Abstract
For decades, researchers have focused primarily on a pathway initiated by beta-amyloid (Aβ) aggregation, amyloid deposition, and accumulation in the brain as the key mechanism underlying the disease and the most important treatment target. However, evidence increasingly suggests that amyloid is deposited early in the course of disease, even prior to the onset of clinical symptoms; thus, targeting amyloid in mild-to-moderate patients, as past failed clinical trials have done, may be insufficient to halt further disease progression. Scientists are investigating other molecular and cellular pathways and processes that contribute to AD pathogenesis. Thus, the Alzheimer’s Association’s Research Roundtable convened a meeting in April 2012 to move beyond amyloid and explore AD as a complex multi-factorial disease, with the goal of using a more inclusive perspective to identify novel treatment strategies.
Introduction
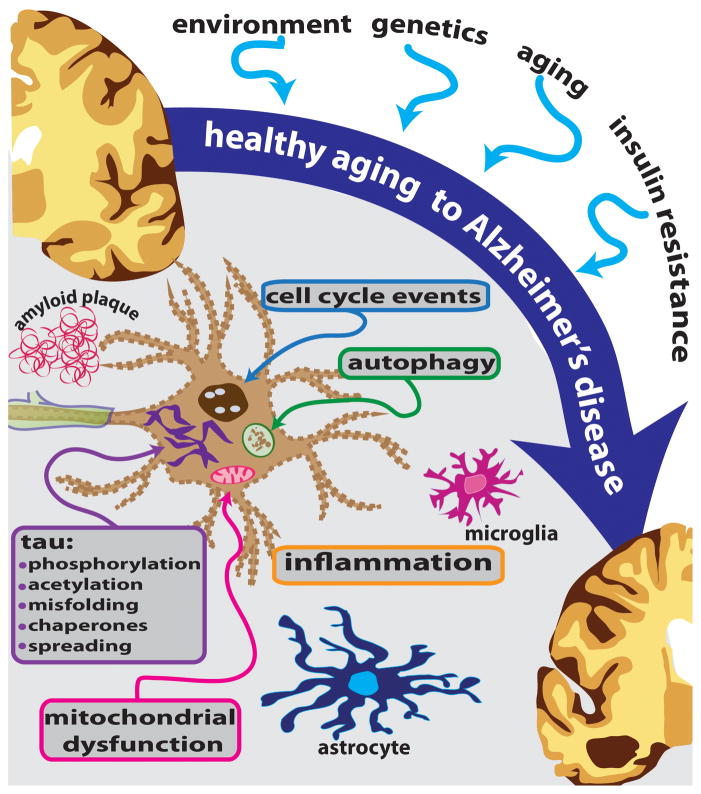
Alzheimer’s disease is currently estimated to affect more than 30 million individuals worldwide. Unless means are found to prevent, slow, or cure the disease, the prevalence is projected to quadruple by 2050 as the world’s population ages. Given the increasingly appreciated multi-factorial nature of the disease, a combination treatment regimen targeting two or more aspects of pathology (e.g., amyloid and tau, autophagy and inflammation, etc.) may be required for effective treatment. Accordingly, scientists are investigating, with heightened urgency, other molecular and cellular pathways and processes that contribute to AD pathogenesis (see Figure 1).
Figure 1.
AD is a complex disease influenced by many factors, and exhibiting alterations in multiple cellular pathways and processes. These mechanisms offer additional therapeutic targets for development of potential disease-modifying strategies.
The Alzheimer’s Association Research Roundtable (AARR) previously addressed non-amyloid targets in 2006 [1], focusing on microtubule (MT) stabilization, primarily achieved by inhibiting the phosphorylation of tau. The action of two kinases -- glycogen synthase kinase 3-β (GSK3β) and cyclin-dependent kinase 5 (CDK5) – received particularly close attention. The therapeutic potential of MT stabilizing drugs had been proposed back in 1994, when Lee et al. presented evidence that the loss of function of tau could have deleterious consequences by depolymerizing MTs, thereby disrupting axonal transport and compromising the function and viability of affected neurons in AD [2]. Since that time, increasing evidence in AD mouse models points to the therapeutic potential of microtubule stabilizing agents [3–5]. Other tau-based approaches discussed in 2006 included preventing the accumulation of phopho-tau and neurofibrillary tangles (NFTs). A few non-tau based strategies were also discussed, including the use of statins, anti-inflammatory agents and antioxidants, and the benefits of environmental enrichment.
As became evident at the 2012 Research Roundtable, tau is still very much alive as an AD therapeutic target, although the picture has become increasingly complex. Moreover, there are many other factors that contribute to the widespread neuronal degeneration and cell death seen in the AD brain. Other aspects of the cell death process that offer additional potential therapeutic targets include autophagy, vesicular trafficking, cell cycle disruption, systemic and cerebral inflammation, mitochondrial dysfunction, calcium homeostasis, insulin resistance, and alterations in chaperone proteins
Multiple factors involved in AD
Since Alois Alzheimer first described the neuropathological features of AD in 1906 [6], a neuropathological diagnosis at autopsy demonstrating the presence of amyloid plaques and NFTs has been the “gold standard” against which other diagnostic tools are compared. Using this gold standard, a recent study conducted by National Alzheimer’s Coordinating Center (NACC) showed that the median sensitivity of clinical diagnosis was about 87% and the median specificity only about 58% [7]. In other words, there is a high rate of misdiagnosis, and the neuropathological criteria fail to classify many persons with dementia. In the clinical trials arena, the implications of clinical misdiagnosis means that large numbers of participants are needed to maintain statistical power. Revised diagnostic guidelines and the increased use of biomarkers should improve early diagnosis [8, 9]. It is also likely that the increased use of biomarkers will lead to segmentation of patient populations to enrich response rates, as has been seen in oncology. However, another implication of these findings is that other mechanisms in addition to the accumulation of plaques and tangles are involved in the neurodegeneration that causes dementia. Current thinking is that amyloid accumulates many years before symptoms become apparent, and that subsequent neurodegeneration involves multiple other mechanisms, including tau abnormalities, microglial activation and neuroinflammation, oxidative stress, and cell cycle abnormalities.
Genetics
Genetic studies point to numerous potential pathogenic pathways. Next to the fully penetrant autosomal dominant mutations in APP, PSEN1, and PSEN2, the ApoEε4 allele is the most powerful genetic factor identified, substantially increasing the risk of developing AD in and decreasing the age of onset [10]. APOE genotype is known to influence Aβ clearance and fibrillogenesis, and may also influence risk through non-amyloid mechanisms. Moreover, genome-wide association studies (GWAS) in people with late onset AD (LOAD) implicate multiple other molecular pathways, including innate immunity, lipid metabolism, and endocytic trafficking [11–13]; and single nucleotide polymorphisms (SNPs) associated with CSF ptau181 appear to modify the rate of progression of dementia [14].
Aging mechanisms
Mechanisms associated with aging also play important roles in AD, which may explain the fact that age is the most critical risk factor for AD. Normal aging is associated with gradual cognitive decline, brain atrophy, glial activation, synaptic loss and changes in lipid metabolism. In AD, the trajectory of synaptic loss diverges from normal after age 60 and is associated with accelerated degeneration in the entorhinal cortex. This accelerated synaptic loss is thought to be driven by many factors including Aβ and NFTs. It is also thought to be affected by brain injury (resulting from trauma, infection, or a vascular event), hormones, lipidemia and midlife obesity [15], and environmental factors including urban air pollution [16].
There is a very close link between aging and autophagy, and between AD and impaired lysosomal proteolysis. Autophagy is critical to neuronal survival because it enables the turnover of organelles and clearance of misfolded proteins. Thus, it is not surprising that neurons are a significant hotspot for genes that affect the lysosomal network and that are associated with familial forms of late-onset neurodegenerative diseases and developmental diseases. Increasing evidence points to impaired lysosome proteolysis and a failure of autophagic mechanisms in AD [17, 18]. And, importantly, many labs have shown that enhancing autophagy efficiency using agents such as rapamycin [19] may have therapeutic effects by reducing amyloidosis, tauopathy, cytotoxicity, synaptic dysfunction, and cognitive impairment.
Cell cycle events
Another key process that may underlie AD neuropathology involves the unscheduled appearance of neuronal cell cycle events (CCEs) [20]. CCEs in neurons, viewed as the increased presence of cell cycle protein expression and evidence for DNA synthesis [21], are tightly correlated with disease state, both anatomically and temporally. And, as was shown 20 years ago, nerve cell death can be driven by forcing mitosis in mature neurons [22]. In addition to neurons, during the progression of AD astrocytes and microglia also undergo transitions that alter their cell cycle activity. Much of this is likely to be in response to the neuronal changes, but the experience in other neurodegenerative diseases suggest that cell:cell interactions act in multiple directions at once. Cell cycling thus has implications for identifying both new biomarkers and new therapeutic targets. Mouse models indicate that markers of cell cycling provide markers of incipient neuronal death early in the disease process, significantly before the deposition of Aβ. These markers, like tau deposition, are not AD-specific, yet still offer important insights into the location and extent of neuronal distress and as such offer significant advantages as outcome measures in pre-clinical trials. For example, non-steroidal anti-inflammatory drugs (NSAIDS) can block new CCEs from appearing but cannot reverse the cell cycle protein expression pattern once it has begun [23]. This could explain both the epidemiological observation that NSAID use lowers the risk of AD as well as the failure to demonstrate a therapeutic effect of NSAIDs when given to people with symptomatic disease. Anti-cycling strategies may be thus be most effective not only early in disease but also when used in combination with anti-aggregation therapies for tau and amyloid [24].
An expanded role for tau
Abnormal phosphorylation of tau has been implicated as a mechanism of AD pathophysiology since the mid-1980s [25]. Today, tau continues to be pursued as a viable target in AD, although we now recognize that tau (MAPT) gene regulation is complex and tau phosphorylation involves multiple kinases and phosphorylation sites. Two recent findings provide insight into tau pathology. The first is identification of conformation specific tau antibodies that recognize some, but not all, pathological forms of tau, suggesting conformational diversity within the tauopathies [26–28]. Second, a biologically active motif was identified in the tau amino terminus that activates a signaling pathway involving protein phosphatase 1 (PP1) and glycogen synthase kinase 3β (GSK3β), providing a molecular basis for altered kinase activities in tauopathies. While these studies suggest potentially druggable targets, there is little clarity about the optimal levels and duration of phosphorylation inhibition. Small molecule inhibitors of GSK3 have been proposed as a therapeutic strategy for inhibiting excessive tau phosphorylation [29]. The attractiveness of GSK3 as a target goes beyond tau, since when abnormally activated, this kinase affects a plethora of signaling cascades that are involved in synaptic plasticity dysfunction [30]. Several candidate drugs with good physicochemical properties and excellent pharmacological profiles have entered Phase I clinical trials; however the challenge has been striking a balance between efficacy and toxicity that allows for sufficient safety margins to test the tau hypothesis.
In addition to phosphorylation, many other tau-based mechanisms of neurodegeneration have been suggested, although it is not clear which targets are relevant. No tau mutations are associated with AD, and abnormal expression of the tau gene has not been implicated with any human disease, although abnormal splicing has been suggested. Hyperphosphorylation of tau leads to increased aggregation and reduces MT affinity, and there are also other post-translational modifications that are being investigated for their roles in pathogenesis. For example, tau acetylation is elevated in patients in the early stages of tauopathy and appears to block the degradation of phospho-tau [31]. Specific sites of tau acetylation have been identified in the brains of transgenic AD mice and patients with AD and other tauopathies, suggesting a potential target for drug discovery and biomarker development [32]. Tau is also found in the mitotic spindle [33], suggesting a possible role for tau in affecting cell cycle regulation.
Befitting its role as a modulator of microtubule structure and function, attention has recently turned to the conformation of the tau protein. Several studies suggest that the pathogenic form of tau is a misfolded protein that exposes a biologically active motif. Indeed, using immunohistochemistry and monoclonal antibodies directed at specific epitopes, tau conformational abnormalities are seen in neurons in the earliest stages of AD, even before filaments are present, and these antibodies have been shown to block the development of, and even reverse pathology in AD mice [34, 35]. Chaperone proteins such as heat shock proteins (Hsps) also influence the conformation of tau and thus represent potential therapeutic targets. For example, inhibition of Hsp90 has been shown to reduce phospho-tau levels in transgenic mice [36], and the heat shock cognate (Hsc) 70 has also been shown to regulate tau polymerization [37]. Work currently underway to develop specific Hsc70 inhibitors has identified the DnaJ family of proteins as critical co-chaperones regulating Hsp70 interaction with tau [38], suggesting that they may represent important targets for drug development targeting tau.
More recently, multiple labs have shown that tau protein aggregation spreads in a prion-like fashion [39–41], possibly along important neural networks, moving from cell to cell in the extracellular space [42]. These results could explain the observation that neurodegenerative diseases target large-scale human brain networks [43] and suggest a mechanism that could explain the effectiveness of monoclonal-antibody- or vaccine-based therapies targeted against phospho-tau or conformer specific epitopes on tau.
Inflammation
Chronic inflammation is a cardinal feature of AD neuropathology and may play a contributing role in AD pathogenesis through a number of pathways. For example, chronic inflammatory stress on results in activation of neuronal cell cycle proteins [44], production of nitric oxide [45] and reactive oxygen species (ROS) [46], reduction in mitochondrial function [47], and impaired dendritic/axonal transport [48]. Significant epidemiological, biochemical, genetic, and neuropathological evidence points to a link between chronic inflammation and AD (reviewed in [49, 50]). Thus, chronic inflammation may create the unique chemistry and cellular physiology that drives AD progression [18–20].
Microglia are the macrophages of the brain and the key effector cells in neuroinflammation. They constantly survey the brain and the integrity of synapses, and are responsible for synaptic pruning. Those salutary microglial actions are compromised in response to locus ceruleus degeneration in the brainstem and subsequent reduction of cortical and hippocampal norepinephrine levels [51]. In the absence of norepinephrine, microglial cells are shifted towards a proinflammatory phenotype which is also characterized by impaired Aβ clearance. Fibrillar Aβ stimulates a classical pro-inflammatory response in the microglia, which can be visualized in MCI and AD patients and may be present even before those stages. Although the details of microglial activation and in particular the complex regulation between different stages of activation remains to be determined, one may hypothesize that drugs which shift microglial phenotype from proinflammatory to surveilling and repair may hold therapeutic potential. Thus, modulating the inflammatory response for therapeutic purposes involves a careful balancing act, requiring a better understanding of inflammatory mechanisms and identification of the appropriate therapeutic window and productive drug targets. Early intervention with drugs that hit multiple complementary targets involved in inflammatory signaling may be necessary.
Mitochondrial dysfunction
Mitochondrial function and bioenergetics are known to be altered in LOAD and have been postulated to be essential factors in AD pathogenesis, as either causes or consequences of amyloid toxicity. The “mitochondrial cascade hypothesis,” proposed by Swerdlow in 2004 [52], posits that mitochondrial function is affected by genetic factors (particularly mitochondrial DNA inherited through the mother) and age, with dysfunction reaching a threshold that results in tau phosphorylation, Aβ production and plaque deposition, synaptic loss, and degeneration. Studies using cybrids – cytoplasmic hybrid cells in which the mitochondrial DNA (mtDNA) has been removed from one cell line and replaced with mtDNA from the cytoplasts of an AD patient -- support the validity of this hypothesis and suggest a number of mitochondrial-based therapeutic approaches for AD and other neurodegenerative diseases. These approaches include manipulating the electron transport chain and cell energy supplies, oxidative stress, apoptosis, mitochondrial mass, mtDNA, and autophagy [53], thus minimizing the downstream consequences of mitochondrial dysfunction.
Brain insulin resistance
Epidemiological studies showing that individuals with type 2 diabetes have as much as a 100% increased risk of developing AD have led to suggestions that insulin resistance may represent an important causal pathway in AD. Recent studies have supported this hypothesis, demonstrating that dysregulation of insulin signaling pathways in the hippocampus and cerebral cortex of mild cognitive impairment (MCI) and AD patients without diabetes correlates with deficits in episodic and working memory and precedes dementia in patients with MCI [54]. These studies suggest brain insulin resistance as another therapeutic target in AD.
Applying lessons from amyloid to the pursuit of non-amyloid targets
Unsuccessful and ongoing clinical trials of amyloid-targeted agents have provided lessons to heed in the clinical development of future non-amyloid based drugs, particularly since continued failures could have a paralyzing effect on the field. Perhaps most urgent is the need to better understand the underlying biology of the disease and particular pathways that will be targeted. With amyloid, for example, it remains unclear precisely what role Aβ plays in disease pathogenesis – is it a trigger or driver of pathology, and/or is there a threshold that must be reached for it to become pathogenic? The answer to these questions may indicate when in the disease process it is best to intervene and whether additional pathways should be targeted simultaneously.
Each hypothetical scenario can and should be modeled and tested in mechanism-relevant preclinical models, with appropriate interpretation that considers the shortcomings of each model. None of the current animal models adequately reflect the complexity of human AD, although they have provided interesting information about different pathways. For example, transgenic models have demonstrated that even high expression of wild-type APP does not produce neurodegeneration or brain atrophy and has no effect on cognition; and that even high expression of mutant APP does not cause NFT pathology, massive neuronal death, or severe behavioral disturbances. Most transgenic models were developed using familial AD genes, alone or in combination, and may not be an accurate reflection of the biology of sporadic AD. On the positive side, animal models accurately reproduce the various forms of amyloid deposition and have been useful in establishing proof of concept for immunotherapy [55], which was subsequently validated in patients [56]. Animal models have also been used to demonstrate safety. Tau transgenic models have elucidated the complexity of the effects of pathological tau on MT stabilization and have been used to establish pre-clinical proof of concept for MT stabilizing agents [4, 57].
Enhancing the predictive value of animal models thus requires selection of the correct model or models; careful study design, including selection of the appropriate time for intervention; careful interpretation of data; and confirmation of results in multiple models. Additional efforts should be devoted to developing new models that incorporate the concepts that emerge as the non-amyloid strategies outlined at this workshop mature. These preclinical studies could then lead to careful design of proof-of-concept studies with a higher likelihood of yielding valid and relevant results. In addition to robust preclinical data, clinical biomarker data are essential prior to initiating a clinical trial. Thus, for example, the Dominantly Inherited Alzheimer Network (DIAN) has recently demonstrated a cascade of pathophysiological changes that begin two decades or more before clinical symptom onset [58]. The DIAN has designed a two-stage secondary prevention trial that will simultaneously test three drugs and share a common placebo group. The first stage will use biomarkers to assess target engagement and effects on downstream markers of neurodegeneration. If a drug reaches the primary endpoints in the first stage, it will proceed to the second larger, longer trial that will assess primary cognitive endpoints in addition to biomarkers.
With DIAN, many of the target engagement biomarkers are amyloid-based -- either CSF analytes or amyloid imaging -- that are translatable from pre-clinical animal models to the clinic. Development of similar translatable pharmacodynamic biomarkers will be especially important for testing hypotheses involving non-amyloid targets, but will take time and effort to develop and validate. For example, if tau targets are to be pursued as therapeutic strategies, there is a clear need for translational science studies that provide evidence for peripheral and central target engagement in the clinic. A fundamental understanding of tau turnover in the CSF is currently lacking, yet attempts to decipher this biology could pave the way for future approaches in tau-based therapies. Measurement of CSF tau or development of pathologic tau imaging tracers may enable dose-finding studies for tau-based therapies, but the pharmacodynamic biomarkers for other treatment modalities are not obvious. Clearly much development work is needed in this area.
Perspectives from industry and the FDA on non-amyloid targets
The issues facing both industry and regulatory agencies with regard to non-amyloid targets mirror those regarding amyloid targets, although the lack of success in developing amyloid-based therapies has made industry more cautious than it was previously. As a result, there is increasing focus on better understanding the pathophysiology of disease and confirming target engagement, with acute molecular and downstream functional effects demonstrated in the preclinical phase of development. Biomarkers will be especially important in this regard.
There is also increased interest in developing external partnerships, novel technologies, and creative early developmental approaches. Aspects of the drug development process that may be more amenable to precompetitive data sharing include studies regarding patient selection, trial duration, and dose selection, as well as further development of novel biomarkers including those that image non-amyloid targets. The Alzheimer’s Disease Neuroimaging Initiative (ADNI) has already provided a model of public-private partnerships, and the National Plan developed in response to the National Alzheimer’s Project Act (NAPA) advocates similar partnerships as a means of moving drug development forward. An industry working group has been convened to promote a strong role for industry in the process of developing national priorities and strategies to compress the discovery pipeline.
Another collaborative project at Harvard University’s Laboratory for Drug Discovery in Neurodegeneration (LDDN) has been designed to stimulate and support academic-industry collaborations aimed at accelerating the discovery of new treatments for neurodegenerative diseases. Using phenotypic assays, LDDN is exploring several speculative, early-stage projects that could lead to the identification of novel targets in multiple pathways involved in AD. LDDN has also initiated a project aimed at collecting compounds already optimized for other uses and exploring repurposing those drugs for CNS diseases.
Conclusions
A tremendous amount of fundamental research and collaboration preceded the identification and preclinical use of amyloid as a marker of AD. Now is the time for the AD community to engage around non-amyloid markers with similar vigor. Barriers to this approach include the plethora of non-amyloid targets and a historical and understandable reluctance to share preclinical data with competitors. The question now is whether industry, academia, and other stakeholders can identify broad areas to explore collaboratively in precompetitive space. Research Roundtable participants agreed that further discussion along these lines could be fruitful in a number of areas, including:
Exploring the possibility of aligning around certain targets and identifying the critical tools necessary to explore these targets.
Examining further whether available models are adequate, and if not, developing a plan to create new models.
Prioritizing how resources should be allocated in a strategic manner.
Acknowledgments
We thank Dr. Adam Bachstetter, University of Kentucky, for preparation of the figure. These studies were supported in part by NIH P30 AG028383 (LVE).
References
- 1.Schenk D, Carrillo MC, Trojanowski JQ. Cytoskeletal modulators and pleiotropic strategies for Alzheimer drug discovery. Alzheimers Dement. 2006;2(4):275–81. doi: 10.1016/j.jalz.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Lee VM, Daughenbaugh R, Trojanowski JQ. Microtubule stabilizing drugs for the treatment of Alzheimer’s disease. Neurobiol Aging. 1994;15(Suppl 2):S87–9. doi: 10.1016/0197-4580(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 3.Brunden KR, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci. 2010;30(41):13861–6. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci. 2012;32(11):3601–11. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc Natl Acad Sci U S A. 2005;102(1):227–31. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Z Psychiatrie Psychisch-Gerichtliche Med. 1907;64:146–148. [Google Scholar]
- 7.Beach TG, et al. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71(4):266–73. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois B, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 9.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 11.Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones L, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5(11):e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruchaga C, et al. SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. PLoS Genet. 2010;6(9) doi: 10.1371/journal.pgen.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitmer RA, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 16.Moulton PV, Yang W. Air pollution, oxidative stress, and Alzheimer’s disease. J Environ Public Health. 2012:472751. doi: 10.1155/2012/472751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141(7):1146–58. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31(21):7817–30. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caccamo A, et al. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–20. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrup K. Reimagining Alzheimer’s disease--an age-based hypothesis. J Neurosci. 2010;30(50):16755–62. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci. 2001;21(8):2661–8. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feddersen RM, et al. Disrupted cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T antigen transgenic mice. Neuron. 1992;9(5):955–66. doi: 10.1016/0896-6273(92)90247-b. [DOI] [PubMed] [Google Scholar]
- 23.Varvel NH, et al. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J Clin Invest. 2009;119(12):3692–702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrup K. The contributions of unscheduled neuronal cell cycle events to the death of neurons in Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:2101–9. doi: 10.2741/527. [DOI] [PubMed] [Google Scholar]
- 25.Grundke-Iqbal I, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83(13):4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanaan NM, et al. Pathogenic Forms of Tau Inhibit Kinesin-Dependent Axonal Transport through a Mechanism Involving Activation of Axonal Phosphotransferases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(27):9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson KR, et al. Characterization of prefibrillar tau oligomers in vitro and in Alzheimer disease. The Journal of biological chemistry. 2011;286(26):23063–76. doi: 10.1074/jbc.M111.237974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson KR, et al. Heat Shock Protein 70 Prevents both Tau Aggregation and the Inhibitory Effects of Preexisting Tau Aggregates on Fast Axonal Transport. Biochemistry. 2011;50(47):10300–10. doi: 10.1021/bi2009147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhat R, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278(46):45937–45. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 30.Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89(6):1313–7. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 31.Min SW, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–66. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen TJ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996;132(3):413–25. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutajangout A, et al. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118(4):658–67. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai X, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286(39):34457–67. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickey CA, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117(3):648–58. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jinwal UK, et al. Hsc70 rapidly engages tau after microtubule destabilization. J Biol Chem. 2010;285(22):16798–805. doi: 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abisambra JF, et al. DnaJA1 Antagonizes Constitutive Hsp70-Mediated Stabilization of Tau. J Mol Biol. 2012 doi: 10.1016/j.jmb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Calignon A, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73(4):685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo JL, V, Lee M. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286(17):15317–31. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kfoury N, et al. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012 doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeley WW, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q, et al. Beta-amyloid activated microglia induce cell cycling and cell death in cultured cortical neurons. Neurobiol Aging. 2000;21(6):797–806. doi: 10.1016/s0197-4580(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 45.Kummer MP, et al. Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation. Neuron. 2011;71(5):833–44. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Fuller S, Steele M, Munch G. Activated astroglia during chronic inflammation in Alzheimer’s disease--do they neglect their neurosupportive roles? Mutat Res. 2010;690(1–2):40–9. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–76. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu HY, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30(7):2636–49. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis. 2010;37(3):503–9. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heneka MT, et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107(13):6058–63. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 53.Swerdlow RH. Role and treatment of mitochondrial DNA-related mitochondrial dysfunction in sporadic neurodegenerative diseases. Curr Pharm Des. 2011;17(31):3356–73. doi: 10.2174/138161211798072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg SM, Bacskai BJ, Hyman BT. Alzheimer disease’s double-edged vaccine. Nat Med. 2003;9(4):389–90. doi: 10.1038/nm847. [DOI] [PubMed] [Google Scholar]
- 57.Barten DM, et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J Neurosci. 2012;32(21):7137–45. doi: 10.1523/JNEUROSCI.0188-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bateman RJ, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3(1):1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]