Abstract
That oestradiol can have both negative and positive feedback actions upon the release of gonadotropin-releasing hormone (GnRH) has been understood for decades. The vast majority of studies have investigated the effects of in vivo oestrogen administration. In the past decade, evidence has accumulated in many neuronal and non-neuronal systems that in addition to traditional genomic action via transcription factor receptors, steroids can also initiate effects rapidly via signaling cascades typically associated with the cell membrane. Here we review work examining the rapid actions of oestradiol on GnRH neurons, addressing the questions of dose dependence, receptor subtypes, signaling cascade and intrinsic and synaptic properties that are rapidly modulated by this steroid.
Introduction
Gonadotropin-releasing hormone (GnRH) neurons form the final common pathway for the central regulation of reproduction. GnRH stimulates release of the pituitary gonadotropins, which in turn activate gonadal steroidogenesis. These steroids complete both homeostatic and non-homeostatic feedback loops centrally to regulate GnRH release (1, 2). In the male and during most of the normal female reproductive cycle, homoeostatic negative feedback upon the frequency of GnRH release is enforced by oestradiol at low levels and, in the female, progesterone. Non-homeostatic feedback is confined to females during the preovulatory period and occurs in response to sustained elevations of oestradiol, which generate positive feedback to induce the preovulatory surge of GnRH release (3, 4).
Our understanding of oestradiol feedback has come largely from studies using in vivo treatments. More recently the availability of identified GnRH neurons has made electrophysiological approaches possible (5, 6). Using in vivo treatment followed by acute brain slice preparation, investigators have shown changes in GnRH neuron firing pattern, neurotransmission to GnRH neurons and function of specific ion channels in these cells in response to oestradiol (7–13). Most evidence indicates the alpha isoforms of the oestrogen receptor (ER) mediates these actions via classical binding to oestrogen response elements (14, 15). Because treatments in these studies were in vivo, it is not possible to parse the mechanisms mediating the effects. In this regard, steroids including oestradiol can engage nuclear transcription factor receptors, via classical and non-classical pathways, and subsequently alter gene expression to bring about effects (16). In addition, very rapid effects of steroids have been observed throughout the central nervous system (17, 18), including in GnRH neurons (19–22) and GT1 cells (23) as well as in the periphery (24). The rapid time course of these effects suggested they did not require the macromolecular synthesis typically associated with classical oestrogen signaling.
Receptors mediating rapid effects of oestradiol
A number of receptor subtypes have been proposed to mediate rapid actions of oestradiol. The classical nuclear subtypes oestrogen receptor alpha (ERα) and beta (ERß) have been demonstrated to engage signaling cascades typically associated with the cell membrane (25). This can occur via lipid modifications of the ERs, such as palmitoylation, allowing association with the lipid bilayer, or binding with caveolins. In addition to classical ERs, however, at least three putative transmembrane oestrogen receptors have been proposed to mediate rapid effects in the central nervous system: G-protein coupled receptor 30 (GPR30, (26)), ER-X (27), and mER (28). GnRH neurons themselves appear to express ERß (29), and GPR30 (30), but could potentially be influenced indirectly by all of these receptor subtypes in a rapid manner.
The rapid effects of high physiologic levels of oestradiol on GnRH neurons
The main approach that has been used to distinguish rapid from classical oestrogen action is one that uses a short treatment. This approach should minimize actions via genomic pathways, allowing non-genomic actions to be revealed. One note of caution is that genomic actions may well occur more quickly than typically currently thought; for example, promoter occupancy changes within one minute of oestradiol exposure on the crh gene (31). Nonetheless, this approach has been used to demonstrate rapid effects of oestrogens on GnRH neurons both in vivo and in vitro (21, 32–37). A majority of these studies demonstrate an increase in firing rate, calcium oscillation frequency or transcription factor phosphorylation, although inhibitory effects have also been reported (21). These studies have rarely examined dose dependence or neurobiological mechanism involved in these responses, however, necessitating further investigation.
To examine the non-genomic actions of oestradiol on GnRH neurons, we used acutely prepared brain slices from adult, short-term ovariectomised mice. The parameter of interest was first recorded under control conditions, and then native oestradiol 17ß was applied via the bath. After a five-minute wash in period, response was recorded during minutes 5–15 after initiation of oestradiol treatment, followed by a return to control solution and recording of responses as they returned to baseline levels. In our initial studies, we wished to focus on effects that might be mediated directly at the GnRH neuron, thus included blockers of ionotropic GABA and glutamate receptors in the bath solution at all times to isolate GnRH neurons from indirect effects mediated by fast synaptic transmission. Of note, this would not block effects via changes in neuromodulation.
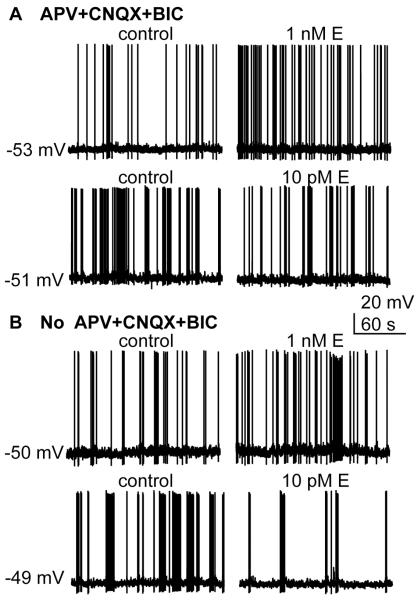
Under these recording conditions, oestradiol induced an increase in firing rate, observable within 2–3 minutes of application (38) (Figure 1A). The percentage of neurons responding and the percent increase in firing rate increased with dose (100 pM to 100 nM). Although steroids are lipophilic, drug penetration in brain slices is typically low due to their thickness. It is thus notable that these responses were observed at treatment levels (100 pM and 1 nM) that would be expected to produce physiological levels of oestradiol in the vicinity of the GnRH neuron based on measured circulating levels (39). Also of note, low physiological levels of oestradiol (10 pM) had no effect on firing rate under these conditions (Figure 1B).
Figure 1.
Rapid effects of oestradiol on GnRH neurons. (A) In the presence of blockers of ionotropic GABA and glutamate receptors (APV+CNQX+BIC) GnRH neurons are excited to fire action potentials at a higher rate by 1 nM oestradiol, but 10 pM oestradiol has no effect. (B) Without blockers , 1 nM oestradiol still increases firing rate but now inhibition is observed with 10 pM oestradiol GnRH neurons, presumably mediated by the upstream fast synaptic transmission network.
To explore further the mechanisms of this effect, a series of pharmacological and electrophysiological studies were done. The excitatory effect of oestradiol was blocked by the pure classical antagonist ICI182780, and mimicked by the ERß agonist DPN, but neither by the ERa agonist PPT, nor the GPR30 agonist G1 had any effect. Oestradiol or DPN also increased excitability of GnRH neurons, and in rare cases overt depolarization of membrane potential was observed in response to oestradiol after action potential firing had been blocked with tetrodotoxin (38). Together these observations suggest a rapid activation of GnRH neurons mediated via ERß expressed in these cells, although as noted above, indirect effects via neuromodulation cannot be eliminated.
In vivo work had indicated that CREB was rapidly phosphorylated in GnRH neurons in an ERß-dependent manner (37). Consistent with this, blockade of protein kinase A (PKA) blocked the ability of oestradiol to increase GnRH neuron firing rate (38). Via this kinase, and potentially others, oestradiol has been shown to rapidly alter conductance of several types of ion channels in GnRH neurons. Oestradiol rapidly increases the current underlying the slow afterdepolarization (sADP), which is carried by sodium (40), decreases calcium-activated potassium currents that underlie the afterhyperpolarization (38) and increased L and R type calcium channels via ERß and GPR30, respectively (41). The above three targets change in a direction that is consistent with increased activity of GnRH neurons and/or potentially increased neurosecretion in response to action potential generation in the case of the high-voltage gated calcium channels. It is important to point out that the story with regard to GRP30 is still evolving and species differences may well exist, with perhaps a more predominant role in the primate (30, 42). A recent study also demonstrated a rapid activation of ATP-sensitive potassium channels (KATP) (32), which would tend to decrease activity of GnRH neurons. Although this latter result may appear contradictory with excitation of GnRH neurons, it is important to point out that although the integrated response of the cell to higher doses of oestradiol appears to be excitatory, that is a net of all the changes that occur. Oestrogen modulation of KATP channels may play a critical role in response to negative energy balance as these channels can serve as energy sensors.
The rapid effects of low physiologic oestradiol levels
Under the recording conditions used above, 10 pM oestradiol had no effect on the firing activity of GnRH neurons. In the intact brain, however, fast synaptic transmission is not blocked as it was in the experimental conditions used above to reduce variables. We next investigated the effects of 10 pM vs 10 nM oestradiol when fast synaptic transmission was allowed to operate within the slice (38). As in the presence of ionontropic GABA and glutamate receptors, high levels of oestradiol excited GnRH neurons. In contrast to the situation in which fast synaptic transmission was blocked, however, low (10 pM) oestradiol now inhibited GnRH neurons in a rapid manner (Figure 1B). This effect was mimicked by the ERa agonist PPT but not the ERß nor GPR30 agonists, suggesting a separate mechanism. Consistent with this, low dose oestradiol had no effect on any GnRH neuron intrinsic property that was examined. This in combination with the need for active fast synaptic transmission suggested this effect was mediated upstream of GnRH neuron. Indeed the ERα agonist PPT was found to rapidly reduce GABAergic transmission. Because activation of GABAA receptors in these cells is primarily excitatory (43–45), this would reduce synaptic drive to fire action potentials. Interestingly, the ERß agonist DPN increased both GABAergic transmission and the postsynaptic response to GABA, both of which would support excitation in response to activation of this receptor. In other work, the alpha isoform of the estradiol receptor was found to mediate the ability of high (100 nM) estradiol to induce a delayed (~15 minute) increase in GABAergic transmission in some GnRH neurons, possibly consistent with the excitatory effects of high estradiol (13). This suggests that even within our current thinking of rapid vs. genomic actions of estradiol, subdivisions of different mechanisms engaged with different time courses and requiring different receptors may exist.
Summary and future directions
After a long period of dispute, there is now little question that steroid hormones can have rapid effects likely both directly on GnRH neurons and on their upstream network via multiple mechanisms (Figure 2). As with long-term effects, these rapid effects are dose-dependent, with higher levels of oestradiol being excitatory and lower inhibitory. A similar dose and receptor dependence of estradiol action was also found in cultured hypothalamic GnRH neurons (46). There are several questions remaining to address. How does endogenous oestradiol level affect the response to acute changes in oestradiol in the vicinity of the GnRH neuron? The vast majority of studies have utilized ovariectomized animals to remove interference from endogenous steroids, however this is clearly not the physiological situation. In the one case in which rapid effects of oestradiol were studied in brain slices from oestradiol-treated mice, the ability of oestradiol to increase high-voltage activated calcium currents was similar in both animal models, suggesting rapid changes in non-genomic estradiol signaling are not precluded in the presence of circulating estradiol (38). The effects on other aspects of this response are not known. How do oestradiol levels change in the vicinity of GnRH neurons? What, if any, is the physiological role of non-genomic estradiol action? And finally, how are the genomic and non-genomic effects integrated in vivo to produce the overall response to this steroid?
Figure 2.
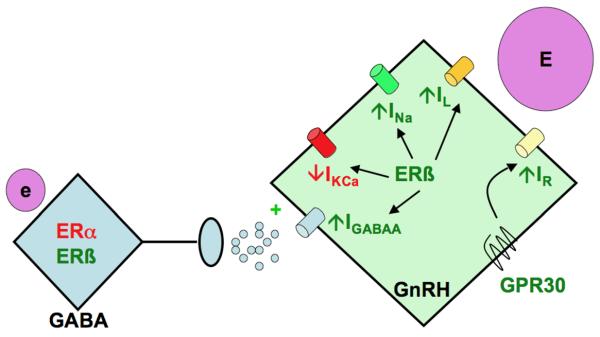
Model summarizing neurobiological mechanisms engaged by oestradiol to regulate GnRH neuron function. Low physiological levels of oestradiol (e) act upstream to alter GABAergic transmission to GnRH neurons. High physiological levels of oestradiol (E) can act directly via ERß and perhaps GPR30. Red font indicates inhibitory action, green font indicates stimulatory or excitatory action.
Abbreviations
- GnRH
gonadotropin-releasing hormone
- OVX
ovariectomised
- LH
luteinizing hormone
- TTX
tetrodotoxin
- ADP
afterdepolarization
- sADP
slow ADP
- AHP
afterhyperpolarization
- DPN
2,3-bis(4-hydroxyphenyl)-propionitrile
- PPT
propylpyrazoletriol
- APV
D(-)2-amino-5-phosphonovaleric acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
References
- 1.Christian CA, Moenter SM. The Neurobiology of Preovulatory and Estradiol-Induced Gonadotropin-Releasing Hormone Surges. Endocr Rev. 2010 doi: 10.1210/er.2009-0023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Docke F, Dorner G. The mechanism of the induction of ovulation by oestrogens. J Endocrinol. 1965;33:491–499. doi: 10.1677/joe.0.0330491. [DOI] [PubMed] [Google Scholar]
- 3.Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129:1175–1182. doi: 10.1210/endo-129-3-1175. [DOI] [PubMed] [Google Scholar]
- 4.Clarke IJ, Thomas GB, Yao B, Cummins JT. GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology. 1987;46:82–88. doi: 10.1159/000124800. [DOI] [PubMed] [Google Scholar]
- 5.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- 6.Spergel DJ, Ulrich D, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neuroscience. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christian CA, Moenter SM. Critical Roles for Fast Synaptic Transmission in Mediating Estradiol Negative and Positive Feedback in the Neural Control of Ovulation. Endocrinology. 2008;149:5500–5508. doi: 10.1210/en.2008-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian CA, Moenter SM. Vasoactive Intestinal Polypeptide Can Excite Gonadotropin-Releasing Hormone Neurons in a Manner Dependent on Estradiol and Gated by Time of Day. Endocrinology. 2008;149:3130–3136. doi: 10.1210/en.2007-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol Suppresses Glutamatergic Transmission to Gonadotropin-Releasing Hormone Neurons in a Model of Negative Feedback in Mice. Biol Reprod:biolreprod. 2009;108:075077. doi: 10.1095/biolreprod.108.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Bosch MA, Rick EA, Kelly MJ, Ronnekleiv OK. 17{beta}-Estradiol Regulation of T-Type Calcium Channels in Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romano N, Lee K, Abraham IM, Jasoni CL, Herbison AE. Nonclassical Estrogen Modulation of Presynaptic GABA Terminals Modulates Calcium Dynamics in Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone H-J, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets? Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 17.Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 18.Kelly MJ, Kuhnt U, Wuttke W. Hyperpolarization of hypothalamic parvocellular neurons by 17 beta-estradiol and their identification through intracellular staining with procion yellow. Experimental Brain Research. 1980;40:440–447. doi: 10.1007/BF00236152. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MJ, Ronnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002;67:447–456. doi: 10.1016/s0039-128x(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 20.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 21.Lagrange AH, Ronnekleiv OK, Kelly MJ. Estradiol-17 beta and mu-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 22.Herbison AE. Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology? The Journal of Physiology. 2009;587:5025–5030. doi: 10.1113/jphysiol.2009.179838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3',5'-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn ME, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Invest. 2010;120:2277–2279. doi: 10.1172/JCI43756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revankar CM, Cimino DF, Skalar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 27.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: A Novel, Plasma Membrane-Associated, Putative Estrogen Receptor That Is Regulated during Development and after Ischemic Brain Injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- 30.Terasawa E, Noel SD, Keen KL. Rapid Action of Oestrogen in Luteinising Hormone-Releasing Hormone Neurones: The Role of GPR30. Journal of Neuroendocrinology. 2009;21:316–321. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalmansingh AS, Uht RM. Estradiol Regulates Corticotropin-Releasing Hormone Gene (crh) Expression in a Rapid and Phasic Manner that Parallels Estrogen Receptor-{alpha} and - Recruitment to a 3',5'-Cyclic Adenosine 5'-Monophosphate Regulatory Region of the Proximal crh Promoter. Endocrinology. 2008;149:346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Kelly MJ, Ronnekleiv OK. 17{beta}-Estradiol Rapidly Increases KATP Activity in GnRH via a Protein Kinase Signaling Pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1 neurons. Endocrinology. 2008;149:5325–5327. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 37.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential Regulation of Gonadotropin-Releasing Hormone Neuron Activity and Membrane Properties by Acutely Applied Estradiol: Dependence on Dose and Estrogen Receptor Subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 40.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26:11961–11973. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Chu Z, Moenter SM. Diurnal In Vivo and Rapid In Vitro Effects of Estradiol on Voltage-Gated Calcium Channels in Gonadotropin-Releasing Hormone Neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G Protein-Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-Type {gamma}-Aminobutyric Acid Receptors Excites Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 44.Moenter SM, DeFazio RA. Endogenous gamma-aminobutyric acid can excite GnRH neurons. Endocrinology. 2005;146:5374–5379. doi: 10.1210/en.2005-0788. [DOI] [PubMed] [Google Scholar]
- 45.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABAA receptor activation on GnRH neurons: towards an emerging consensus. J Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02145.x. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu L, Gustofson RL, Feng H, Ki Leung P, Mores N, Krsmanovic LZ, Catt KJ. Converse Regulatory Functions of Estrogen Receptor-{alpha} and -{beta} Subtypes Expressed in Hypothalamic Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol. 2008;22:2250–2259. doi: 10.1210/me.2008-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]