Abstract
Purpose
Adherence to guideline-consistent chemotherapy-induced nausea and vomiting (CINV) prophylaxis is suboptimal. The primary aim of this study was to evaluate the magnitude of compliance to institutional guideline-directed antiemetic prophylaxis using a computerized physician order entry system at a single tertiary care institution. A nurse survey was also performed to evaluate how oncology practices, within a cooperative group, managed clinician orders for the prevention of CINV.
Methods
The electronic medical records of 100 consecutive patients were evaluated. The primary endpoint was the incidence of compliance to provide all aspects of scheduled institutional guideline-directed antiemetic prophylaxis for acute (day 1) and delayed (days 2–4) CINV. A descriptive analysis was performed on the convenience sample. Logistic regression was completed to determine the predictors of noncompliance.
Results
The incidence of compliance on days 1–4 was 94 %. Half of the noncompliant events (three of six, 50 %) occurred on day 1 alone and involved patients receiving low-emetogenic chemotherapy. There was a high degree of compliance to institutional guidelines for the treatment of delayed CINV (97 %). Patients receiving minimally emetogenic and moderately emetogenic chemotherapy (N =70) were observed to be 100 % compliant. Patients receiving doxorubicin/cyclophosphamide were numerically less likely to receive institutional guidelines, compared to patients receiving other chemotherapy regimens (OR, 0.24 (0.04, 1.36), p value, 0.05). The nurse survey suggested significant variability amongst the involved institutions with regards to antiemetic prescribing practices.
Conclusions
Computerized physician order entry is associated with impressive adherence to clinician-prescribing practices, according to institutional guidelines, for acute and delayed CINV.
Keywords: Chemotherapy-induced nausea and vomiting, Acute CINV, Delayed CINV, Antiemetics, Computerized physician order entry
Introduction
Various evidence-based guidelines have been published regarding the prevention of CINV [1–3], a common problem that adversely impacts patients’ quality of life [4]. Despite guideline statements and information that patients who do not receive adequate antiemetic prophylaxis have poorer outcomes and utilize greater healthcare resources, adherence to guideline-consistent CINV prophylaxis has often been reported to be suboptimal [5–9]. Methods to improve the implementation of antiemetic guidelines have included the use of education outreach visits [10], audit and feedback strategies [11], pharmacist-driven programs [12], and noncomputerized protocol-based antiemetic order sets [13, 14].
Evidence supports that computerized physician order entry for chemotherapy administration decreases medication errors and improves user satisfaction, completeness of documentation, and adherence to guideline-based therapies [15–17]. Currently, Mayo Clinic, Rochester utilizes an electronic clinical document manager, a form of computerized physician order entry, to prescribe inpatient and outpatient chemotherapy. Since 1995, institutional guideline-directed antiemetic prophylaxis order sets have automatically been provided when chemotherapy orders are chosen [14]. The clinician ordering chemotherapy has the option to take the guidelines as they are suggested or can deviate from the recommended antiemetic therapies per his/her discretion.
A literature review did not reveal any published evidence that computerized physician order entry improves antiemetic prescribing practices, leading to the development of the current project. The primary aim of this retrospective study was to evaluate the magnitude of compliance to institutional guideline-directed antiemetic prophylaxis using the current computerized physician order entry system at a single tertiary care institution. Given the ease of use of computerized physician order entry, it was hypothesized that there would be substantial compliance with the institutional recommendations.
To obtain further information regarding this topic, a nurse survey was also performed to evaluate how oncology practices within a cooperative group managed clinician orders for the prevention of CINV.
Site
Currently, the outpatient oncology practice at Mayo Clinic, Rochester consists of approximately 135 clinicians who prescribe approximately 5,650 doses of chemotherapy for 1,300 patients each month.
Patients and methods
The first 100 consecutive eligible outpatients (aged ≥18) receiving chemotherapy for solid tumor malignancies, starting from January 1, 2012, were studied. Evaluated patients must have been receiving their first ever chemotherapy dose at Mayo Clinic, Rochester, have only been receiving a single day of chemotherapy (no additional planned doses for at least 5 days), and must not have been receiving concurrent radiotherapy.
The study employed a single-institution retrospective design. All data were obtained from the comprehensive electronic medical records (EMR). EMR data included inpatient and outpatient clinic visit information, all prior chemotherapy and supportive medication administration logs, and all prescribed medications since a patient’s initial visit to the institution. Variables collected and analyzed included patient age and gender, primary cancer diagnosis, the presence of metastatic disease, chemotherapy regimen and protocol number, trial participation, and the respective chemotherapy regimens’ emetogenicity: highly emetogenic chemotherapy (HEC), moderately emetogenic chemotherapy (MEC), low emetogenic chemotherapy (LEC), or minimally emetogenic chemotherapy (MinEC).
The primary endpoint was the incidence of compliance to provide all aspects of scheduled institutional guideline-directed antiemetic prophylaxis for acute (day 1) and delayed (days 2–4) CINV. This included evidence of administration of antiemetics on the day of chemotherapy infusion as well as documentation of antiemetic prescriptions that were provided to the patient to be taken on days 2–4. Noncompliance was defined if a recommended antiemetic was not prescribed per the guidelines or if additional antiemetics were given beyond those recommended within the institutional guidelines. Table 1 illustrates the Mayo CINV guidelines in use at the time the studied patients were being treated. Secondary endpoints included subset analyses to determine if demographic or clinical characteristics had any significant association with compliance to institutional recommendations.
Table 1.
Mayo CINV guidelines in place at the study time
| Day 1 (acute phase) | Days 2–4 (delayed phase) | |
|---|---|---|
| Mayo CINV guidelinesa | ||
| HEC | Granisetron + Dex + aprepitant/fosaprepitant | Dex (days 2–4) + aprepitant (days 2–3) |
| MEC | Granisetron + Dex | Optional dex (days 2–3) |
| LEC | Prochlorperazine + optional Dex | None |
| MinEC | Optional pre-chemotherapy Prochlorperazine or Dex | None |
CINV chemotherapy-induced nausea and vomiting, HEC highly emetogenic chemotherapy, MEC moderately emetogenic chemotherapy, LEC low-emetogenic chemotherapy, MinEC minimally emetogenic chemotherapy, Dex dexamethasone
Based on Mayo CINV guidelines, last updated in January 2011. All day 1 antiemetics are given prior to chemotherapy administration. All prescriptions to be taken on days 2–4 are given to the patient on day 1. Antiemetics denoted as optional are given at the discretion of the prescribing clinician
In addition, a survey, conducted to identify how individual cooperative group oncology practices prescribed antiemetic prophylaxis, consisted of a five-item, relatively open-ended questionnaire was offered to nurse participants at the Alliance for Clinical Trials in Oncology Group Meeting on June 28, 2012. They were given the option to complete and return the survey at the end of the meeting.
The Mayo Clinic Institutional Review Board, according to US federal guidelines, approved all aspects of this study.
Statistical analysis
Descriptive statistics formed the basis for statistical analyses in this retrospective study with a convenience sample. Mean (standard deviation), median (range), frequency (percentage), and histograms were used to summarize data. Univariate logistic regression was performed to explore the association between potential factors and the compliance. Odds ratio with 95 % confidence interval and p values were reported. The sample size of this convenience sample was determined by interval estimation. Based on a noncompliance rate of 39 % on a previous report [7], a standard error of 4.9 % would be achieved with a sample size of 100 patients.
Results
A total of 290 patients from January 1, 2012 to March 7, 2012 were reviewed until 100 eligible patients were reached. Clinical and patient demographics, by overall compliance, are presented in Table 2.
Table 2.
Clinical and patient demographics by overall compliance
| Total (N =100) | Yes (N =94) | NO(N =6) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 61.4 (12.3) | 61.4 (12.4) | 61.7(11.9) |
| Median | 62.0 | 62.0 | 57.0 |
| Q1, Q3 | 53.0, 71.0 | 53.0, 71.0 | 53.0, 71.0 |
| Range | (31.0–90.0) | (31.0–90.0) | (51.0–81.0) |
| Gender | |||
| Male | 42 | 42 (100 %) | 0 (0 %) |
| Female | 58 | 52 (90 %) | 6 (10 %) |
| Primary cancer | |||
| Gynecological | 17 | 17 (100 %) | 0 (0 %) |
| Breast | 16 | 13 (81 %) | 3 (19 %) |
| Lung | 16 | 15 (94 %) | 1 (6 %) |
| CRC | 13 | 13 (100 %) | 0 (0 %) |
| Other | 38 | 36 (95 %) | 2 (0 %) |
| Chemotherapy emetogenicity | |||
| MinEC | 6 | 6 (100 %) | 0 (0.0 %) |
| LEC | 12 | 9 (75 %) | 3 (25 %) |
| MEC | 64 | 64 (100 %) | 0 (0.0 %) |
| HEC | 18 | 15 (83 %) | 3 (17 %) |
| Chemotherapy | |||
| Paclitaxel, carboplatin | 20 | 20 (100 %) | 0 (0.0 %) |
| Doxorubicin, cyclophosphamide | 13 | 10 (77 %) | 3 (23 %) |
| FOLFOX6 | 12 | 12 (100 %) | 0 (0 %) |
| Pemetrexed, Carboplatin | 10 | 10 (100 %) | 0 (0 %) |
| Other | 45 | 42 (93 %) | 3 (7 %) |
| Trial regimen | |||
| No | 93 | 87 (94 %) | 6 (6 %) |
| Yes | 7 | 7 (100 %) | 0 (0 %) |
| Metastatic disease | |||
| Yes | 46 | 44 (96 %) | 2 (4 %) |
| No | 54 | 50 (93 %) | 4 (7 %) |
SD standard deviation, CRC co-lorectal cancer, MinEC minimally emetogenic chemotherapy, LEC low-emetogenic chemotherapy, MEC moderately emetogenic chemotherapy, HEC highly emetogenic chemotherapy, FOLFOX6 folonic acid/5-fluoro-uracil/oxaliplatin
For the primary outcome, the incidence of compliance on days 1–4 was 94 %. Compliance rates separated by day are shown in Fig. 1. Table 3 presents compliance rates by chemotherapy emetogenicity for each day. Half of the noncompliant events occurred on day 1 alone and involved the omission of pre-chemotherapy prochlorperazine in patients receiving LEC (three of six, 50 %). Patients receiving MinEC and MEC were observed to be 100 % compliant with institutional guideline-directed antiemetic prophylaxis (N =70).
Fig. 1.
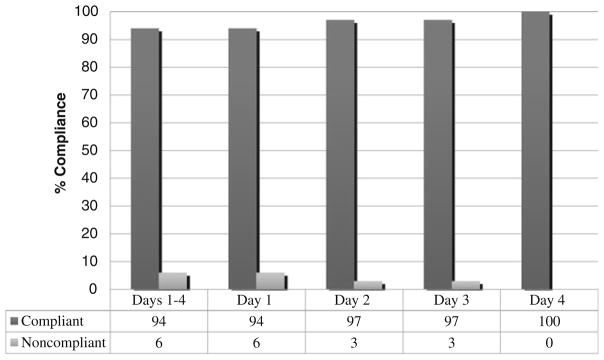
Compliance for scheduled antiemetics by day
Table 3.
Antiemetic compliance rates and reasons for noncompliance by chemotherapy emetogenicity and day
| Emetic grade | Days | Reasons for noncompliance | Compliance
|
|||
|---|---|---|---|---|---|---|
| Yes
|
No
|
|||||
| N | % | N | % | |||
| MinEC | 1–4 | 6 | 100 % | 0 | 0 % | |
| LEC | 1–4 | 9 | 75 % | 3 | 25 % | |
| 1 | 9 | 75 % | ||||
| Pre-chemotherapy ondansetron instead of prochlorperazinea | 2 | 17 % | ||||
| Pre-chemotherapy lorazepam instead of prochlorperazineb | 1 | 8 % | ||||
| 2 | 12 | 100 % | ||||
| 3 | 12 | 100 % | ||||
| 4 | 12 | 100 % | ||||
| MEC | 1–4 | 64 | 100 % | 0 | 0 % | |
| HEC | 1–4 | 15 | 83 % | 3 | 17 % | |
| 1 | 15 | 83 % | ||||
| Palonosetron (instead of granisetron) + Dex, no aprepitantc | 2 | 11 % | ||||
| Palonosetron (instead of granisetron) + Dex + Pre-chemotherapy lorazepam, no aprepitantc | 1 | 6 % | ||||
| 2 | 15 | 83 % | ||||
| No aprepitantc | 3 | 17 % | ||||
| 3 | 15 | 83 % | ||||
| No aprepitantc | 3 | 17 % | ||||
| 4 | 18 | 100 % | ||||
CINV chemotherapy-induced nausea and vomiting, HEC highly emetogenic chemotherapy, MEC moderately emetogenic chemotherapy, LEC low-emetogenic chemotherapy, MinEC minimally emetogenic chemotherapy, Dex dexamethasone
One of the two events was considered noncompliant due the omission of pre-chemotherapy prochlorperazine due to a documented medication interaction (tramadol). No reason for the other deviation was documented
This patient received pre-chemotherapy lorazepam, instead of prochlorperazine. No reason for this deviation was documented
These noncompliant events constituted three different patients that were not prescribed aprepitant on day 1 and days 2–3 by the same provider. These patients received all other Mayo CINV guideline antiemetics on day 1 and days 2–4
A univariate logistic regression was performed with results summarized in Table 4. There was a trend towards patients receiving HEC or MEC to be more likely to receive institutional guideline-directed antiemetic prophylaxis than those receiving LEC or MinEC (Odds Ratio [OR], 5.27)(0.97, 28.62), p value, 0.06). Patients receiving doxorubicin/ cyclophosphamide were numerically less likely to receive institutional guideline-directed antiemetic prophylaxis compared to patients receiving other chemotherapy regimens (OR, 0.24 (0.04, 1.36), p value, 0.05).
Table 4.
Univariate logistic odds ratios for predictors of noncompliance
| Variables | Odds ratio | Lower 95 % confidence limit for odds ratio | Upper 95 % confidence limit for odds ratio | Standard error | Pr>ChiSq | p value |
|---|---|---|---|---|---|---|
| Age | 1.00 | 0.93 | 1.07 | 0.03 | 0.96 | 0.96 |
| Doxorubicin, cyclophosphamide vs. other | 0.24 | 0.04 | 1.36 | 59.62 | 0.90 | 0.05 |
| Male vs. female | 3.2 | 0.64 | 15.91 | 0.41 | 0.16 | 0.12 |
| HEC and MEC vs. LEC and MinEC | 5.27 | 0.97 | 28.62 | 0.43 | 0.05 | 0.06 |
| Metastatic disease, yes vs. no | 1.76 | 0.31 | 10.08 | 0.45 | 0.53 | 0.52 |
| Primary cancer, breast vs. other | 0.24 | 0.04 | 1.61 | 45.47 | 0.91 | 0.15 |
| Primary cancer, CRC vs. other | >999.9 | <0.001 | >999.9 | 140.10 | 0.96 | |
| Primary cancer, gynecological vs. other | >999.9 | <0.001 | >999.9 | 124.50 | 0.96 | |
| Primary cancer, lung vs. other | 0.83 | 0.07 | 9.90 | 45.48 | 0.93 | |
| Trial regimen, yes vs. no | >999.9 | <0.001 | 1 | 192.3 | 0.98 | 0.34 |
MinEC minimally emetogenic chemotherapy, LEC low-emetogenic chemotherapy, MEC moderately emetogenic chemotherapy, HEC highly emetogenic chemotherapy, CRC colorectal cancer
What were the reasons for the deviations observed in six patients who were not strictly compliant with institutional guidelines? Three of these patients, receiving LEC, were to be given pre-chemotherapy prochlorperazine by the institutional guidelines but were not given such; each of them, however, was given a prescription of PRN prochlorperazine for breakthrough CINV. One patient was given pre-chemotherapy ondansetron (rather than prochlorperazine) due to the fact that the patient was taking tramadol, which has a known drug interaction with prochlorperazine. Another two patients receiving LEC were given pre-chemotherapy ondansetron or lorazepam rather than the recommended prochlorperazine, without an apparent explanation in the clinical notes. Lastly, one provider chose to administer, to three patients, pre-chemotherapy palonosetron on day 1 and dexamethasone on days 1–4 instead of institutional guidelines for a HEC regimen (doxorubicin/ cyclophosphamide). These three patients accounted for the only observed noncompliant events on days 2–3, as they were not given oral aprepitant on days 2–3.
With regards to the nurse survey information, evaluable data were obtained from 19 surveys. There was significant variability amongst the involved institutions with regards to antiemetic prescribing practices, which included the use of institutional-directed protocols, nursing or pharmacist-directed protocols, provider discretion alone, or a combination of these. Individuals from 15 of 19 institutions stated that their chemotherapy and supportive medications were ordered via an electronic order system. Of these institutions, nine stated that they utilized automated antiemetic order sets that were imported with each chemotherapy regimen. Only 3 of 19 institutions stated that the cancer provider, independent of any particular antiemetic protocol, prescribed antiemetics at his/her own discretion.
Discussion
Among patients receiving MinEC, MEC, and HEC regimens in this single-institution retrospective study, significant compliance to scheduled institutional guideline-directed antiemetic prophylaxis was observed. These observational data support that a well-organized and simple-to-use computerized physician order entry system does standardize the prescribing practices for antiemetics and should, thereby, improve the management of acute and delayed CINV compared to clinicians prescribing antiemetics at their own discretion.
The actual guideline variations in the reviewed experience were relatively mild ones. The omission of pre-chemotherapy prochlorperazine for LEC was the most common deviation from institutional guidelines and does not appear to be very egregious as the majority of patients also received PRN prochlorperazine for breakthrough CINV. The use of pre-chemotherapy ondansetron, instead of prochlorperazine, for LEC was uncommon and was very appropriate in one patient who was receiving tramadol. The decision by one provider to use palonosetron on day 1 and dexamethasone on days 1–4 instead of using granisetron on day 1, plus dexamethasone on days 1–4, plus aprepitant on days 1–3, for HEC was a reasonable alternative to the institutional guidelines. Previously reported studies support, by cross-study comparisons, that palonosetron might give similar protection as does aprepitant plus granisetron [18–20].
A notable finding in the current study was the high degree of compliance to antiemetic prophylaxis for delayed CINV (days 2–4) for all emetogenic grades of chemotherapy. This is in stark contrast to what has been observed in the literature where adherence to delayed CINV prophylaxis has been reported to be poor [5–8, 21]. The utilized system minimizes the need for most providers to independently consider the specific combination and dosing of antiemetics for each chemotherapy regimen and decreases the underuse of antiemetic prophylaxis for both acute and delayed CINV. It, however, does allow an individual provider to “opt-out” of the recommended anti-emetics if the provider favors an alternative approach. It should not be expected that there would be 100 % compliance with guidelines as there may be clinical situations that dictate that an alternative approach is appropriate for some patients.
Multiple studies have noted the undertreatment of patients receiving HEC and the overtreatment of patients receiving LEC/MinEC [6, 22–24]. It appears that the currently described system minimizes these types of events.
A computerized physician order entry system to promote standardized administration of both chemotherapy and supportive medications requires a significant multidisciplinary effort to initiate, maintain, and minimize errors within such a system [15–17, 25]. As such, many university and community oncology practices have yet to establish such systems.
Recent data from the United Kingdom suggest significant heterogeneity in antiemetic prescription practices amongst cancer providers there [26]. In this study of 154 oncologists and oncology nurse prescribers, there was a significant deviation from evidence-based consensus guidelines as well as marked variability amongst individual prescribing practices. Interestingly, a significant proportion of the institutions that completed the survey were noted to have established EMRs with electronic ordering systems.
The main limitations of this study are inherent to the retrospective design. As data were abstracted from the EMR, no information on patient adherence or outcomes can be established. As there was a high degree of provider compliance to institutional guideline-directed antiemetic recommendations, further research within a setting utilizing a similar system might help to determine if these prescribing practices translate into improved patient outcomes.
Though the most efficacious and cost-effective antiemetic regimens for CINV continue to be elucidated [27], the need to minimize physician-related barriers to guideline implementation remains. The barriers to guideline implementation have been divided into issues related to attitudes (lack of belief and/or disagreement), behavior (practice characteristics and/or patient preferences), and knowledge (lack of awareness and/or familiarity) [11, 28]. Evidence-based consensus guidelines linked to a computerized physician order entry system remove many of these barriers and can improve clinician-prescribing practices. The current study provides evidence to support that such a system can improve adherence to guideline-based CINV prophylaxis.
Acknowledgments
Research Grants: This work was supported by the United States National Institutes of Health Grant-CA 124477 (PI Charles Loprinzi MD).
Contributor Information
Kunal C. Kadakia, Email: kunkadak13@gmail.com, Division of Internal Medicine, Mayo Clinic, 200 1st Street S.W., Rochester, MN 55905, USA
Alexis D. Leal, Division of Internal Medicine, Mayo Clinic, 200 1st Street S.W., Rochester, MN 55905, USA
Drew K. Seisler, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA
Rui Qin, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA.
Kelliann C. Fee-Schroeder, Department of Oncology, Mayo Clinic, Rochester, MN, USA
Darryl C. Grendahl, Division of Medical Oncology, Mayo Clinic, 200 1st Street S.W., Rochester, MN 55905, USA
Kristine M. Sorgatz, Department of Oncology, Mayo Clinic, Rochester, MN, USA
Charles L. Loprinzi, Email: cloprinzi@mayo.edu, Division of Medical Oncology, Mayo Clinic, 200 1st Street S.W., Rochester, MN 55905, USA
References
- 1.NCCN. [Accessed 19 May 2012];Antiemesis. 2012 http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Version 1. 2012.
- 2.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(31):4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2010;21(Suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, Suarez C, Zorrilla I, Gomez J, Zabaleta P, Nocea G, Llombart-Cussac A. Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer. 2012;20(12):3141–3148. doi: 10.1007/s00520-012-1448-1. [DOI] [PubMed] [Google Scholar]
- 5.Drug Utilization Review Team in Oncology (DURTO) . Antiemetic prescription in Italian breast cancer patients submitted to adjuvant chemotherapy. Support Care Cancer. 2003;11(12):785–789. doi: 10.1007/s00520-003-0478-0. [DOI] [PubMed] [Google Scholar]
- 6.Aapro M, Molassiotis A, Dicato M, Pelaez I, Rodriguez-Lescure A, Pastorelli D, Ma L, Burke T, Gu A, Gascon P, Roila F. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER) Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012 doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 7.Burmeister H, Aebi S, Studer C, Fey MF, Gautschi O. Adherence to ESMO clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting. Support Care Cancer. 2012;20(1):141–147. doi: 10.1007/s00520-010-1079-3. [DOI] [PubMed] [Google Scholar]
- 8.Fabi A, Barduagni M, Lauro S, Portalone L, Mauri M, Marinis F, Narduzzi C, Tonini G, Giampaolo M, Pacetti U, Paoloni F, Cognetti F. Is delayed chemotherapy-induced emesis well managed in oncological clinical practice? An observational study. Support Care Cancer. 2003;11(3):156–161. doi: 10.1007/s00520-002-0427-3. [DOI] [PubMed] [Google Scholar]
- 9.Grunberg SM. Obstacles to the implementation of antiemetic guidelines. J Natl Compr Cancer Netw JNCCN. 2009;7(5):601–605. doi: 10.6004/jnccn.2009.0040. [DOI] [PubMed] [Google Scholar]
- 10.Roila F. Transferring scientific evidence to oncological practice: a trial on the impact of three different implementation strategies on antiemetic prescriptions. Support Care Cancer. 2004;12(6):446–453. doi: 10.1007/s00520-003-0553-6. [DOI] [PubMed] [Google Scholar]
- 11.Mertens WC, Higby DJ, Brown D, Parisi R, Fitzgerald J, Benjamin EM, Lindenauer PK. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(7):1373–1378. doi: 10.1200/JCO.2003.08.118. [DOI] [PubMed] [Google Scholar]
- 12.Dranitsaris G, Leung P, Warr D. Implementing evidence based antiemetic guidelines in the oncology setting: results of a 4-month prospective intervention study. Support Care Cancer. 2001;9(8):611–618. doi: 10.1007/s005200100273. [DOI] [PubMed] [Google Scholar]
- 13.Nolte MJ, Berkery R, Pizzo B, Baltzer L, Grossano D, Lucarelli CD, Kris MG. Assuring the optimal use of serotonin antagonist antiemetics: the process for development and implementation of institutional antiemetic guidelines at Memorial Sloan-Kettering Cancer Center. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16(2):771–778. doi: 10.1200/JCO.1998.16.2.771. [DOI] [PubMed] [Google Scholar]
- 14.Loprinzi CL, Alberts SR, Christensen BJ, Hanson LJ, Farley DR, Broers JK, Betcher DL, Grady RE, Southorn PA, Johnson TM, Perez EA. History of the development of antiemetic guidelines at Mayo Clinic Rochester. Mayo Clinic Proc Mayo Clinic. 2000;75(3):303–309. doi: 10.4065/75.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Chen AR, Lehmann CU. Computerized provider order entry in pediatric oncology: design, implementation, and outcomes. J Oncol Pract Am Soc Clin Oncol. 2011;7(4):218–222. doi: 10.1200/JOP.2011.000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harshberger CA, Harper AJ, Carro GW, Spath WE, Hui WC, Lawton JM, Brockstein BE. Outcomes of computerized physician order entry in an electronic health record after implementation in an outpatient oncology setting. J Oncol Pract Am Soc Clin Oncol. 2011;7(4):233–237. doi: 10.1200/JOP.2011.000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sklarin NT, Granovsky S, O’Reilly EM, Zelenetz AD. Electronic chemotherapy order entry: a major cancer center’s implementation. J Oncol Pract Am Soc Clin Oncol. 2011;7(4):213–218. doi: 10.1200/JOP.2011.000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer. 2011;19(6):823–832. doi: 10.1007/s00520-010-0908-8. [DOI] [PubMed] [Google Scholar]
- 19.Celio L, Denaro A, Agustoni F, Bajetta E. Palonosetron plus 1-day dexamethasone for the prevention of nausea and vomiting due to moderately emetogenic chemotherapy: effect of established risk factors on treatment outcome in a phase III trial. J Support Oncol. 2012;10(2):65–71. doi: 10.1016/j.suponc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10(2):115–124. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- 21.De Angelis V, Roila F, Patoia L, Lopez M, Cetto GL, Sabbatini R, Carreca I, Manente P, Del Favero A. Impact on antiemetic prescriptions of the Consensus Conference (CC) and of an expert’s visit to oncological centers. Proc Am Soc Clin Oncol. 2000;19:606a. (abstr 2386) [Google Scholar]
- 22.Italian Group for Antiemetic Research . Transferability to clinical practice of the results of controlled clinical trials: the case of antiemetic prophylactic treatment for cancer chemotherapy-induced nausea and vomiting. Ann Oncol Off J Eur Soc Med Oncol ESMO. 1998;9(7):759–765. [PubMed] [Google Scholar]
- 23.Italian Group for Antiemetic Research . Cancer patients submitted to innovative chemotherapeutic agents of intermediate emetogenic potential: antiemetic prescriptions and incidence of emesis. Tumori. 2004;90:103–106. doi: 10.1700/210.2345. [DOI] [PubMed] [Google Scholar]
- 24.Glaus A, Knipping C, Morant R, Bohme C, Lebert B, Beldermann F, Glawogger B, Ortega PF, Husler A, Deuson R. Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer. 2004;12(10):708–715. doi: 10.1007/s00520-004-0662-x. [DOI] [PubMed] [Google Scholar]
- 25.Mertens MC, Cassells LJ, Brown DE, Koertge V, Cabana L, Parisi R, Naglieri-Prescod D, Higby DJ. Chemotherapy ordering in a computerized physician order entry (CPOE) environment: a longitudinal analysis of defects from oncologist to patient. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006;24 (June 20 Supplement):18S. [Google Scholar]
- 26.Molassiotis A, Brearley SG, Stamataki Z. Use of antiemetics in the management of chemotherapy-related nausea and vomiting in current UK practice. Support Care Cancer. 2011;19(7):949–956. doi: 10.1007/s00520-010-0909-7. [DOI] [PubMed] [Google Scholar]
- 27.Roscoe JA, Heckler CE, Morrow GR, Mohile SG, Dakhil SR, Wade JL, Kuebler JP. Prevention of delayed nausea: a university of Rochester cancer center community clinical oncology program study of patients receiving chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(27):3389–3395. doi: 10.1200/JCO.2011.39.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA J Am Med Assoc. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]


