Abstract
The clinical benefits of BRAF inhibition in patients with advanced-stage BRAF-mutant melanoma are now well established. Although the emergence of cutaneous squamous cell carcinomas (SCCs) and secondary melanomas in patients on BRAF-inhibitor therapy have been well described, reports are emerging of additional secondary premalignant and malignant events, including RAS-mutant leukaemia, the metastatic recurrence of RAS-mutant colorectal cancer and the development of gastric and colonic polyps. In most cases, paradoxical MAPK activation—resulting from the BRAF-inhibitor-mediated homodimerization and heterodimerization of nonmutant RAF isoforms—seems to underlie the development of these secondary tumours. Although evidence supports that therapy with the simultaneous administration of BRAF and MEK inhibitors mitigates the onset of treatment-induced SCCs, whether combination treatment will limit the emergence of all BRAF-inhibitor-driven pathologies is unclear. In this Review, we describe the clinical and mechanistic manifestations of secondary cancers that have thus far been observed to arise as a consequence of BRAF inhibition. We discuss the concept of pre-existing populations of partly transformed cells with malignant potential that might be present in various organ systems and the rationale for novel therapeutic strategies for the management of BRAF-inhibitor-induced neoplasia.
Introduction
One of the most compelling examples of targeted therapy has been the development of BRAF inhibitors for the treatment of advanced-stage BRAF-mutant melanoma.1–4 Mutations in exon 15 of the BRAF gene are known to drive the tumorigenic behaviour of over 50% of all cutaneous melanomas.5 These mutations are not restricted to melanoma; they are also found in 10% of colorectal carcinomas, 40% of papillary thyroid carcinomas and nearly all hairy cell leukaemias.5–8 BRAF is a member of the RAF kinase family of signal transduction protein kinases, which are involved in cell growth (Figure 1). In its oncogenic form, BRAF increases the activity of the MAPK/ERK pathway independently of RAS.9 When active in melanoma cells, constitutive MAPK signalling drives tumour progression through increased cell-cycle entry (via regulation of cyclin D1), suppression of apoptosis (through the negative regulation of Bcl-2-like protein 11 [BIM] and Bcl-2-modifying factor [BMF]) and through the enhancement of cell motility and invasion (via the control of the cytoskeleton, integrin expression and matrix metalloproteinase expression).10–15 Inhibition of either RAF or MEK in BRAF ‘addicted’ cancers is associated with inhibition of cell-cycle entry, decreased cell survival and rapid tumour regression.4, 13, 16–18
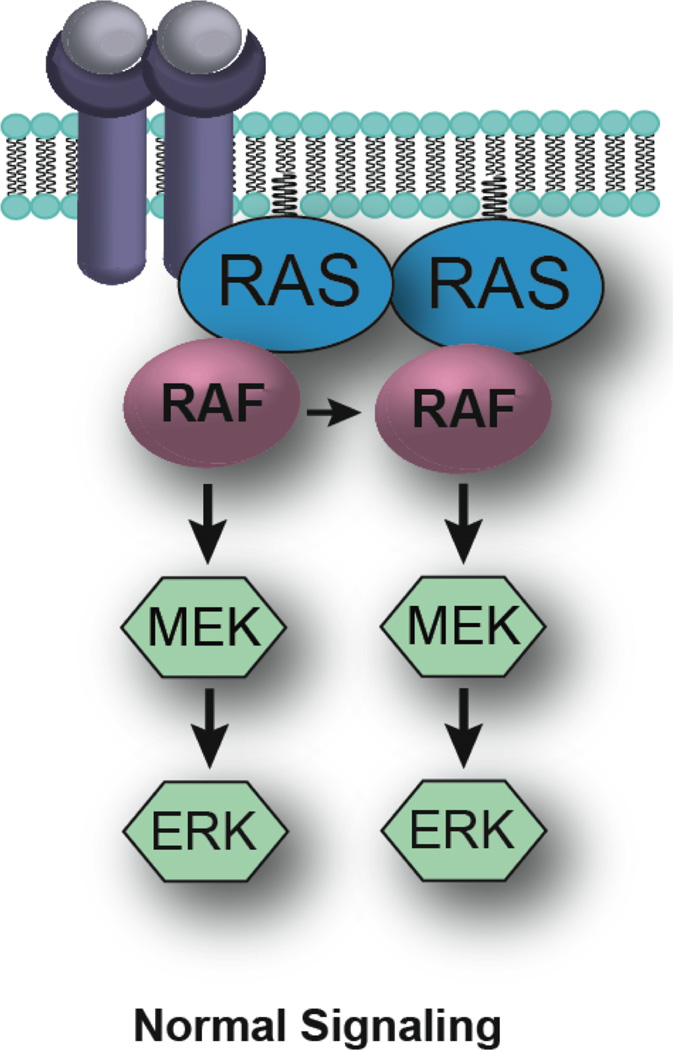
Figure 1.
RAF activation of the MAPK/ERK pathway. Under normal conditions, the growth signalling cascades are initiated through binding of growth factors to growth factor receptor tyrosine kinase receptors at the cell surface. The GTPase RAS is then recruited to the plasma membrane, leading to its activation. RAS binds to and promotes dimer formation of the RAF family of kinases, a process crucial for kinase activation and downstream signal transduction.
Small-molecule BRAF inhibitors have proven highly effective at inhibiting the BRAFV600E mutant form of the kinase, which accounts for approximately 80% of the BRAF mutations found in melanoma.4, 18, 19 The two selective inhibitors most extensively studied thus far—vemurafenib and dabrafenib—are potent inhibitors of BRAFV600E, with half maximal inhibitory concentration (IC50) values of 31 nM and 0.6 nM, respectively.4, 20 Despite some selectivity for the BRAF mutation, both agents also inhibit wild-type BRAF and CRAF, albeit less potently (vemurafenib IC50: BRAFWT, 100nM, CRAF 48nM. Dabrafenib IC50: BRAFWT, 3.2 nM, CRAF, 5nM).4, 20 In addition, MEK inhibitors—such as selumetinib, MEK162 and trametinib—have shown good efficacy in preclinical models of BRAF-mutant melanoma and have advanced to phase II and III clinical trials.16, 17, 21–24 In patients whose melanomas harbour oncogenic BRAF, both BRAF and MEK inhibitors are associated with good clinical activity and objective response rates ranging from 20% (trametinib, MEK-162) to 50% (dabrafenib, vemurafenib).3, 23, 25, 26
Although patients with BRAFV600-mutant melanoma show clear benefits on BRAF-inhibitor or MEK-inhibitor therapy, nearly all eventually show signs of disease progression.10, 25, 27 For example, median progression-free survival has been reported to be 5.1 months and 5.3 months in the phase III evaluations of dabrafenib and vemurafenib, respectively.3, 25 Multiple mechanisms of acquired drug resistance have been reported to explain these findings, including the development of concurrent NRAS or MEK mutations, increased receptor tyrosine kinase (RTK) signalling and mutant BRAF gene-splice variants or amplification.10, 28–31 A convergent event at the time of BRAF-inhibitor resistance is the reactivation of the MAPK pathway. In preclinical studies, MAPK signalling has been shown to rapidly recover following inhibition of BRAF, a result of depressed feedback inhibition and adaptive signalling through RTKs (including the HER family kinases).13, 32 Further experimental studies have suggested that responses to BRAF inhibitors are potentiated by combination with MEK inhibitors, and this strategy is being actively pursued in the clinic.13, 32 Published phase I–II data on the combination of the BRAF inhibitor dabrafenib with the MEK inhibitor trametinib seem very promising, with an overall response rate as high as 76% compared with 54% in patients receiving dabrafenib monotherapy, and a progression-free survival hazard ratio of 0.39 favouring the combination therapy.33
Although properly selected patients with melanoma generally benefit from receiving BRAF or MEK targeted therapies, adverse events can occur on treatment, including the emergence of secondary malignancies. The current data suggests that unintended or paradoxical activation of MAPK signalling might underlie the majority of the secondary malignancies, hence dual BRAF and MEK inhibition might abrogate these issues.33 In this Review, we discuss the available data on the toxicities observed in patients receiving BRAF inhibitors, with a special focus on the role of paradoxical MAPK activation in the development of secondary malignancies. We further outline the possible long-term consequences of chronic BRAF inhibitor treatment and explore consideration of combination strategies for patients receiving long-term therapy.
Paradoxical activation of MAPK
The paradoxical activation of MAPK signalling in cell lines with either RAS mutations or upstream RTK activity was an unexpected observation that emerged during the development of small-molecule BRAF inhibitors.34–36 Under normal conditions, cell growth is initiated through the binding of growth factors to their respective cell-surface growth-factor receptors. This event, in turn, leads to the recruitment and activation of the GTPase RAS, which then binds to and induces the dimerization of the downstream RAF family of serine/threonine kinases—a three-member family consisting of ARAF, BRAF and CRAF (Figure 1).37, 38 In the case of the best-studied RAF isoform CRAF (also known as Raf-1), dimerization occurs following the binding of GTP-bound RAS to CRAF,39, 40 which induces CRAF to adopt an open conformation that exposes dimerization sites. Dimerization of CRAF then leads to its phosphorylation at Ser338 and activation of CRAF kinase activity.41, 42 Some evidence suggests that CRAF (when dimerized) mediates its own transphosphorylation at Ser338.43
The importance of dimerization for the RAF activation process is illustrated by the observation that BRAF mutants with impaired kinase activity (and even those that are ‘kinase-dead’) can still stimulate the MAPK pathway through dimerization with CRAF, leading to RAS-independent activation.44 In a similar manner, the elimination of the RAS-binding domain of BRAF—owing to alternate splicing—leads to BRAF dimerization and MAPK activation, which constitutes an important mechanism of acquired resistance to BRAF inhibitors.28 One surprise early finding was the ability of kinase-dead BRAF mutants to activate MAPK signalling in cell-culture models, but not in isolated kinase assays.44 Biochemical studies revealed these effects to be mediated through the formation of heterodimers between impaired BRAF kinase mutants and nonmutant RAF isoforms, leading to downstream MAPK pathway activation (Figure 2).44 The process of RAF dimerization is also thought to be important in determining substrate specificity, with the BRAF/CRAF heterodimer known to be more efficient at phosphorylating MEK than BRAF and CRAF monomers or BRAF/BRAF and CRAF/CRAF homodimers.45
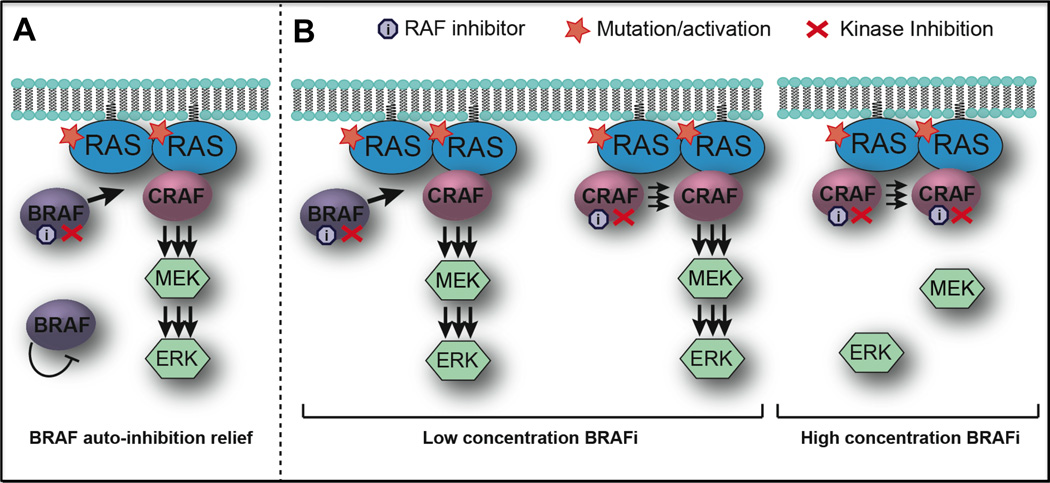
Figure 2.
Paradoxical activation of the MAPK/ERK pathway in tumours treated with RAF inhibitors. a | In cells with mutant RAS, BRAF is typically sequestered in the cytosol and is kept inactive either through autophosphorylation or by phophorylating another protein that keeps it in an inactive state. One study has demonstrated that inhibiting BRAF in the presence of a mutated or growth-factor-activated RAS leads to relief of BRAF autoinhibition and, consequently, its recruitment to the plasma membrane where it dimerizes with and hyperactivates CRAF.46 b | Another suggested mechanism is focused around conformational changes in BRAF and CRAF caused by physical binding of the RAF inhibitor, promoting dimer formation between an uninhibited CRAF protomer and an inhibitor-bound BRAF or CRAF. At low concentrations, the drug binds only one RAF protomer and leads to transactivation of the other. At high concentrations, the drug binds and inhibits both RAF members of the dimer, blocking the signalling complex entirely.35, 47
The formation of RAF dimers also seems to underlie the paradoxical activation of the MAPK signalling pathway that occurs when cells with upstream RAS activity are treated with BRAF inhibitors.35, 46, 47 This process mirrors the dimerization between low-activity BRAF mutants and wild-type BRAF, whereby the binding of drug to one RAF protomer induces the binding to and transactivation of its dimerization partner, a process that results in MAPK activation.44 This ability to induce dimerization and activation of BRAF seems to be independent of which conformation the inhibitor stabilizes (active or inactive), with the binding of the BRAF inhibitor to one of the protomers being the prerequisite for dimerization to occur.
Two subtly different mechanistic explanations for the phenomenon of paradoxical MAPK activation have been offered.46–48 The first postulates that BRAF is trapped in the cytosol in an autoinhibited state and that the binding of the BRAF inhibitor to wild-type BRAF releases the protein, enabling it to dimerize with CRAF (Figure 2a).46 The alternate model suggests that, at low concentrations, the BRAF inhibitor binds to one of the RAF protomers, changes the protein conformation that drives the dimerization and transactivation of the inhibitor-free protomer and ultimately stimulates the MAPK pathway (Figure 2b).35 In both of these cases, the paradoxical MAPK signalling that occurs following BRAF inhibition depends on the upstream activity at the level of RAS, that can arise from either increased RTK signalling or directly as a result of activating mutations in Ras.34, 35, 46, 49 Furthermore, in addition to its effects upon MAPK signalling, BRAF inhibition can also drive the invasion and survival of NRAS-mutant melanoma cells through the activation of focal adhesion kinase (FAK) signalling and the maintenance of Mcl-1 (induced myeloid leukaemia cell differentiation protein Mcl-1) expression, respectively.34, 50
Adverse effects of BRAF inhibition
Compared with the considerable off-target effects of cytotoxic chemotherapies, such as damage to rapidly dividing cells in the bone marrow, gut and hair follicles,51 the toxicities observed with dabrafenib and vemurafenib are relatively mild, with both drugs being well tolerated. Common grade 2 and grade 3 toxicities include skin events, gastrointestinal symptoms, arthralgia, fatigue, headache, and pyrexia.3, 25 In the respective phase III trials, these adverse effects led to dose interruptions or modifications in 28% of patients taking dabrafenib and 38% of patients taking vemurafenib.2, 3 However, patients will typically continue on therapy after improvement in symptoms after dose modification and/or interruption, with only 3% of patients discontinuing the drug (in the case of dabrafenib) because of off-target effects.3
Cutaneous adverse effects
Some of the more-serious adverse events associated with BRAF inhibitors are cutaneous in origin (Figure 3) and include photosensitivity, rash, hyperkeratosis, verrucous keratosis, papillary lesions, keratoacanthomas and SCCs.52–54 These effects are not unique to the current generation of BRAF inhibitors; similar events have been reported following the administration of earlier generation (less-specific) RAF kinase inhibitors, such as sorafenib, RAF265 and XL281.52, 55 In the BRAF Inhibitor in Melanoma (BRIM) trials of vemurafenib, most of the SCCs (76%) that developed in patients were well-differentiated cutaneous keratoacanthoma-type carcinomas.54 In the phase III trial, 18% of patients developed cutaneous SCCs, keratoacanthomas or both, with a slightly lower incidence of cutaneous SCCs or keratoacanthomas being reported in the phase III trial of dabrafenib (Figure 3a–d).52, 54 The SCCs that developed as a result of RAF inhibition are generally more differentiated than SCCs that resulted from chronic sun damage.52 In most cases, the SCCs developed rapidly after the initiation of RAF inhibition, with some lesions appearing as soon as 2 weeks on drug (median 8 weeks).52 The lesions were managed by simple excision and did not require drug interruption; as yet, no case of metastasis has been reported.52
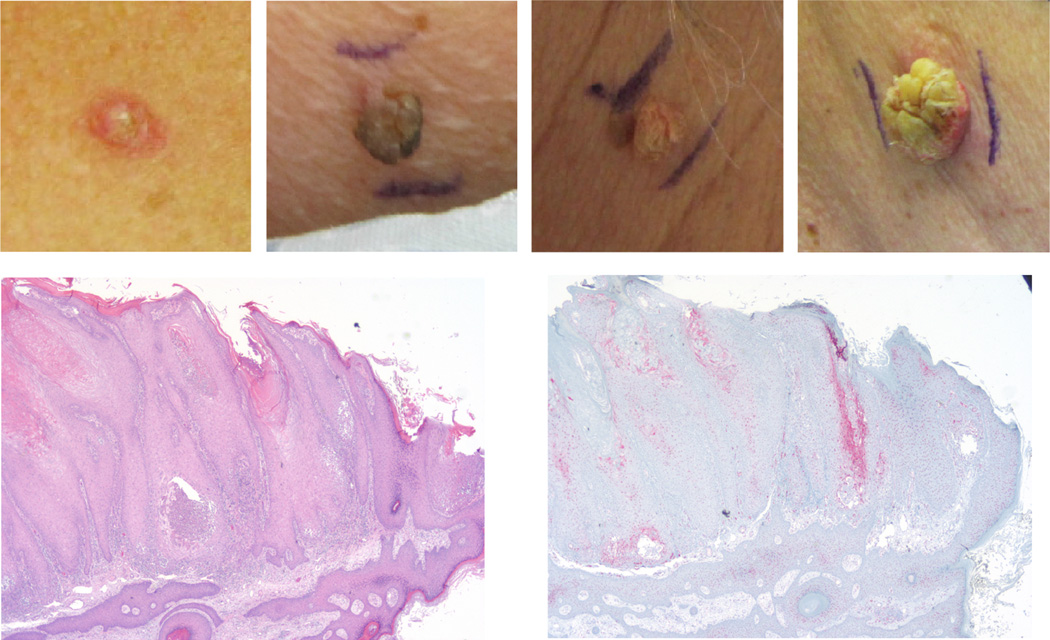
Figure 3.
Secondary cutaneous skin changes in patients receiving vemurafenib treatment. a | Excoriated and ruptured folliculitis with surrounding granulomatous inflammation. b | Inflamed verruca vulgaris with overlying cutaneous horn. c | Inflamed verruca vulgaris. d | Superficial invasive squamous cell carcinoma arising in association with an inflamed verruca vulgaris. e | Immunohistochemical staining (haematoxylin and eosin)of the lesion from panel d. f | Immunohistochemical staining of the lesion shown in d, indicating expression of phosphorylated ERK in the verruca vulgaris.
In approximately 80% of cases, treatment-related SCCs emerge on skin showing signs of chronic ultraviolet radiation damage, as indicated by the presence of solar elastosis.52, 53, 56 Although the association between chronic sun damage and treatment-related SCC development remains to be convincingly demonstrated, ultraviolet radiation is known to be a potent environmental mutagen; skin with sun damage might be a pre-existing source of cell clones that harbour oncogenic mutations.57 In a recent series of three studies, the keratoacanthomas and SCCs emerging in patients on BRAF-inhibitor therapy were subjected to genetic analysis.54, 58, 59 Activating mutations in RAS—particularly the HRAS isoform—were noted to be a common event in the sequenced keratoacanthomas and SCCs. In the first of these studies, 35 keratoacanthoma specimens were screened for mutations in HRAS, KRAS, CDKN2A and TP53; 21 samples harboured RAS mutations and an additional two of 18 samples tested exhibited mutations in TP53.58 As part of the same study, RAS mutations (four in HRAS and four in KRAS) were identified in a validation set of 14 samples.58 Representative immunohistochemical staining of these treatment-derived lesions showed increased expression of phosphorylated ERK in the keratoacanthoma cells compared with the surrounding skin, suggesting paradoxical MAPK signalling only occurred in “initiated” clones of keratinocytes.58
The second study compared the mutational profiles of spontaneous and immunosuppression-related SCCs to those developing in patients on vemurafenib or sorafenib.59 Use of the OncoMap platform, (Dana-Faber Cancer Center, Boston, MA) which could analyse 396 mutations in 33 known oncogenes, showed that 21% of the BRAF-inhibitor-mediated SCCs harboured activating RAS mutations (10 mutations in HRAS, one in KRAS).59 Much lower levels of RAS mutations (3%) were observed in both spontaneous and immune-suppression-mediated SCCs, whereas the overall mutation rates of the three SCC sample groups did not significantly differ.59 In the third study, performed under the auspices of the Vemurafenib Dermatology Working Group, a total of 29 SCCs and keratoacanthomas that developed on vemurafenib therapy were sequenced for mutations in HRAS, KRAS, NRAS and BRAF on the MassARRAY® platform (Sequenom, San Diego, USA).54 Of these, 11 of the SCCs analysed (41%) harboured mutations in HRAS, with eight mutations observed at exon 2 and three mutations identified at exon 3.54
SCCs are not the only keratinocyte hyperproliferations to emerge in patients on BRAF inhibitor therapy. Veruccous keratoses are distinctive, papillomatous keratotic lesions that can show histologic atypia and are thought to be premalignant.53 In an Australian cohort of patients undergoing dabrafenib treatment, 49% of patients presented with drug-induced verrucous keratoses, with a median time to emergence of 11.6 weeks.53 Although these lesions did not seem to be fully malignant, mutations in HRAS and KRAS were observed in eight of 11 cases.52, 53 Veruccous keratoses, like keratoacanthomas, also showed increased expression of phosphorylated ERK on immunohistochemical staining compared with the surrounding skin (Figure 3e and f). Although RAS mutations are common in BRAF-inhibitor-associated keratinocyte events, these results suggest that factors in addition to mutant RAS might be required for full malignant development.
The role of paradoxical MAPK signalling in BRAF-inhibitor-induced keratoacanthoma and SCC development is strongly supported by preclinical studies. Experiments have shown that treatment of the HRASQ61L mutant B9 mouse SCC cell line with the BRAF inhibitor PLX4720 led to increased colony formation on soft agar, which was associated with a MAPK pathway activation gene signature (Spry2, Dusp6, Fos, Fos11 and Egfr1).58 Similar growth-promoting effects were also noted when human A431 SCC cells engineered to express mutant HRAS were treated with PLX4720.58 In an in vivo mouse model, in which the topical application of 7,12-dimethylbenz(a)anthracene (DMBA) and 12-O-tetradecanolyphorbol-13-acetate (TPA) induces the development of HRASQ61L-mutant cutaneous SCCs, the tumour promoting effects of PLX4720 were found to be more subtle than would be expected from the in vitro experiments. Under these conditions, PLX4720 did not increase the number of SCCs that emerged following treatment with DMBA and TPA, but shortened the latency of tumour development.58 It was further noted that mice given DMBA and PLX4720 alone did not develop tumours, again suggesting that BRAF inhibition was not directly carcinogenic. However, the role of paradoxical MAPK signalling was demonstrated by the ability of the MEK inhibitor PD18352 to suppress SCC development following DMBA, TPA and PLX4720 treatment.58 Interestingly, inhibition of MEK was not found to be effective against established SCC in the two-step carcinogenesis model, suggesting that although MAPK signalling was an initiating factor for SCC development, sustained activity was not required for tumour maintenance.
Nevi and secondary melanomas
Although SCCs are the most common cutaneous malignancies to arise on BRAF-inhibitor therapy, melanocytic hyperproliferations and new primary melanomas have also been reported.25, 56, 60, 61 For example, from the phase II and III studies of vemurafenib, 10 cases of new primary melanomas and one case of a new dysplastic nevus were reported in patients receiving vemurafenib.61 Similarly, three new primary melanomas were reported in patients receiving dabrafenib in the BREAK-3 phase III study.3 In the largest case series to date, 22 new melanocytic lesions were identified in 19 patients on selective BRAF-inhibitor therapy at seven institutions.60 Of these, 12 were newly detected primary melanomas and 10 were nevi, with nine of the nevi being dysplastic. Although the time to onset of these melanomas is comparable to BRAF-inhibitor-induced SCCs (median 8 weeks), the incidence rate of primary melanoma seems to be approximately 10-fold lower than that of SCC. However, five of the new melanoma cases identified in the phase III vemurafenib study were observed during total-body skin dermoscopy examination at a single centre;60 consequently, the actual incidence of dysplastic nevi and new primary melanomas occurring on selective BRAF inhibitors is not yet clear.
Available genotyping data on a limited number of new primary melanomas and dysplastic nevi developing in patients on selective BRAF-inhibitor therapy have routinely demonstrated the lesions to have wild-type BRAF status.60, 61 Interestingly, one melanoma was found to harbour an NRASQ61R mutation.60 In another report, a new primary melanoma arising on BRAF-inhibitor therapy was found to be wild-type for both BRAF and NRAS.56 Immunohistochemical analysis of signalling in the new primary melanomas have shown nonsignificant increases in staining of phosphorylated ERK, and an increase in phosphorylated-AKT signalling that was statistically significant60. Although the lack of BRAF mutations and the trend towards increased ERK signalling is suggestive of a role for paradoxical MAPK activation in the development of new primary melanomas, further study will be required to establish this link.
Noncutaneous RAS-mutant malignancies
Although secondary cutaneous malignancies during selective BRAF-inhibitor therapy are most commonly reported, rare cases of noncutaneous malignant development have also been reported. In one recent case report, a 76-year old man with recurrent BRAFV600K mutant melanoma was initiated on vemurafenib therapy.62 The patient experienced clinical improvement on the BRAF inhibitor and had signs of disease regression. However, following 11 days of treatment, he developed worsening fatigue with a concurrent elevation in his white blood cell count, predominantly of monocytes.62 A diagnosis of myelomonocytic leukaemia was made, presumably existing but undiagnosed prior to the initiation of BRAF-inhibitor therapy, with subsequent genotyping studies showing the presence of an activating an NRASG12R mutation in the monocyte (CD14+), erythroid (CD71+) and megakaryocyte (CD41a+) lineages. The increased ratio of phosphorylated ERK to total ERK expression observed in the monocytes of the patient while on vemurafenib therapy was suggestive of paradoxical MAPK signalling.62 A series of ex vivo experiments revealed that the patients’ NRAS-mutant leukaemia could be stimulated by the BRAF inhibitor PLX4720—an effect that could be reversed on co-treatment with the MEK inhibitor PD0325901. The dependency of the leukaemia on vemurafenib was demonstrated by the marked reduction in the white-cell count and reduced staining of phosphorylated ERK in the monocytic cell fraction seen upon cessation of PLX4720. Although not yet extensively explored in the clinical setting it is possible that the careful titration of the BRAF and MEK inhibitor combination may abrogate the development of secondary Ras mutant tumours.
Although the growth-promoting activity of selective BRAF inhibitors potentially presents a risk for patients with occult RAS-mutant cancers, the situation is less clear for individuals who have previously undergone successful treatment for RAS-mutant tumours. Recently, a case was detailed in which a patient with previously resected Duke B KRASG12D-mutant adenocarcinoma of the colon presented with widely disseminated BRAF-mutant melanoma.63 He was started on BRAF inhibition with dabrafenib and MEK inhibition with trametinib in combination. After 12 weeks of treatment, he experienced objective regression of his metastatic melanoma but the development of a new brain metastasis not seen at initial staging. The patient underwent resection of the brain metastasis, which was characterized as KRAS-mutant colon cancer rather than melanoma. Correlative studies performed on a cell line derived from the brain metastasis showed the BRAF inhibitor stimulated growth, whereas the MEK inhibitor was growth-inhibitory. After a temporary drug hold, the patient was restarted on trametinib monotherapy. Interestingly, worsening pleural disease and increasing carcinoembryonic antigen levels, presumably from metastatic colon cancer, stabilized with MEK inhibitor monotherapy. It is clear that patients with a prior history of Ras mutant cancer need careful monitoring when receiving any BRAF inhibitor-based therapeutic regimen.
Colonic adenomas and gastric polyps
Although the majority of patients receiving BRAF-inhibitor monotherapy experience disease progression after a median of 5–6 months, some individuals do show a significant long-term survival benefit.3, 25 Of the 32 patients treated in the expansion cohort of the phase I study of vemurafenib, eight individuals showed long-term responses (median follow-up duration of 35.9 months, range 34.6–38.2 months).64 With chronic BRAF inhibitor treatment, paradoxical MAPK pathway activation might encourage malignant transformation of other precancerous non-BRAF-mutant cell types. Indeed, early data suggest a possible link between prolonged BRAF-inhibitor treatment and gastrointestinal pathologies. The first case to be reported involved a patient with a negative colonoscopy prior to initiating BRAF inhibitor therapy.65 Following long-term (>2 years) vemurafenib treatment, the patient developed haematochezia and underwent upper endoscopy and colonoscopy, which revealed four colonic adenomas, one hyperplastic colonic polyp and six gastric polyps.65 Similarly, endoscopic analysis on a cohort of four long-term vemurafenib responders revealed the presence of multiple colonic adenomas or gastric polyps in three patients. None of these patients had a familial history of colorectal cancer or polyposis. Although colorectal adenomas are common in individuals >50 years of age (incidence of 20–50%), the emergence of new adenomas within 3 years of a negative endoscopy is unusual.66 Furthermore, gastric polyps are also relatively rare (routinely found in 6% of all endoscopies) and the regrowth of multiple gastric polyps following resection (as was observed in one patient in the cohort) is also an uncommon occurrence, so the likelihood of these lesions being observed by chance alone is low.67, 68
Despite KRAS mutations being found in 10–15% of adenomas <1 cm in size and in 30–60% of adenomas >1 cm, no evidence of RAS mutations was reported in any of the adenomas identified in the long-term vemurafenib treatment cohort.69–71 Instead, seven of the eight lesions assessed harboured mutations in the tumour suppressor gene APC, which is known to be associated with sporadic and hereditary colorectal cancer.65, 69 The tumour-suppressive function of the APC protein depends on its ability to bind to β-catenin (Figure 4).72 This interaction in turn leads to the phosphorylation of β-catenin by GSK3β and casein kinase-1 (CKI) and the subsequent degradation of the APC/β-catenin complex by the proteasome.73, 74 Stabilized β-catenin primarily mediates its oncogenic effects through formation of β-catenin–Tcf4 transcriptional complexes that induce the expression of target genes such as MYC and CCND1.
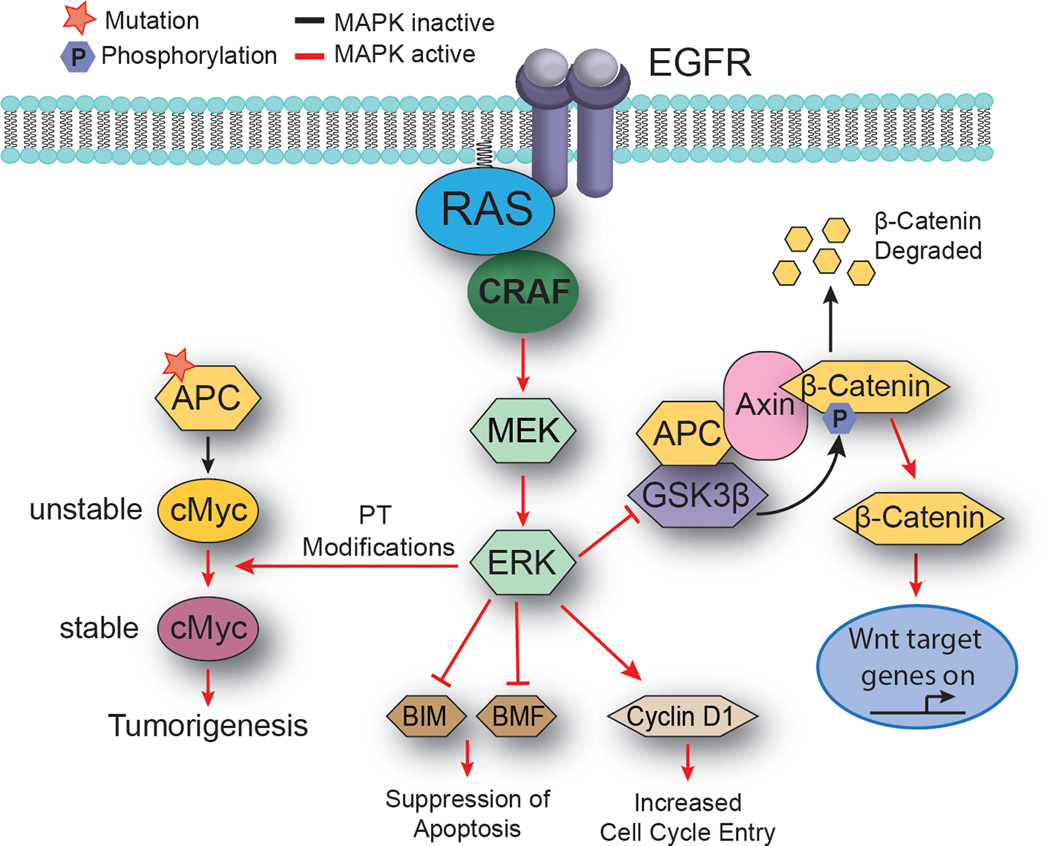
Figure 4.
MAPK signalling cooperates with loss of APC to promote adenoma development. The tumour suppressive function of APC lies in its ability to bind β-catenin, leading to phosphorylation of β-catenin by GSK3β and its subsequent degradation.72 Activation of the MAPK/ERK pathway can mediate β-catenin stabilization through ERK-mediated inhibition of GSK3β.96 Stabilized β-catenin primarily mediates its oncogenic effects through transcriptional activation of target genes such as MYC.75 Pro-oncogenic MYC is vital for APC-mediated tumorigenesis. ERK can further drive tumour formation in the context of APC loss by stabilizing Myc though post-translational modifications.75 Abbreviations: MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; EGFR, epidermal growth factor receptor; APC, adenomatous polyposis coli; GSK3β, Glycogen synthase kinase-3 beta; Axin, axis inhibition protein.
Although cooperation between mutant APC and paradoxical MAPK signalling in human adenoma development has not yet been demonstrated, some evidence suggests that APC loss and MAPK signalling are required for the development of colorectal carcinoma in genetically engineered mouse models. For example, in the APCmin/+ mouse, a model system in which intestinal neoplasia occurs spontaneously following the loss of the second APC allele, MAPK signalling cooperates to drive tumour progression through the phosphorylation and stabilization of pro-oncogenic MYC (Figure 4).75 In this system, MAPK signalling is mediated through the EGFR receptor; evidence supports that BRAF inhibition activates paradoxical MAPK signalling in cell lines with genetic amplification of EGFR receptor family members (for example, HER2).49, 75 Furthermore, BRAF and MEK inhibition can also increase adaptive EGFR and ERBB3 signalling in a variety of cancer cell lines,76–79 which suggests that BRAF inhibitors could activate EGFR signalling in APC-mutant colonic epithelial cells. However, a mechanistic link between these preclinical observations and adenoma development remains to be determined. Together these data suggest the potential for oncogenesis even in cells lacking Ras mutations, raising the possibility that the paradoxical signalling induced following BRAF inhibition may be more complex than initially thought. The true extent of this will only be established through the further analysis of patients showing long-term responses to BRAF inhibitor therapy.
Preventing secondary malignancies
If the primary driver for secondary malignancies during BRAF-inhibitor therapy is the paradoxical activation of the MAPK pathway, a practical approach to prevent these events is concurrent downstream inhibition of either MEK or ERK. Indeed, in the phase I–II study of dabrafenib and trametinib in patients with BRAF-mutant melanoma, the number of patients who developed cutaneous SCCs was lower in the combination arms (five out of 99 patients; 5%) than in the dabrafenib monotherapy arm (10 out of 53 patients; 19%).33 Results from the ongoing phase III trial of dabrafenib plus trametinib versus single-agent dabrafenib (Combi-D) or single-agent vemurafenib (Combi-V) will help determine if concurrent MEK inhibition is an effective strategy for preventing SCCs and, possibly, other secondary malignancies (NCT01584648). However, this strategy is unlikely to resolve the issue completely—SCCs still can occur in patients taking concurrent MEK or ERK inhibitors, and recurrent KRAS-mutant colorectal cancer was noted in a patient on dabrafenib plus trametinib combination therapy.33, 63
An alternative approach to prevent paradoxical activation of the MAPK pathway might be through the use of a new class of pan-RAF inhibitors, so-called paradox breakers. These agents, exemplified by the compound PB04 (PLX7904), were empirically selected for their lack of paradoxical MAPK activation in NRAS-mutant melanoma cells despite retaining the ability to potently block the signalling of phosphorylated ERK in BRAF-mutant melanoma cells.80 Studies have shown that the paradox breakers are proapoptotic and inhibit the anchorage-independent growth of melanoma cell lines; acquired resistance to PLX4720 is mediated through mutant NRASQ61K.80 Further studies will be required to determine if these agents have similar activity to the BRAF and MEK inhibitor combination in preventing the onset of HRAS-mutant SCCs and keratoacanthomas.
In addition to strategies targeting the MAPK pathway, a case report has demonstrated efficacy of the synthetic retinoid acitretin in the management of vemurafenib-induced hyperkeratotic papules and SCCs.53, 81 Indeed, topical and systemic retinoids have been used for various dermatological conditions and have shown efficacy in the prevention of nonmelanoma skin cancers in solid-organ transplant recipients taking immunosuppressive agents.82 These retinoids activate the retinoic acid receptor to regulate gene transcription, ultimately promoting cell maturation and differentiation, as well as decreasing cell growth and malignant transformation.83 In the case reported by Anforth and colleagues,81 a patient with melanoma developed numerous hyperkeratotic lesions after receiving vemurafenib for 10 months. After 10 weeks of acitretin, no additional hyperkeratotic lesions developed. Since retinoid administration can be associated with substantial toxicity, further prospective evaluation will be necessary before this treatment strategy is recommended for general patient care.
Similar to the use of retinoids, inhibition of cyclooxygenase (COX)-2 has been evaluated as a strategy to prevent BRAF-inhibitor-mediated cutaneous SCC development.84 Experimental and preclinical investigations of SCC carcinogenesis induced by ultraviolet light have shown the increase in COX-2 expression and prostaglandin production can be abrogated by the use of the COX-2 inhibitors, such as celecoxib and diclofenac, mitigating SCC development.85–87 In patients with chronic ultraviolet-radiation-mediated skin damage, oral celecoxib (200 mg twice daily) was shown to be an effective chemopreventive strategy to reduce SCC development.88
Importantly, COX-2 inhibition with celecoxib has also been explored as a strategy to reduce the development of colorectal adenomas in individuals at high risk for colorectal polyps or cancers high-risk patients, showing a modest reduction in adenomas detected on colonoscopy.89, 90 Indeed, mouse model experiments suggest that celecoxib treatment delays the onset of SCC development that is mediated by DBMA, TPA and PLX4720, reducing the eventual tumour burden by >90%.84 Whether this regimen could be effective and safe in patients receiving selective BRAF inhibitors remains to be determined.
Insights gained
The ability of BRAF-kinase inhibitors to stimulate the growth of clinically occult, pre-existing mutant cells that have been dormant can provide important new insights into the process of oncogenic transformation. As individuals age, their prolonged exposure to environmental carcinogens increases the likelihood of acquiring potentially deleterious mutations in cells. Most of these will never undergo full oncogenic transformation and are destined to remain in an initiated—but growth-arrested—state throughout the lifetime of the individual. The scale of this phenomenon has been clearly illustrated in postmortem studies of the thyroid and prostate glands; although the incidence of clinically apparent thyroid carcinoma in individuals aged 50–70 years is about 0.1%, careful postmortem analyses of the thyroid glands of a cohort of patients who died following trauma revealed small thyroid tumours in up to 36% of cases.91 Similarly, although the lifetime incidence of prostate cancer in men of all ages is 16%, up to 65% of men aged 61–70 years and 83% of men aged 71–80 years harbour occult prostate cancers that are only detectable postmortem.92, 93 In normal sun-exposed skin, keratinocyte clones can be readily identified that harbour persistent oncogenic TP53 mutations.94
The data to date suggest that, in situations where paradoxical activation of the MAPK pathway as a consequence of selective BRAF inhibition occurs, an oncogenic event might ‘awaken’ pre-existing mutant cells within the body. Although thousands of patients have now received BRAF inhibitor therapy, these drugs have mostly been used in individuals with advanced-stage melanoma. As long-term follow-up data are often lacking for this group of patients, it is currently difficult to judge the full spectrum of potential secondary malignancies that can occur in patients on BRAF-inhibitor therapy. Depending on the results of ongoing trials, single-agent BRAF inhibition may well be superseded by BRAF and MEK inhibitor combination.33, 95 The ability of this combination to abrogate paradoxical MAPK activation is expected to reduce the incidence of secondary malignancies, but might not entirely prevent it.
Conclusions
Selective BRAF inhibitors provide clear clinical benefit to patients with advanced BRAF-mutant melanoma. The major off-target effects of this drug class are associated with the paradoxical activation of the MAPK pathway, leading to the development of secondary malignancies. Although cutaneous SCCs are the best-documented of these, the emergence of other (nonkeratinocyte) secondary tumours is a growing concern. Although the significance of the colonic and gastric polyps associated with chronic BRAF-inhibitor therapy is still unclear, questions remain over whether these lesions will eventually undergo malignant transformation. Long-term follow-up studies and surveillance will be necessary to determine the actual incidence of these events and whether specific screening procedures are warranted for patients undergoing therapy with BRAF inhibitors. As BRAF inhibitors are currently under investigation in the adjuvant setting for patients with resected stage II and III melanoma and are being explored in other BRAF-mutant malignancies, these considerations will become increasingly critical. Indeed, concerns of secondary malignancies have led to the exclusion of patients with a family history of colorectal carcinoma syndromes from the trial of adjuvant vemurafenib in those with resected cutaneous BRAF-mutant melanoma (NCT01667419). Despite these concerns, the development of BRAF inhibitors represents a major milestone in the therapeutic management of disseminated melanoma. With careful patient surveillance and rationally designed drug combinations, the future for patients with advanced BRAF-mutant melanoma and other malignancies continues to look increasingly optimistic.
Key points.
BRAF inhibitors are commonly used in patients with BRAF-mutant melanoma and their use is potentially expanding to other patient populations
Paradoxical activation of the MAPK pathway can occur in cells wild-type for BRAF through the BRAF-inhibitor-mediated formation of RAF dimers
Secondary skin changes, including hyperkeratosis, keratoacanthomas and squamous cell carcinomas, can occur in patients treated with BRAF inhibitors
Secondary melanomas, gastric and colonic polyps and recurrences of pre-existing malignancies have also been reported in patients receiving BRAF inhibitors
Potential strategies to limit the development of or manage treatment-induced cancers include combination therapy with inhibitors of BRAF and MEK, and use of retinoids, topical 5-fluorouracil and cyclooxygenase 2 inhibitors
Acknowledgements
K. S. M. Smalley is supported by NIH/National Cancer Institute grants (R01 CA161107-02 and U54 CA143970-03).
Biographies
Geoffrey T. Gibney
Geoffrey T. Gibney, MD is a medical oncologist in the Department of Cutaneous Oncology at Moffitt Cancer Center, and Assistant Professor of Medicine in the Department of Oncologic Sciences at the University of South Florida Morsani College of Medicine. He has a particular interest in targeted therapy for metastatic melanoma and systemic treatment of melanoma metastatic to the brain and central nervous system. He joined Moffitt in 2011 after completing a fellowship in Medical Oncology at Yale University.
Jane L. Messina
Jane L. Messina, MD is a dermatopathologist and Professor of Pathology in the Department of Pathology and Cell Biology and the Department of Dermatology and Cutaneous Surgery at the University of South Florida Morsani College of Medicine. She is also staff dermatopathologist for the Cutaneous Oncology Program at Moffitt Cancer Center. Her research interests include cutaneous lesions arising after BRAF inhibitor therapy and the diagnosis of melanocytic lesions in children and adolescents.
Inna V. Fedorenko
Inna V. Fedorenko, BS is a graduate student in the laboratory of Dr Keiran Smalley at the Moffitt Cancer Center. Her research interests include targeted therapy for melanoma and how host-tumor interactions mediate drug resistance.
Vernon K. Sondak
Vernon K. Sondak, MD is a surgical oncologist and Chair of the Department of Cutaneous Oncology at Moffitt Cancer Center and Professor of Surgery in the Departments of Oncologic Sciences and Surgery at the University of South Florida Morsani College of Medicine. His research interests include surgical and adjuvant therapy for melanoma and other aggressive cutaneous malignancies, and the treatment of melanocytic tumors in children and adolescents.
Keiran S.M. Smalley
Keiran S. M. Smalley, PhD is the Scientific Director of the Melanoma Research Center of Excellence and an Associate Member at the Moffitt Cancer Center in the Departments of Molecular Oncology and Cutaneous Oncology. He is also an Associate Professor in the Department of Oncologic Sciences at the University of South Florida Morsani College of Medicine. His research interests include the development of new precision medicine approaches for the treatment of melanoma.
Footnotes
Competing interests [Print version]
G. T. Gibney declares an association with the following companies: Genentech and Roche. See the article online for full details of the relationships. All other authors declare no competing interests.
Competing interests [Online version]
G. T. Gibney acts as a consultant or advisory board member for following companies: Genentech and Roche. All other authors declare no competing interests.
Review criteria
A search was performed of all records from PubMed from 2002–2013 of the melanoma and BRAF literature. In addition, searches were made of abstracts from the American Society of Clinical Oncology (ASCO) Annual Meetings, The Society for Melanoma Research (SMR) Annual Meetings and the American Association for Cancer Research (AACR) Annual Meetings.
Author contributions
All authors researched the data for the manuscript, discussed its contents, wrote the article and edited it before submission.
Contributor Information
Geoffrey T. Gibney, Department of Cutaneous Oncology, The Moffitt Cancer Center & Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
Jane L. Messina, Department of Pathology and Cell Biology, University of South Florida College of Medicine, 12901 Bruce B. Downs Boulevard, MDC 11, Tampa, FL 33612, USA
Inna V. Fedorenko, The Department of Molecular Oncology, The Moffitt Cancer Center & Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
Vernon K. Sondak, Department of Cutaneous Oncology, The Moffitt Cancer Center & Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
Keiran S.M. Smalley, The Department of Molecular Oncology, The Moffitt Cancer Center & Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA
References
- 1.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman PB, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011 doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauschild A, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 4.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Tiacci E, et al. BRAF Mutations in Hairy-Cell Leukemia. New England Journal of Medicine. 2011;364:2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura ET, et al. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Research. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 8.Tol J, Nagtegaal ID, Punt CJA. BRAF Mutation in Metastatic Colorectal Cancer. New England Journal of Medicine. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 9.Wellbrock C, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–2342. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 10.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–2233. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartlidge RA, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paraiso KH, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–6681. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arozarena I, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Haass NK, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–239. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- 17.Solit DB, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai J, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long GV, et al. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. Journal of Clinical Oncology. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 20.Menzies AM, Long GV, Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug design, development and therapy. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greger JG, et al. Combinations of BRAF, MEK, and PI3K/mTOR Inhibitors Overcome Acquired Resistance to the BRAF Inhibitor GSK2118436 Dabrafenib, Mediated by NRAS or MEK Mutations. Molecular Cancer Therapeutics. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 22.Kirkwood JM, et al. Phase II, Open-Label, Randomized Trial of the MEK 1/2 Inhibitor Selumetinib as Monotherapy versus Temozolomide in Patients with Advanced Melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ascierto PA, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. The lancet oncology. 2013;14:249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 24.Robert C, et al. METRIC phase III study: Efficacy of trametinib (T), a potent and selective MEK inhibitor (MEKi), in progression-free survival (PFS) and overall survival (OS), compared with chemotherapy (C) in patients (pts) with BRAFV600E/K mutant advanced or metastatic melanoma (MM) J Clin Oncol. 2012;30:LBA8509. [Google Scholar]
- 25.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty KT, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 27.Falchook GS, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulikakos PI, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387-U144. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nazarian R, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagle N, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi HB, et al. Melanoma whole-exome sequencing identifies B-V600E-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nature Communications. 2012;3 doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lito P, et al. Relief of Profound Feedback Inhibition of Mitogenic Signaling by RAF Inhibitors Attenuates Their Activity in BRAFV600E Melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty KT, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halaban R, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the ongenic B-RAF inhibitor, PLX4720, in mutant N-Ras melanoma cell lines. Oncogene. 2010;30:366–371. doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nature reviews. Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 38.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 39.Luo Z, et al. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- 40.Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 2005;20:963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Cho KJ, et al. Raf Inhibitors Target Ras Spatiotemporal Dynamics. Current Biology. 2012;22:945–955. doi: 10.1016/j.cub.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 42.Zang MW, et al. Characterization of SeR338) Phosphorylation for Raf-1 Activation. Journal of Biological Chemistry. 2008;283:31429–31437. doi: 10.1074/jbc.M802855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baljuls A, Kholodenko BN, Kolch W. It takes two to tango--signalling by dimeric Raf kinases. Molecular bioSystems. 2013;9:551–558. doi: 10.1039/c2mb25393c. [DOI] [PubMed] [Google Scholar]
- 44.Wan PT, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 45.Rushworth LK, Hindley AD, O'Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Molecular and Cellular Biology. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heidorn SJ, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatzivassiliou G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 48.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph EW, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan FM, Shao Y, Mayberry MM, Aplin AE. Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene. 2010 doi: 10.1038/onc.2010.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keefe DM, Bateman EH. Tumor control versus adverse events with targeted anticancer therapies. Nature reviews. Clinical oncology. 2012;9:98–109. doi: 10.1038/nrclinonc.2011.192. [DOI] [PubMed] [Google Scholar]
- 52.Anforth R, Fernandez-Penas P, Long GV. Cutaneous toxicities of RAF inhibitors. The lancet oncology. 2013;14:e11–e18. doi: 10.1016/S1470-2045(12)70413-8. [DOI] [PubMed] [Google Scholar]
- 53.Anforth RM, et al. Cutaneous manifestations of dabrafenib (GSK2118436): a selective inhibitor of mutant BRAF in patients with metastatic melanoma. British Journal of Dermatology. 2012;167:1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 54.Lacouture ME, et al. Analysis of Dermatologic Events in Vemurafenib-Treated Patients With Melanoma. The oncologist. 2013 doi: 10.1634/theoncologist.2012-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. Journal of the American Academy of Dermatology. 2009;60:299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 56.Rinderknecht JD, et al. RASopathic skin eruptions during Vemurafenib therapy. PloS One. 2013 doi: 10.1371/journal.pone.0058721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. Journal of photochemistry and photobiology. B, Biology. 2001;63:88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 58.Su F, et al. RAS Mutations in Cutaneous Squamous-Cell Carcinomas in Patients Treated with BRAF Inhibitors. New England Journal of Medicine. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oberholzer PA, et al. RAS Mutations Are Associated With the Development of Cutaneous Squamous Cell Tumors in Patients Treated With RAF Inhibitors. Journal of Clinical Oncology. 2012;30:316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmer L, et al. Atypical Melanocytic Proliferations and New Primary Melanomas in Patients With Advanced Melanoma Undergoing Selective BRAF Inhibition. Journal of Clinical Oncology. 2012;30:2375–2383. doi: 10.1200/JCO.2011.41.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dalle S, Poulalhon N, Thomas L. Vemurafenib in Melanoma with BRAF V600E Mutation. New England Journal of Medicine. 2011;365:1448–1449. doi: 10.1056/NEJMc1108651. [DOI] [PubMed] [Google Scholar]
- 62.Callahan MK, et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. The New England journal of medicine. 2012;367:2316–2321. doi: 10.1056/NEJMoa1208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrews M, et al. Colorectal cancer promoted in a patient receiving dabrafenib (GSK2118436) in combination with MEK1/2 inhibitor trametinib (GSK1120212) Pigment Cell & Melanoma Research. 2012;25:842. [Google Scholar]
- 64.Kim K, et al. Significant long-term survival benefit demonstrated with vemurafenib in ongoing phase I study. Pigment Cell & Melanoma Research. 2012;25:866. [Google Scholar]
- 65.Chapman PB, et al. Development of colonic adenomas and gastric polyps in BRAF mutant melanoma patients treated with vemurafenib. Pigment Cell & Melanoma Research. 2012;25:847. [Google Scholar]
- 66.Heitman SJ, et al. Prevalence of Adenomas and Colorectal Cancer in Average Risk Individuals: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology. 2009;7:1272–1278. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 67.Carmack SW, Genta RM, Schuler CM, Saboorian MH. The Current Spectrum of Gastric Polyps: A 1-Year National Study of over 120,000 Patients. American Journal of Gastroenterology. 2009;104:1524–1532. doi: 10.1038/ajg.2009.139. [DOI] [PubMed] [Google Scholar]
- 68.Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nature Reviews Gastroenterology & Hepatology. 2009;6:331–341. doi: 10.1038/nrgastro.2009.70. [DOI] [PubMed] [Google Scholar]
- 69.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 70.Kressner U, et al. Ki-ras mutations and prognosis in colorectal cancer. European Journal of Cancer. 1998;34:518–521. doi: 10.1016/s0959-8049(97)10111-3. [DOI] [PubMed] [Google Scholar]
- 71.Brink M, et al. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2003;24:703–710. doi: 10.1093/carcin/bgg009. [DOI] [PubMed] [Google Scholar]
- 72.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 73.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. The EMBO journal. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda S, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. The EMBO journal. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SH, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nature Medicine. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prahallad A, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 77.Turke AB, et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Research. 2012;72:3228–3237. doi: 10.1158/0008-5472.CAN-11-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corcoran RB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discovery. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abel EV, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. The Journal of clinical investigation. 2013 doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le K, Blomain E, Aplin AE. Selective RAF inhibitor impairs ERK1/2 phosphorylation and growth in mutant NRAS, vemurafenib-resistance melanoma cells. Pigment Cell & Melanoma Research. 2013 doi: 10.1111/pcmr.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anforth R, Blumetti TC, Mohd Affandi A, Fernandez-Penas P. Systemic retinoid therapy for chemoprevention of nonmelanoma skin cancer in a patient treated with vemurafenib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:e165–e167. doi: 10.1200/JCO.2011.39.8594. [DOI] [PubMed] [Google Scholar]
- 82.Lien MH, Fenske NA, Glass LF. Advances in the chemoprevention of non-melanoma skin cancer in high-risk organ transplant recipients. Seminars in oncology. 2012;39:134–138. doi: 10.1053/j.seminoncol.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 83.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nature reviews. Drug discovery. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 84.Escuin-Ordinas H, et al. COX2 inhibition prevents the appearance of cutaneous squamous cell carcinomas accelerated by BRAF inhibitors. Pigment Cell & Melanoma Research. 2012;25:854. doi: 10.1016/j.molonc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.An KP, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochemistry and Photobiology. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 86.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 87.Burns EM, et al. Preventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh-1 mice in a model of UVB-induced cutaneous squamous cell carcinoma. Carcinogenesis. 2013;34:370–377. doi: 10.1093/carcin/bgs349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elmets CA, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. Journal of the National Cancer Institute. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bertagnolli MM, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer prevention research. 2009;2:310–321. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arber N, Moshkowitz M. Small bowel polyposis syndromes. Current gastroenterology reports. 2011;13:435–441. doi: 10.1007/s11894-011-0218-4. [DOI] [PubMed] [Google Scholar]
- 91.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 92.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 93.Delongchamps NB, de la Roza G, Jones R, Jumbelic M, Haas GP. Saturation biopsies on autopsied prostates for detecting and characterizing prostate cancer. BJU international. 2009;103:49–54. doi: 10.1111/j.1464-410X.2008.07900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ling G, et al. Persistent p53 mutations in single cells from normal human skin. The American journal of pathology. 2001;159:1247–1253. doi: 10.1016/S0002-9440(10)62511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smalley KS, Sondak VK. Skin cancer: Targeted therapy for melanoma: is double hitting a home run? Nature reviews. Clinical oncology. 2012 doi: 10.1038/nrclinonc.2012.215. [DOI] [PubMed] [Google Scholar]
- 96.Ding Q, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Molecular cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]