneumeth.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friocourt G, Liu JS, Antypa M, Rakic S, Walsh CA, Parnavelas JG. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. J Neurosci. 2007;27:3875–3883. doi: 10.1523/JNEUROSCI.4530-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10:2–8. doi: 10.12965/jer.140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 24.Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behav Brain Res. 2011;221:505–514. doi: 10.1016/j.bbr.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 25.Park SJ, Kim DH, Jung JM, Kim JM, Cai M, Liu X, et al. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur J Pharmacol. 2012;676:64–70. doi: 10.1016/j.ejphar.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Elvander E, Schott PA, Sandin J, Bjelke B, Kehr J, Yoshitake T, et al. Intraseptal muscarinic ligands and galanin: influence on hippocampal acetylcholine and cognition. Neuroscience. 2004;126:541–557. doi: 10.1016/j.neuroscience.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 27.Sharma D, Puri M, Tiwary AK, Singh N, Jaggi AS. Antiamnesic effect of stevioside in scopolamine-treated rats. Indian J Pharmacol. 2010;42:164–167. doi: 10.4103/0253-7613.66840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 29.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 30.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 32.Yancey SL, Overton JM. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol (1985) 1993;75:1334–1340. doi: 10.1152/jappl.1993.75.3.1334. [DOI] [PubMed] [Google Scholar]
- 33.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uslaner JM, Eddins D, Puri V, Cannon CE, Sutcliffe J, Chew CS, et al. The muscarinic M1 receptor positive allosteric modulator PQCA improves cognitive measures in rat, cynomolgus macaque, and rhesus macaque. Psychopharmacology (Berl) 2013;225:21–30. doi: 10.1007/s00213-012-2788-8. [DOI] [PubMed] [Google Scholar]