Abstract
Potassium uptake by guard cells represents part of the osmotic motor which drives stomatal opening. Patch-clamp measurements have identified inward rectifying K+ channels capable of mediating K+ uptake in guard cells and various other plant cell types. Here we report the molecular cloning and characterization of a voltage-dependent K+ channel (KST1) from potato (Solanum tuberosum L.) guard cells. In situ hybridization shows expression of kst1 in guard cells. Two-electrode voltage-clamp and patch-clamp studies of the gene product after cRNA injection into Xenopus oocytes identified KST1 as a slowly activating, voltage-dependent, inward rectifying K+ channel. The single channel current voltage curve was linear in the range -160 to +20 mV, with a deduced single channel conductance of 7 pS in symmetrical 100 mM K+. This channel type, modulated by pH changes within the physiological range, required ATP for activation. In line with the properties of a K(+)-selective channel, KST1 was permeable to K+, Rb+ and NH4+ and excluded Na+ and Li+. Cs+ at submillimolar concentrations blocked the channel in a voltage-dependent manner. Related studies on potato guard cell protoplasts confirmed the biophysical characteristics of the kst1 gene product (KST1) in the heterologous expression system. Therefore, KST1 represents a major K+ uptake channel in potato guard cells.
Full text
PDF


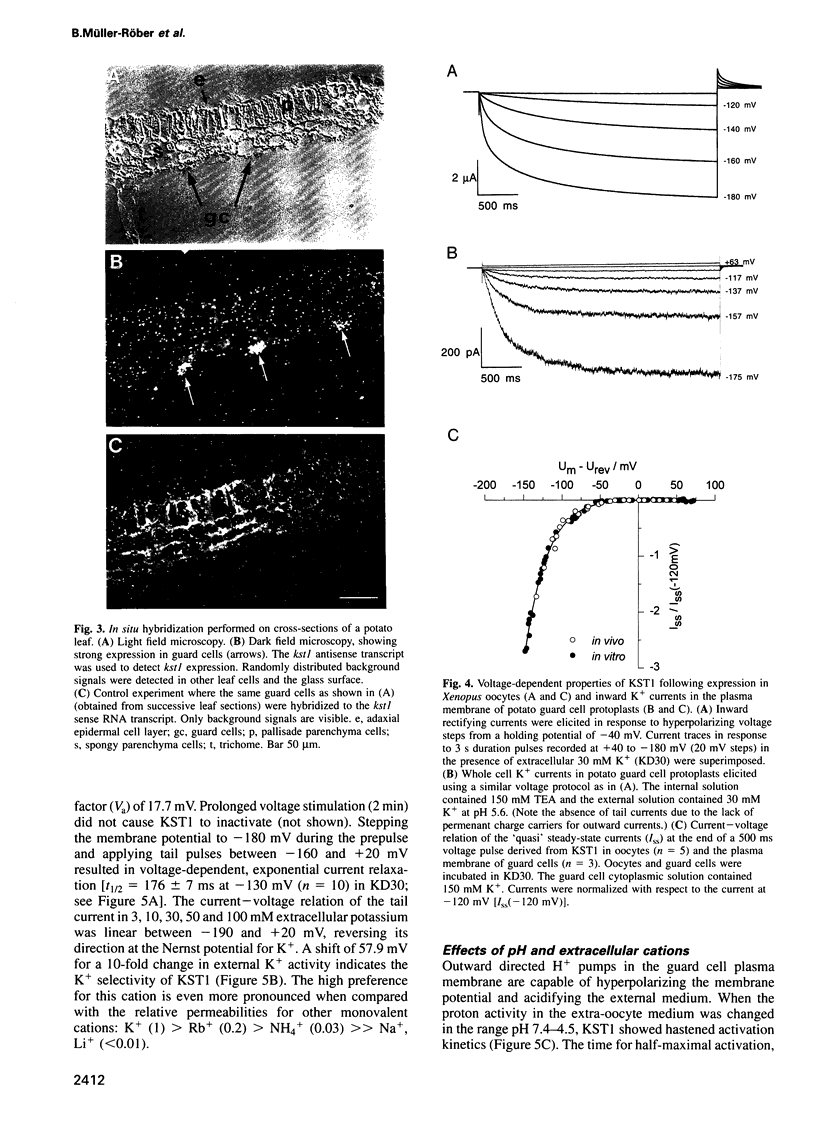
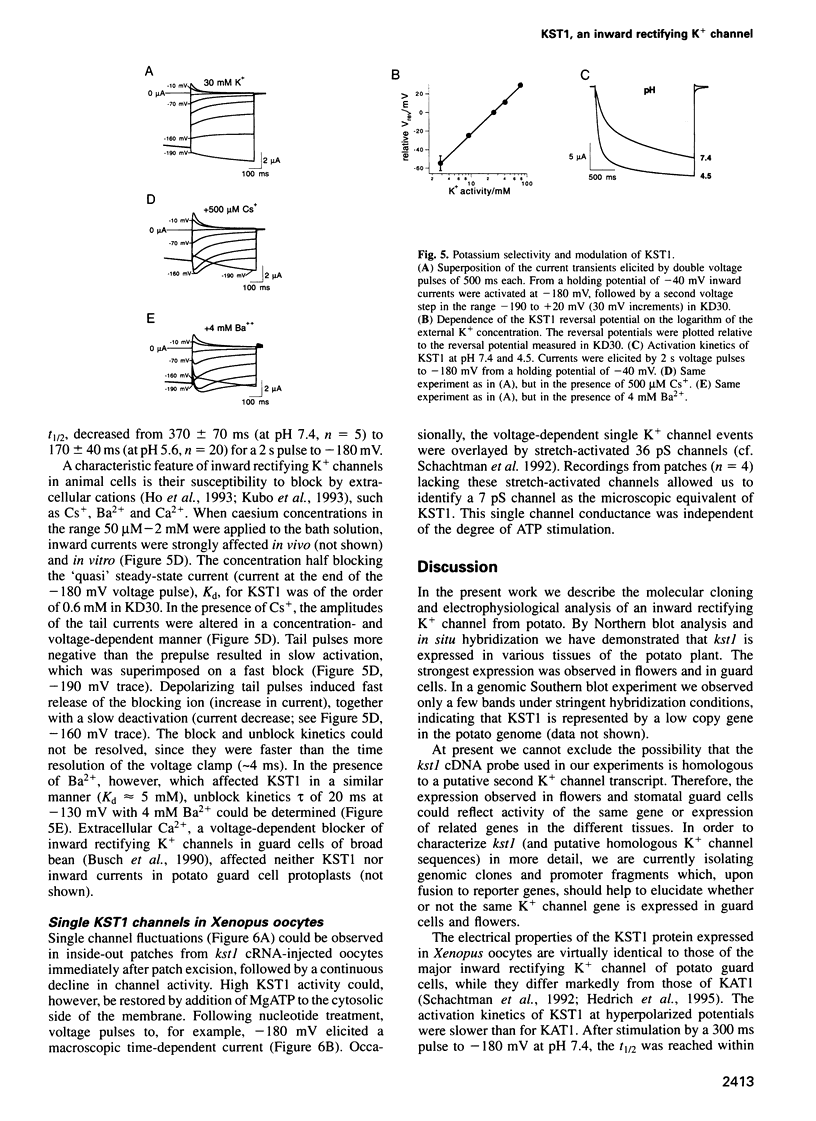
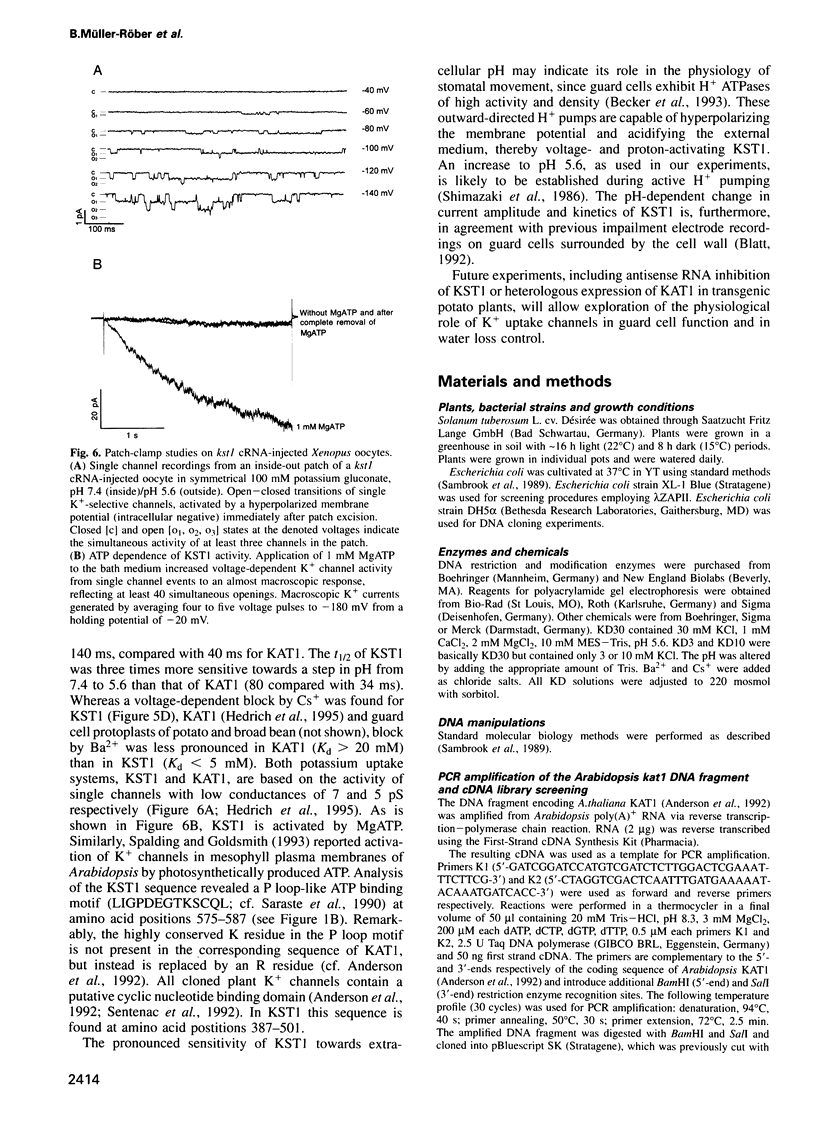


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. Potassium channels. Advent of a new family. Nature. 1993 Mar 11;362(6416):107–108. doi: 10.1038/362107a0. [DOI] [PubMed] [Google Scholar]
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Huprikar S. S., Kochian L. V., Lucas W. J., Gaber R. F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. H., Lynch J. W. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991 Apr;121(2):101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Blatt M. R. K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH. J Gen Physiol. 1992 Apr;99(4):615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Becker D. Green circuits--the potential of plant specific ion channels. Plant Mol Biol. 1994 Dec;26(5):1637–1650. doi: 10.1007/BF00016494. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Busch H., Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990 Dec;9(12):3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Liman E. R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992 Nov;9(5):861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Milkman R. An Escherichia coli homologue of eukaryotic potassium channel proteins. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3510–3514. doi: 10.1073/pnas.91.9.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Jan Y. N., Jan L. Y. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987 Aug 14;237(4816):749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Schroeder J. I., Lucas W. J., Anderson J. A., Gaber R. F. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992 Dec 4;258(5088):1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Schroeder J. I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994 Aug 25;370(6491):655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Schmelzer E., Kruger-Lebus S., Hahlbrock K. Temporal and Spatial Patterns of Gene Expression around Sites of Attempted Fungal Infection in Parsley Leaves. Plant Cell. 1989 Oct;1(10):993–1001. doi: 10.1105/tpc.1.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J. M., Gaymard F., Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992 May 1;256(5057):663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Siddik Z. H., Boxall F. E., Harrap K. R. Flameless atomic absorption spectrophotometric determination of platinum in tissues solubilized in hyamine hydroxide. Anal Biochem. 1987 May 15;163(1):21–26. doi: 10.1016/0003-2697(87)90087-x. [DOI] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Kawalleck P., Hahlbrock K. Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. Mol Gen Genet. 1988 Jul;213(1):93–98. doi: 10.1007/BF00333403. [DOI] [PubMed] [Google Scholar]
- Spalding E. P., Goldsmith MHM. Activation of K+ Channels in the Plasma Membrane of Arabidopsis by ATP Produced Photosynthetically. Plant Cell. 1993 Apr;5(4):477–484. doi: 10.1105/tpc.5.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Methfessel C., Sakmann B., Noda M., Numa S. Patch clamp characterization of sodium channels expressed from rat brain cDNA. Eur Biophys J. 1987;14(3):131–138. doi: 10.1007/BF00253837. [DOI] [PubMed] [Google Scholar]
- Véry A. A., Gaymard F., Bosseux C., Sentenac H., Thibaud J. B. Expression of a cloned plant K+ channel in Xenopus oocytes: analysis of macroscopic currents. Plant J. 1995 Feb;7(2):321–332. doi: 10.1046/j.1365-313x.1995.7020321.x. [DOI] [PubMed] [Google Scholar]