Abstract
Antagonists of the NOP receptor have antidepressant effects in rodent models, suggesting that the N/OFQ-NOP system may play an important role in affective disorders. Furthermore, multiple lines of experimental evidence link N/OFQ neurotransmission with physiological and behavioral responses to stress. One possibility is that disregulated expression of the N/OFQ peptide neurotransmitter and/or the NOP receptor may participate in the etiology of stress-induced psychopathology. In the present set of experiments, we compared gene expression for prepro-N/OFQ and NOP receptor in groups of rats that were exposed to differing regimens of social defeat stress. Male Long-Evans rats were exposed to no social defeat, a single, acute social defeat or to repeated social defeats with or without an acute defeat on the final day. In situ hybridization was conducted with 35S-labelled riboprobes aimed at prepro-N/OFQ mRNA or NOP receptor mRNA. Expression was analyzed by quantification of optical density in limbic and extra-limbic forebrain regions. There were no statistically significant changes in prepro-N/OFQ mRNA expression after stress exposure in any of the brain regions analyzed. However, the rats that were exposed to acute social defeat displayed elevations in NOP receptor mRNA expression in the central and basomedial nuclei of the amygdala and in the paraventricular nucleus of the hypothalamus. Additionally, the rats that were acutely stressed after a history of repeated social defeat also displayed elevated levels of NOP receptor mRNA expression in the paraventricular nucleus of the hypothalamus. These results suggest that the N/OFQ-NOP receptor system is affected by acute stress exposure, particularly in limbic regions. This stress-induced upregulation of NOP receptor gene expression further supports the possibility that disregulation of the N/OFQ-NOP system may contribute to behavioral and hormonal disregulation following stress.
Keywords: Nociceptin, Orphanin FQ, N/OFQ, NOP, Stress, Amygdala, Limbic system, paraventricular nucleus, hypothalamus, PVN
1. Introduction
There is extensive evidence that the peptide neurotransmitter, nociceptin/orphanin FQ (N/OFQ) and its cognate receptor, NOP, play important roles in expression of emotionally-relevant behaviors and in activation of the hypothalamic-pituitary-adrenal (HPA) axis. Many studies report that intracerebroventricular (icv) injections of N/OFQ (Jenck et al., 1997; Gavioli et al., 2002; Vitale et al., 2006) or systemic injections of the synthetic agonist Ro 64-6198 (Jenck et al., 2000; Dautzenberg et al., 2001; Varty et al., 2005) produce decreases in expression of anxiety-related behaviors of rodents. However, we found increases in anxiety-related behaviors after icv or intra-limbic injections of N/OFQ in rats (Fernandez et al., 2004; Green et al., 2007), and Kamei and colleagues (2004) reported both anxiolytic and anxiogenic-like effects after icv injections in mice. Griebel and colleagues (1999) found that N/OFQ decreases anxiety-related behaviors only in conditions where the mice are exposed to unavoidable severe stress (forced contact with a threatening stimulus). Furthermore, Gavioli and colleagues (2007) found both heightened and suppressed expression of anxiety-related behaviors in NOP receptor knockout mice, depending upon the type of test used. Thus, it appears that N/OFQ neurotransmission is implicated in regulation of anxiety states, but the specific effect may depend upon currently unidentified characteristics of the test.
More consistent results have been obtained in tests of the antidepressant-like effects of NOP receptor antagonists. Although N/OFQ did not alter immobility measures in forced swim and tail suspension tests after icv administration, the NOP receptor antagonists [Nphe1]-nociceptin (1-13)-NH2 (Redrobe et al., 2002), UFP-101 (Gavioli et al., 2003, 2004), J-113397 (Redrobe et al., 2002), and SB-612111 (Rizzi et al., 2007) each exerted antidepressant-like effects in these tests.
Studies of the effects of N/OFQ on activity of the HPA axis have uniformly revealed that N/OFQ elevates circulating adrenocorticotropic hormone (ACTH) and corticosterone (CORT) concentrations after injection into the lateral ventricles (Devine et al., 2001; Nicholson et al., 2002; Fernandez et al., 2004; Leggett et al., 2006) and limbic structures (Green et al., 2007) in unstressed and mildly stressed rats. Furthermore, the N/OFQ-induced activation of the HPA axis is accompanied by increased expression of corticotrophin releasing hormone (CRH) mRNA in the paraventricular hypothalamus (PVN) and by increased proopiomelanocortin (POMC) mRNA expression in the pituitary (Leggett et al., 2006). These effects of N/OFQ administration resemble the effects of acute stress exposure (see Harbuz and Lightman, 1989) and are blocked by concurrent icv administration of the NOP receptor antagonist UFP-101 (Leggett et al., 2006).
Interestingly, N/OFQ content of the basal forebrain is reduced by acute restraint stress, and these neuronal stores are replenished within 24 h (Devine et al., 2003). This suggests that stress causes release of endogenous N/OFQ, and that biosynthetic activity returns N/OFQ stores to normal levels after release.
Since N/OFQ and the NOP receptor are implicated in regulation of affect and in HPA axis responses, we investigated the possibility that exposure to emotional stress may alter gene expression in this peptide neurotransmitter system. In these experiments, we used the social defeat model of emotional stress. In this widely used model, a young naïve male rat (the “intruder”) is placed into the cage of an experienced and larger male rat (the “resident”). The resident male rat characteristically exerts dominance by pinning the intruder, and the intruder characteristically submits, by displaying a supine posture in response (see Miczek et al., 2004). Accordingly, the social defeat procedure emulates natural social hierarchies and takes advantage of social stressors that rats may experience in their natural habitats.
Social defeat exposure activates cortical and limbic circuits (Matsuda et al., 1996; Martinez et al., 1998; Chung et al., 1999; Nikulina et al., 2004) that are thought to be important in processing of emotional stressors (Herman and Cullinan, 1997). Furthermore, regional forebrain expression of c-fos is modified by repeated exposure to social defeat. Some limbic areas (e.g. bed nucleus of stria terminalis, medial amygdala) continue to have elevated c-fos expression, whereas other areas (e.g. septum, central amygdala) no longer express elevated c-fos after repeated defeat (Martinez et al., 1998). In addition, repeated defeat produces substantial increases in the duration of limbic c-fos activation (Matsuda et al., 1996). Thus, alterations in responding during repeated exposure to social defeat may reveal interesting stress-induced plasticity in neural circuits that process emotional regulation (Koolhaas et al., 1997; Miczek et al., 2004; Buwalda et al., 2005). Accordingly, we examined prepro-N/OFQ and NOP receptor mRNA after acute and repeated social defeat using in situ hybridization histochemistry.
2. Methods
All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and were pre-approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida.
2.1 Animals
Thirty male and 14 female Long-Evans rats (Harlan, Indianapolis) were housed in 43 × 21.5 × 25.5 cm polycarbonate cages on a 12–12 h light-dark cycle (lights on at 7:00 am) in a climate-controlled vivarium (temperature 21–23 °C, humidity 55–60%). Standard laboratory chow and tap water were available ad libitum.
Fourteen of the male rats (weighing 600–700 g at the time of the experiment) were vasectomized and pair-housed with the 14 female rats (200–225 g at arrival). These male rats were trained to exhibit dominant behaviors in the home cage (by repeated exposure to naïve smaller “intruder” males), and were used as “residents” in the social defeat procedure (see below). The remaining 16 males, weighing approximately 300 g each, were used as experimental intruder rats. The intruder rats were pair-housed throughout the course of the experiment.
2.2 Social Defeat Procedure
The experimental rats were randomly assigned to 4 treatment groups. The rats of Group 1 were not exposed to social defeat (no stress controls). Group 2 rats were exposed to a social defeat session on the 6th day of the experiment only (no repeated stress, acute stress). Group 3 was exposed to a social defeat session on each of the first 5 days, but did not experience a defeat session on the 6th day (repeated stress, no acute stress), and Group 4 rats were defeated on each of the 6 experimental days (repeated stress, acute stress). This experimental design allowed us to examine the effects of acute defeat on the N/OFQ-NOP system (in comparison with unstressed controls), to evaluate whether the tone of the system changed over repeated exposures (i.e. habituation or sensitization), and to assess whether the response to an acute defeat changed with repeated stress experience. The group assignments and stress exposures of the rats are summarized in Table 1. In all cases of repeated social defeat sessions, the intruder rat was exposed to a new resident for each encounter, in order to prevent habituation in the interactions between the resident and intruder rats.
Table 1.
Summary of rat group assignments: The rats were assigned to groups that experienced differing regimens of repeated and acute social defeat exposure, and all the brains were harvested at equivalent times on day 6.
| Group | day 1 | day 2 | day 3 | day 4 | day 5 | day 6 |
|---|---|---|---|---|---|---|
| 1 (no stress) | no stress | no stress | no stress | no stress | no stress | no stress |
| 2 (acute stress) | no stress | no stress | no stress | no stress | no stress | soc. defeat |
| 3 (repeated stress) | soc. defeat | soc. defeat | soc. defeat | soc. defeat | soc. defeat | no stress |
| 4 (repeated + acute stress) | soc. defeat | soc. defeat | soc. defeat | soc. defeat | soc. defeat | soc. defeat |
The social defeat procedure was conducted in 2 stages. During the first stage, the resident female was removed from the home cage, and 10 min later, the intruder was placed directly into the cage with the male resident. The encounter continued for 5 min or until the intruder was defeated 3 times (whichever occurred first). Each defeat was counted when the intruder displayed submissive behavior (supine posture for at least 2 s) with the resident standing over the intruder, maintaining physical contact. This first stage was also terminated if the intruder exhibited freezing behavior for 90 s. Immediately after stage 1, each intruder rat was removed from the residents cage and placed individually into a 10 × 10 × 15 cm (inner dimensions) double-walled wire mesh cage. The intruder within the wire cage was then placed back into the resident’s cage until 10 min had passed from the start of stage 1. This allowed for equalization of the duration of stress exposure of the intruder rats, and limited the number of defeats per session to a maximum of three. Following the 10-min session, the intruder rat was returned to its home cage and the female was returned to the resident’s cage. All social defeat sessions were videotaped and the frequency and duration of defeats were scored. On the 6th day, all of the intruder and control rats were rapidly decapitated at 3 h after the start of the defeat session, or at the equivalent time in the groups that were not acutely defeated before termination. Immediately after termination of the rats, each brain was removed, frozen in 2-methylbutane at −40 °C, and stored at −80 °C until use.
2.3 In situ Hybridization
Each brain was sectioned into 15 μm slices in the coronal plane, mounted onto polylysine-subbed slides, and hybridized with riboprobes directed at prepro-N/OFQ or NOP receptor mRNA. The 504 base cDNA fragment corresponding to the 5′ end of the coding region of the rat prepro-N/OFQ was cloned into plasmid pAMP (provided by Dr. Olivier Civelli). The 529 base cDNA fragment corresponding to 102 bases in the 3′ untranslated region through 427 bases of the open reading frame of the NOP receptor was cloned into BSSK (provided by Dr. Huda Akil). Antisense and sense riboprobes were generated and labeled with 35S-UTP (>1000 Ci/nmole; GE Healthcare). The pAMP plasmid was linearized with EcoR1 and transcribed with SP6 polymerase to generate the antisense prepro-N/OFQ riboprobe. This plasmid was also linearized with HindIII and transcribed with T7 to yield the sense probe. The BSSK plasmid was linearized with EcoR1 and transcribed with T7 to generate the antisense riboprobe for NOP receptor mRNA, and this plasmid was also linearized with Xho1 and transcribed with T3 to yield the sense probe for the NOP receptor. The brain sections were processed for hybridization according to methods described by Kabbaj et al. (2000). Briefly, the sections were fixed in 4% formaldehyde for 1 h, then rinsed in 3 washes of 2x SSC and one wash of ddH2O all at room temperature (RT). The sections were then immersed in 0.1 M Triethanolamine plus 0.25% vol/vol acetic anhydride for 10 min., rinsed in ddH2O, and dried. The 35S-labelled riboprobes were prepared in 50% hybridization buffer and were hybridized to the mounted sections overnight at 55 °C. Then, the sections were treated with RNAse A, washed in 2x SSC (RT), 1x SSC (RT), and 0.1x SSC (60 min, 67 °C) to remove excess label. The sections were then dried and apposed to Kodak X-OMAT film in X-ray cassettes (RT) for 1 week (NOP receptor) or 2 weeks (N/OFQ). The films were developed, then photographed and digitized with an MCID camera and MCID Basic Image Analysis software system (Imaging Research Inc., St. Catharines, Canada).
Optical densities were measured in selected limbic and extra-limbic forebrain regions that are known to participate in physiological stress responses and/or emotional regulation. The forebrain sections included cingulate cortex and subnuclei of the septum, bed nucleus of stria terminalis (BNST), amygdala, and hypothalamus.
2.4 Densitometry
Densitometric analysis of the radioactive signal was performed using the MCID Basic software. A standard outline was determined for each region of interest using the Paxinos and Watson Rat Brain Atlas (1998). Bilateral optical density measures were then sampled from each region. If multiple sections were used per region, the optical densities for these sections were integrated to create one data point per rat per region per probe. External background measures were taken from outside the section, and sense background measures were taken from the respective forebrain structures in the sense sections. Final optical density values were calculated as a function of specific signal relative to external background minus nonspecific signal relative to external background. The calculations were performed with the following formula:
2.5 Statistics
Potential differences in the number of daily defeats between Group 3 (repeated stress) and Group 4 (repeated plus acute stress) were analyzed over the 5 repeated stress days with a 2 × 5 repeated measures analysis of variance (RM-ANOVA). Similarly, differences in the total daily duration of the defeats were analyzed with a 2 × 5 RM-ANOVA for these groups. In addition, potential differences in the number of defeats and in the total duration of defeats on the 6th day were analyzed using T-tests comparing scores for Group 2 (acute stress) and Group 4 (repeated plus acute stress).
Potential between-groups differences in expression of prepro-N/OFQ mRNA were analyzed with one-way ANOVAs for each region comparing optical density scores across all groups. Similarly, differences in NOP receptor mRNA expression were analyzed with one-way ANOVAs. All significant effects (p < 0.05) were further analyzed using Dunnet’s post-tests.
3. Results
3. 1 Social defeats
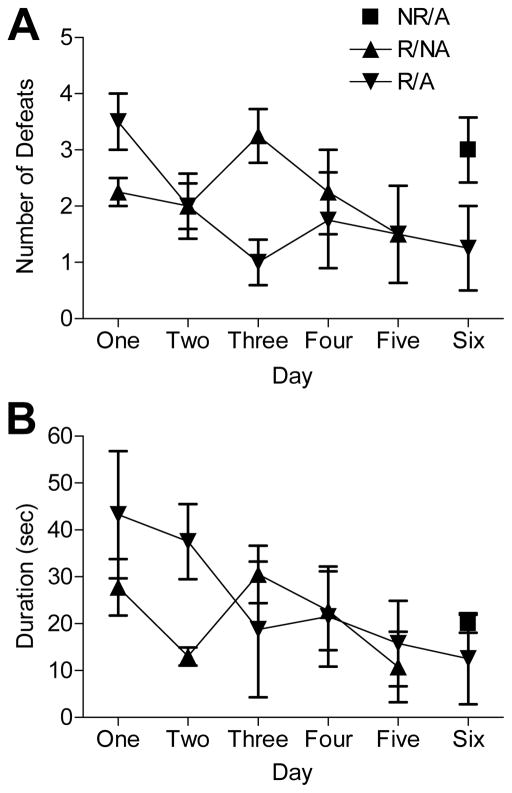
The groups that were exposed to 5 repeated social defeat sessions (Groups 3 and 4) experienced equivalent amounts of defeat. There were no significant differences in the total number (F(1,24) = 1.385, p > 0.05) or duration (F(1,24) = 2.796, p > 0.05) of daily defeats between these groups . Additionally, there were no significant changes in the number of defeats across days 1 through 5 in these groups (F(4,24) = 1.068, p > 0.05) or in the interaction between stress group and day (F(4, 24) = 1.748, p > 0.05). There were also no significant changes in the durations of defeats across days 1 through 5 (F(4,24) = 1.286, p > 0.05) or in the interaction between stress group and day (F(4,24) = 1.016, p > 0.05) in Groups 3 and 4. Furthermore, there were no significant differences in the numbers of defeats (t(6) = 1.849, p > 0.05) or in the duration of the defeats (t(5) = 0.756, p > 0.05) between the two groups (Groups 2 and 4) that were defeated on the 6th day (Fig. 1).
Figure 1.
(A) Number of social defeats per group per day and (B) total amount of time the rats were defeated per day. Values shown are group means ± SEM (n = 4 rats per group). NR/A = no repeated, acute stress group, R/NA = repeated, no acute stress group, R/A = repeated plus acute stress group.
3. 2 In situ Hybridization
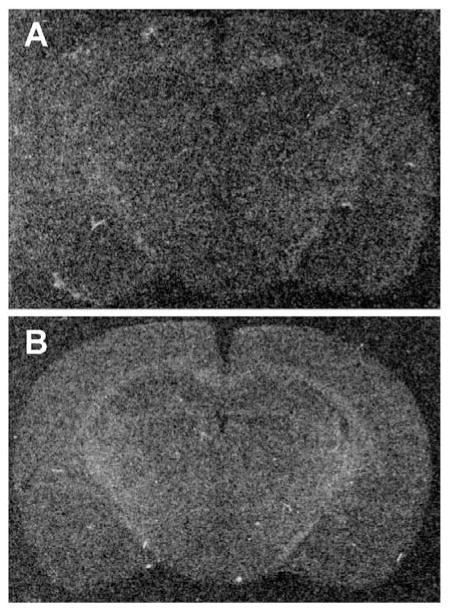
There was no specific signal detected in the control sections that were treated with sense riboprobes for prepro-N/OFQ and for NOP receptor mRNAs. In these sections, the optical densities of the limbic and extra-limbic regions did not differ from the optical densities of the external backgrounds (Fig. 2).
Figure 2.
Representative x-ray images of brain sections treated with sense riboprobes. Sections treated with (A) N/OFQ sense strands and (B) NOP receptor sense strands did not display specific signal, and background levels were comparable to those of antisense-treated sections.
3.2.1 Prepro-N/OFQ
There were no statistically significant between-groups differences in prepro-N/OFQ mRNA expression in any of the regions examined (Table 2). Darkfield images are presented for representative sections hybridized with antisense probes for prepro-N/OFQ mRNA in Fig 3.
Table 2.
Summary of between-groups differences in optical densities for prepro-N/OFQ mRNA in selected limbic and extra-limbic forebrain regions after differing histories of acute and repeated social defeat stress. Values expressed are mean optical densities (±SEM) after in situ hybridization with riboprobes aimed at prepro-N/OFQ mRNA. OD scores were obtained by calculating the specific OD relative to external background minus the nonspecific OD relative to external background.
| prepro N/OFQ mRNA optical densities | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | NR/NA | NR/A | R/NA | R/A | F value, df = (3,15) | p value | sig. | |
| medial prefrontal cortex | 18.8 ± 9.6 | 16.7 ± 6.2 | 29.5 ± 10.6 | 16.3 ± 6.0 | 0.548 | 0.659 | n.s. | |
| caudate | 9.9 ± 8.4 | 1.2 ± 1.7 | 19.7 ± 11.8 | 74.0 ± 39.4 | 2.428 | 0.116 | n.s. | |
| septum | dorsolateral | 110.6 ± 11.0 | 125.5 ± 15.7 | 127.3 ± 15.2 | 125.5 ± 20.2 | 0.240 | 0.867 | n.s. |
| infralimbic | 45.4 ± 8.1 | 40.5 ± 6.5 | 56.0 ± 13.4 | 42.0 ±12.4 | 0.445 | 0.725 | n.s. | |
| ventrolateral | 62.0 ± 12.3 | 75.9 ± 8.5 | 75.9 ± 13.3 | 74.4 ± 15.5 | 0.066 | 0.837 | n.s. | |
| BNST | medial anterior | 36.3 ± 10.1 | 41.5 ± 3.4 | 50.1 ± 17.8 | 39.0 ± 7.0 | 0.298 | 0.826 | n.s. |
| medial posterior | 142.8 ± 29.6 | 170.4 ± 11.4 | 192.3 ± 9.8 | 182.8 ± 11.5 | 1.502 | 0.264 | n.s. | |
| lateral | 92.4 ± 14.8 | 129.4 ± 12.3 | 126.7 ± 10.6 | 125.9 ± 5.5 | 2.392 | 0.120 | n.s. | |
| medial ventral | 20.2 ± 16.8 | 54.6 ± 4.5 | 60.7 ± 24.1 | 67.5 ± 14.6 | 1.619 | 0.237 | n.s. | |
| amygdala | central | 118.5 ± 8.3 | 129.1 ± 16.8 | 124.5 ± 17.0 | 100.7 ± 10.6 | 0.827 | 0.504 | n.s. |
| medial | 155.5 ± 6.9 | 162.3 ± 11.7 | 172.5 ± 24.4 | 136.9 ± 22.7 | 0.695 | 0.573 | n.s. | |
| basomedial | 22.7 ± 5.1 | 10.6 ± 5.6 | 21.5 ± 14.1 | 10.3 ± 5.6 | 0.636 | 0.606 | n.s. | |
| lateral | 14.1 ± 6.2 | 6.6 ± 5.2 | 13.4 ± 14.2 | 13.0 ±9.8 | 0.406 | 0.751 | n.s. | |
| basolateral | 21.4 ± 6.4 | 9.3 ± 5.4 | 20.2 ±15.4 | 7.8 ± 7.7 | 0.549 | 0.658 | n.s. | |
| hypothalamus | medial preoptic | 39.1 ± 14.3 | 45.4 ± 10.6 | 55.1 ± 10.9 | 56.5 ± 8.1 | 0.543 | 0.662 | n.s. |
| paraventricular | 38.5 ± 18.8 | 22.5 ± 7.8 | 20.8 ± 15.1 | 12.0 ± 7.9 | 0.691 | 0.575 | n.s. | |
| ventromedial | 24.2 ± 6.7 | 11.1 ± 3.1 | 27.9 ± 9.2 | 8.3 ± 8.5 | 1.747 | 0.211 | n.s. | |
| arcuate | 42.9 ± 4.6 | 33.2 ± 8.8 | 41.9 ± 6.2 | 38.9 ± 8.6 | 0.395 | 0.759 | n.s. | |
NR/NA= no repeated, no acute social defeat; NR/A = no repeated, acute social defeat; R/NA = repeated, no acute social defeat; R/A = repeated plus acute social defeat.
p<0.05,
p< 0.01 for ANOVAs,
p<0.05,
p< 0.01 for Dunnett’s post comparisons between specific groups and the unstressed controls.
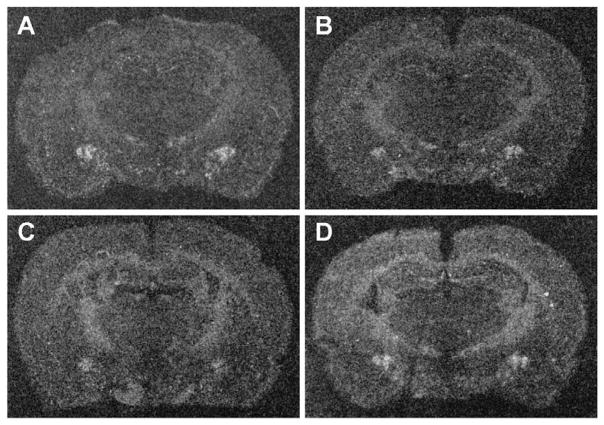
Figure 3.
Representative x-ray images of brain sections showing prepro-N/OFQ mRNA expression in the amygdala. Sections were exposed to 35S-labelled riboprobes complimentary to segments of prepro-N/OFQ mRNA. There were no significant differences in expression between (A) no stress control rats, (B) rats exposed to acute social defeat, (C) rats exposed to repeated, no acute social defeat, and (D) rats exposed to repeated plus acute social defeat for any of the regions sampled.
3.2.2 NOP receptor
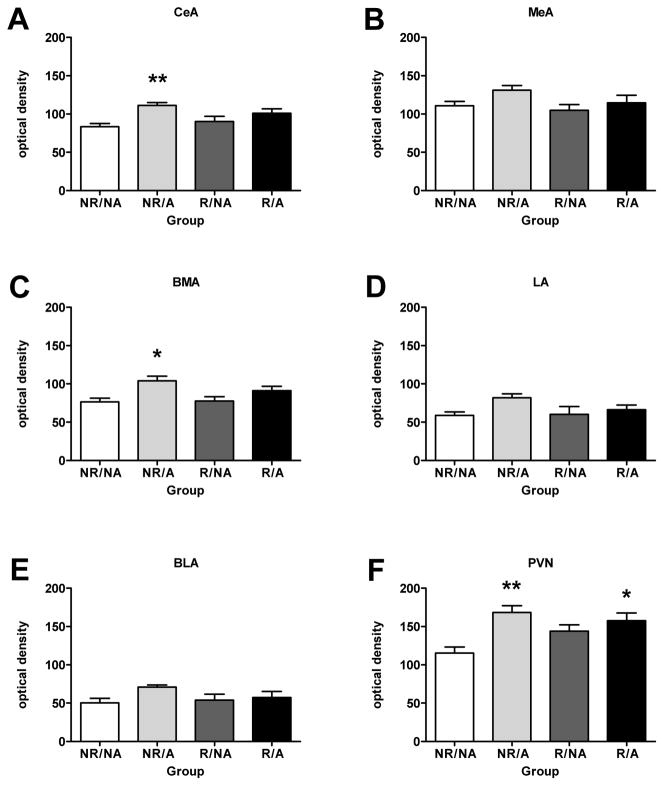
There were significant stress-induced increases in NOP receptor mRNA expression in several important limbic regions. In the amygdala, NOP receptor mRNA expression was elevated in the central (CeA; F(3, 15) = 5.376, p < 0.05) and the basomedial nuclei (BMA; F(3, 15) = 5.391, p < 0.05) after exposure to acute social defeat exposure (i.e. Group 2). NOP receptor mRNA expression did not differ between groups for any of the other regions (medial, lateral and basolateral) of the amygdala, although there was a trend toward elevated expression in the acutely stressed rats of Group 2 for all three regions (Fig. 4). In the PVN, there were significant elevations in NOP receptor mRNA in both acutely stressed groups (Groups 2 and 4; F(3, 15) = 6.683, p < 0.01; Fig. 4). There were no significant between-groups differences in NOP receptor mRNA expression in any of the other regions sampled (Table 3). Darkfield images are presented for representative sections hybridized with antisense probes for NOP receptor mRNA in Fig 5.
Figure 4.
NOP receptor mRNA expression in the amygdala and PVN. mRNA expression was significantly greater in (A) the central amygdala and (C) the basomedial amygdala after exposure to acute social defeat. Similar patterns were seen in (B) the medial amygdala, (D) the lateral amygdala, and (E) the basolateral amygdala, although these were not statistically significant. NOP mRNA expression was also elevated in (F) the PVN after acute and repeated plus acute social defeat. Values expressed are group means in optical density ± SEM (n = 4 rats per group). Significant differences between the stress-exposed rats and the no stress controls are expressed as * p < 0.05 and ** p < 0.01. NR/NA= no repeated, no acute social defeat; NR/A = no repeated, acute social defeat; R/NA = repeated, no acute social defeat; R/A = repeated plus acute social defeat.
Table 3.
Summary of between-groups differences in optical densities for NOP receptor mRNA in selected limbic and extra-limbic forebrain regions after differing histories of acute and repeated social defeat stress. Values expressed are mean optical densities (±SEM) after in situ hybridization with riboprobes aimed at NOP receptor mRNA. OD scores were obtained in the same manner as in Table 2.
| NOP receptor mRNA optical densities | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | NR/NA | NR/A | R/NA | R/A | F value, df = (3,15) | p value | sig. | |
| medial prefrontal cortex | 49.3 ± 3.6 | 47.2 ± 3.7 | 54.0 ± 7.1 | 60.3 ± 5.9 | 1.199 | 0.352 | n.s. | |
| caudate | 8.4 ± 0.8 | 0.4 ± 4.6 | 14.6 ± 7.8 | 17.4 ± 3.3 | 2.406 | 0.118 | n.s. | |
| septum | dorsolateral | 28.5 ± 4.3 | 48.8 ± 6.7 | 39.0 ± 10.7 | 53.4 ± 3.0 | 2.615 | 0.099 | n.s. |
| infralimbic | 19.2 ± 5.6 | 32.8 ± 8.2 | 31.2 ± 9.2 | 45.3 ± 2.4 | 2.399 | 0.119 | n.s. | |
| ventrolateral | 38.2 ± 3.1 | 53.8 ± 7.9 | 50.1 ± 9.0 | 58.0 ± 6.1 | 0.274 | 0.262 | n.s. | |
| BNST | medial anterior | 41.3 ± 3.2 | 59.8 ± 8.0 | 55.7 ± 6.7 | 62.8 ± 5.9 | 2.351 | 0.124 | n.s. |
| medial posterior | 101.4 ± 12.3 | 103.9 ± 7.7 | 107.8 ± 3.7 | 104.2 ± 11.1 | 0.080 | 0.970 | n.s. | |
| lateral | 40.9 ± 2.1 | 49.0 ± 9.7 | 51.0 ± 2.7 | 49.4 ± 5.3 | 0.606 | 0.624 | n.s. | |
| medial ventral | 37.1 ± 7.4 | 38.6 ± 12.4 | 77.0 ± 12.8 | 47.6 ± 16.2 | 2.184 | 0.148 | n.s. | |
| amygdala | central | 83.6 ± 4.0 | 111.3 ± 3.6 b | 90.3 ± 6.8 | 100.9 ± 5.9 | 5.376 | 0.014 | * |
| medial | 110.7 ± 5.8 | 131.1 ± 6.2 | 104.9 ±7.4 | 114.7 ± 9.8 | 2.269 | 0.133 | n.s. | |
| basomedial | 76.4 ± 4.8 | 104.0 ± 6.1 a | 77.5 ± 5.8 | 91.1 ± 5.7 | 5.391 | 0.014 | * | |
| lateral | 59.0 ± 4.5 | 82.0 ± 4.9 | 60.2 ± 10.2 | 66.2 ± 6.2 | 2.384 | 0.120 | n.s. | |
| basolateral | 50.2 ± 6.1 | 70.8 ± 3.0 | 53.7 ± 8.0 | 57.3 ± 7.7 | 1.908 | 0.182 | n.s. | |
| hypothalamus | medial preoptic | 102.3 ± 7.4 | 104.1 ± 9.9 | 108.4 ± 4.8 | 111.0 ± 8.9 | 0.246 | 0.862 | n.s. |
| paraventricular | 115.2 ± 8.1 | 168.5 ± 8.7 b | 143.9 ± 8.3 | 157.5 ± 10.3 a | 6.683 | 0.007 | ** | |
| ventromedial | 159.5 ± 6.0 | 201.9 ± 18.3 | 155.8 ± 7.7 | 176.8 ± 16.8 | 2.481 | 0.111 | n.s. | |
| arcuate | 90.7 ± 8.0 | 119.6 ± 16.5 | 92.5 ± 11.9 | 108.1 ± 17.8 | 0.948 | 0.448 | n.s. | |
NR/NA= no repeated, no acute social defeat; NR/A = no repeated, acute social defeat; R/NA = repeated, no acute social defeat; R/A = repeated plus acute social defeat.
p<0.05,
p< 0.01 for ANOVAs,
p<0.05,
p< 0.01 for Dunnett’s post comparisons between specific groups and the unstressed controls.
Figure 5.
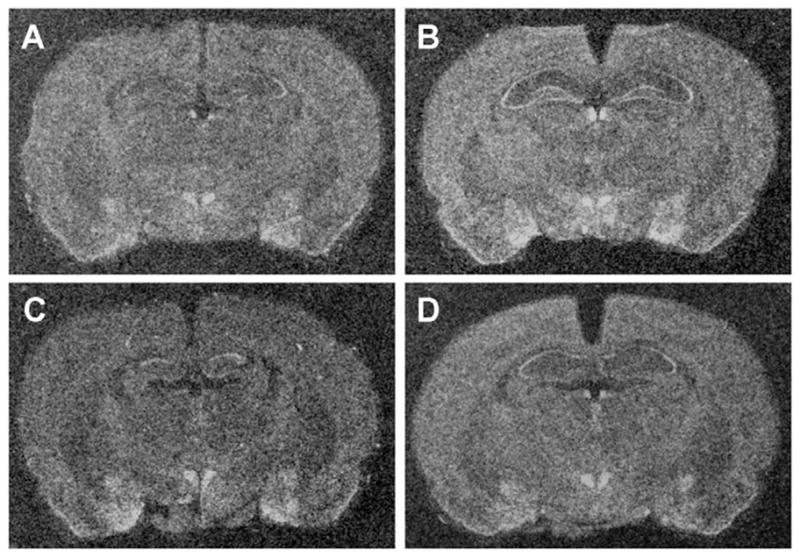
Representative x-ray images of brain sections showing NOP receptor mRNA expression in the amygdala and paraventricular nucleus of the hypothalamus. Sections were exposed to 35S-labelled riboprobes complimentary to segments of NOP receptor mRNA. (A) No stress control rats display moderate signal in the PVN and in the subnuclei of the amygdala. Rats exposed to (B) acute social defeat displayed elevated expression in the PVN, CeA, and BMA. In addition, the rats exposed to (D) repeated plus acute social defeat displayed elevated mRNA expression in the PVN. The rats exposed to (C) repeated, no acute social defeat did not display any differences in NOP mRNA expression as compared to the expression in unstressed control rats.
4. Discussion
Social defeat is a potent stressor that produces substantial physiological changes in intruder rats. The HPA axis is activated in intruder rats following single social defeat exposures, as evidenced by transient increases in circulating ACTH (Heinrichs et al., 1992; Ebner et al., 2005) and CORT (Heinrichs et al., 1992; Covington, III and Miczek, 2001; Ebner et al., 2005). After exposure to repeated social defeat, there are enduring physiological changes, such as increases in basal CORT (de Goeij et al., 1992), decreases in thymus and seminal vesicle masses (Buwalda et al., 2001), and decreases in the amplitude of circadian cardiac and thermoregulatory rhythms (Tornatzky and Miczek, 1993).
Repeated social defeat also induces long-term behavioral changes. This includes increased anxiety-related behaviors in the elevated plus maze (Heinrichs et al., 1992; Calfa et al., 2006) and increased behavioral despair in the Porsolt test (Rygula et al., 2005). All of these results suggest that repeated social defeat may be a valuable tool for studying potential long-term effects of chronic stress exposure.
Using the social defeat procedure, we compared prepro-N/OFQ and NOP receptor gene expression between rats in basal conditions, and rats that were exposed to acute and/or repeated stress. In general, basal expression of prepro-N/OFQ and NOP receptor mRNA (i.e. from Group 1) agreed with previous anatomical examinations, with more abundant expression in a variety of limbic and limbic-associated forebrain regions than in surrounding regions. Those regions included cortex, septum, BNST, and subnuclei of the hypothalamus and amygdala (Neal et al., 1999a, b).
4.1 Prepro-N/OFQ mRNA
Devine and colleagues (2003) previously found decreased neuronal content of N/OFQ from dissected forebrain following acute stress exposure, and the N/OFQ content was replenished within 24 h. In order for the peptide content to be replenished, it is reasonable to presume that elevated transcription and protein synthesis may occur. If so, then acute exposure to social defeat (i.e. Group 2) should produce increases in prepro-N/OFQ mRNA. However, we did not observe such an increase in the present experiment. It is possible that in the present study the time-point selected was not during the peak of the mRNA upregulation. It is also possible that there is a relatively small upregulation of mRNA in selected brain regions, (e.g. subnuclei of septum and BNST) and that N/OFQ peptide stores are slowly replenished over an extended portion of that 24-h period in these areas.
4.2 NOP receptor mRNA
The finding that NOP mRNA is increased in central and basomedial amygdaloid subnuclei after acute social defeat stress (Group 2) complements the findings from other studies in which N/OFQ neurotransmission has been implicated in anxiety-related behaviors. In fact, although conflicting anxiogenic and anxiolytic actions have been observed after icv injections of N/OFQ (Jenck et al., 1997; Gavioli et al., 2002; Fernandez et al., 2004; Kamei et al., 2004; Vitale et al., 2006; Green et al., 2007), we consistently find increases in anxiety-related behaviors when rats are tested for neophobia in an open field after acute intraparenchymal injections directly into the amygdala (Green et al., 2007).
The lack of elevation in amygdaloid NOP receptor mRNA after the last social defeat session in those rats that were exposed to repeated social defeat (Group 4), suggests that the rats developed tolerance in this biochemical response to the stressor. This is somewhat surprising, since efforts were made to assure that the rats were exposed to a different resident on each day of the stress regimen, including the final day. However, the data suggest that social stress-induced changes in expression of amygdaloid NOP receptor mRNA are a transient response that undergoes habituation during repeated exposure to the stressor. This finding is redolent of the fact that c-fos expression is activated in amygdaloid nuclei after acute social defeat and in some of those nuclei (e.g. central amygdala), it is no longer activated by stress exposure after repeated defeat (Martinez et al., 1998).
The finding that NOP receptor mRNA expression was elevated in the PVN following acute social defeat, both in rats that were previously not exposed to stress (Group 2) and in rats that had a previous history of stress (Group 4), complements previous reports that icv N/OFQ administration activates the HPA axis in rats (Devine et al., 2001; Fernandez et al., 2004; Green et al., 2007) and produces increases in CRH mRNA in the PVN (Leggett et al., 2006). Furthermore, the persistent elevations in PVN NOP receptor mRNA even after repeated defeat is reminiscent of the fact that PVN c-fos expression is elevated by social defeat in both naïve and repeatedly defeated rats (Martinez et al., 1998). The findings raise the interesting possibility that stress-induced plasticity in PVN NOP receptor expression could result in increased sensitivity of the HPA axis to N/OFQ. This potential will need to be investigated further with studies of HPA axis function in repeatedly stressed rats.
4.3 Conclusions
We have presented evidence of plasticity in expression of NOP receptor mRNA in response to acute stress, particularly in the amygdala and the PVN, two regions that are important in behavioral and physiological responses to stress. It remains evident that N/OFQ neurotransmission is an important modulator of normal responses to emotional stressors (Jenck et al., 1997; Devine et al., 2001; Gavioli et al., 2002; Nicholson et al., 2002; Fernandez et al., 2004; Kamei et al., 2004; Leggett et al., 2006; Vitale et al., 2006; Green et al., 2007), and it may be implicated in the pathophysiology of depression (Redrobe et al., 2002; Gavioli et al., 2003, 2004; Rizzi et al., 2007). The potential involvement of this system in stress-induced psychopathology is further suggested by stress-induced changes in NOP receptor mRNA expression.
Acknowledgments
The authors than Dr. Huda Akil for her support of this project. This research was supported by Grant # 0515136 from the National Science Foundation, and by Grant # P01 DA021633 from the National Institutes of Health (NIDA).
References
- Buwalda B, Felszeghy K, Horvath KM, Nyakas C, de Boer SF, Bohus B, Koolhaas JM. Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Physiol Behav. 2001;72:349–354. doi: 10.1016/s0031-9384(00)00414-5. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Calfa G, Volosin M, Molina VA. Glucocorticoid receptors in lateral septum are involved in the modulation of the emotional sequelae induced by social defeat. Behav Brain Res. 2006;172:324–332. doi: 10.1016/j.bbr.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. Central serotonin depletion modulates the behavioural, endocrine and physiological responses to repeated social stress and subsequent c-fos expression in the brains of male rats. Neurosci. 1999;92:613–625. doi: 10.1016/s0306-4522(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacol. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, Kilpatrick GJ, Jenck F. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther. 2001;298:812–819. [PubMed] [Google Scholar]
- de Goeij D, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992;577:165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- Devine DP, Hoversten MT, Ueda Y, Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurons during acute stress. J Neuroendocrinol. 2003;15:69–74. doi: 10.1046/j.1365-2826.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- Devine DP, Watson SJ, Akil H. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic-pituitary-adrenal axis. Neurosci. 2001;102:541–553. doi: 10.1016/s0306-4522(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm Behav. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behaviour and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacol. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Caló G. Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci. 2003;17:1987–1990. doi: 10.1046/j.1460-9568.2003.02603.x. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rae GA, Caló G, Guerrini R, De Lima TC. Central injections of nocistatin or its C-terminal hexapeptide exert anxiogenic-like effect on behaviour of mice in the plus-maze test. Br J Pharmacol. 2002;136:764–772. doi: 10.1038/sj.bjp.0704739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli EC, Rizzi A, Marzola G, Zucchini S, Regoli D, Calo’ G. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28:1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo’ G. Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:547–553. doi: 10.1007/s00210-004-0939-0. [DOI] [PubMed] [Google Scholar]
- Green MK, Barbieri EV, Brown BD, Chen KW, Devine DP. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides. 2007;41:399–410. doi: 10.1016/j.npep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Orphanin FQ, a novel neuropeptide with anti-stress-like activity. Brain Res. 1999;836:221–224. doi: 10.1016/s0006-8993(99)01684-4. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker HP, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: Anxiolytic profile in the rat. Proc Natl Acad Sci U S A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6986. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Matsunawa Y, Miyata S, Tanaka S, Saitoh A. Effects of nociceptin on the exploratory behavior of mice in the hole-board test. Eur J Pharmacol. 2004;489:77–87. doi: 10.1016/j.ejphar.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Leggett JD, Harbuz MS, Jessop DS, Fulford AJ. The nociceptin receptor antagonist [Nphe1,Arg14,Lys15]nociceptin/orphanin FQ-NH2 blocks the stimulatory effects of nociceptin/orphanin FQ on the HPA axis in rats. Neurosci. 2006;141:2051–2057. doi: 10.1016/j.neuroscience.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Miczek KA, Covington HE, III, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid RK, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with 125I-[14Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid RK, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Nicholson JR, Akil H, Watson SJ. Orphanin FQ-induced hyperphagia is mediated by corticosterone and central glucocorticoid receptors. Neurosci. 2002;115:637–643. doi: 10.1016/s0306-4522(02)00290-7. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neurosci. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Redrobe JP, Caló G, Regoli D, Quirion R. Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:164–167. doi: 10.1007/s00210-001-0511-0. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calo G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther. 2007;321:968–974. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, Xu X, Korfmacher WA, Tulshian D, Parker EM, Higgins GA. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 2005;182:132–143. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- Vitale G, Arletti R, Ruggieri V, Cifani C, Massi M. Anxiolytic-like effects of nociceptin/orphanin FQ in the elevated plus maze and in the conditioned defensive burying test in rats. Peptides. 2006;27:2193–2200. doi: 10.1016/j.peptides.2006.04.003. [DOI] [PubMed] [Google Scholar]