Abstract
A long-held view has been that interest of male mice in female body odors reflects an activation of reward circuits in the male brain following their detection by the vomeronasal organ (VNO) and processing via the accessory olfactory system. We found that adult, sexually naïve male mice acquired a conditioned place preference (CPP) after repeatedly receiving estrous female urine on the nose and being placed in an initially non-preferred chamber with soiled estrous bedding on the floor. CPP was not acquired in control mice that received saline on the nose before being placed in a non-preferred chamber with clean bedding. Robust acquisition of a CPP using estrous female odors as the reward persisted in separate groups of mice in which VNO-accessory olfactory function was disrupted by bilateral lesioning of the accessory olfactory bulb (AOB) or in which main olfactory function was disrupted by zinc sulfate lesions of the main olfactory epithelium (MOE). By contrast, no CPP was acquired for estrous odors in males that received combined AOB and MOE lesions. Either the main or the accessory olfactory system suffices to mediate the rewarding effects of estrous female odors in the male mouse, even in the absence of prior mating experience. The main olfactory system is part of the circuitry that responds to chemosignals involved in motivated behavior, a role that may be particularly important for humans which lack a functional accessory olfactory system.
Keywords: vomeronasal organ, conditioned place preference, reward circuits
Chemosignals exuded in urine and tears control social communication in several rodent species. It is now widely accepted (e.g., Lin et al., 2004; Brennan and Zufall, 2006; Kang et al., 2011) that volatile chemosignals, which are detected by the main olfactory epithelium (MOE) and processed via the main olfactory bulb, as well as non-volatile chemosignals, which are detected by the vomeronasal organ (VNO) and processed via the accessory olfactory bulb (AOB), both make essential contributions to aspects of social communication in mice of both sexes. These chemosignal-induced social behaviors reflect the integration of inputs from the main and accessory olfactory systems in the medial (‘vomeronasal’) amygdala (Licht and Meredith, 1987; Lehman and Winans, 1982; Kang et al., 2011) followed by transmission of these inputs to the hypothalamus and basal forebrain. In humans, chemosignals produced in male underarm sweat (Wyart et al., 2007) and in female tears (Gelstein et al., 2011) modulate sexual attraction/arousal to sexually relevant visual cues in opposite-sex subjects. In so far as humans lack a functional VNO-accessory olfactory system (Frasnelli et al, 2011; Trotier, 2011), these observed effects of chemosignals presumably reflect their detection and processing via the main olfactory system.
The neural circuits that mediate behavioral effects of sexually relevant chemosignals in human as well as other adult mammals are not well understood. Evidence establishing an important role of the main olfactory system in the processing of social chemosignals in mice would further support the notion that social odor communication in humans may be subserved entirely by this system. Male rodents are hard-wired to seek out and investigate opposite-sex bedding/non-volatile urinary odors; however, a rigorous behavioral assessment of the reward salience of female chemosignals for male rodents (as well as humans) is lacking. In male rodents (Bressler & Baum, 1996; Pankevich et al. 2006b) exposure to soiled bedding from an estrous female augmented Fos expression in segments of the mesolimbic dopamine pathway. However, the relative contribution of the main vs. the accessory olfactory system to the detection and processing of sexually rewarding chemosignals is unknown.
We examined whether processing of rewarding chemosignals occurs via the main and/or the accessory olfactory system of mice using the conditioned place preference (CPP) paradigm, which has been widely used to establish the reward salience of natural incentive stimuli (mating, aggression, water, sucrose, food) in addition to abused drugs. After first establishing that a CPP was reliably acquired by males exposed to estrous female urine and estrous bedding, we compared the ability of bilateral electrolytic lesions of the AOB, zinc sulfate lesions of the MOE, or combined AOB and MOE lesions to block males’ acquisition of a CPP to estrous female chemosignals. Numerous studies (e.g., Stowers et al., 2002) have used mice with a null mutation of the TRP-2c cation channel (which is expressed only in the VNO) to study the effects of disrupting the function of the accessory olfactory system in mice. We elected to use a surgical (AOB lesions) instead of a genetic method to disrupt accessory olfactory function because previous studies (Kelliher et al., 2006; Hasen and Gammie, 2009) have shown that aspects of accessory olfactory function (e.g., pregnancy block in females) persist in TRP-2c mutant mice. This implies that signaling mechanisms in addition to TRP-2c exist in mice, a finding recently confirmed by two different studies (Kim et al., 2012; Yang and Delay, 2010). We administered AOB lesions to mice instead of surgically removing the VNO because of our previous observation (Martel and Baum, 2007) that volatile opposite-sex urinary chemosignals, detected by the main olfactory system, specifically stimulated Fos expression in the AOB, acting via centrifugal inputs to the AOB that originate in the medial amygdala (Martel and Baum, 2009). We elected to use a chemical (intranasal zinc sulfate) instead of a genetic method to disrupt main olfactory function because previous research (Lin et al., 2007; Thompson et al., 2012; Pacifico et al., 2012) suggests that there are multiple signaling pathways in main olfactory epithelium sensory neurons that are difficult to eliminate using single gene mutations.
Method
Subjects
Testes-intact male Swiss Webster mice were purchased from Charles River at 6–8 weeks of age and housed in groups of 4 under a reversed 12L/12D light/dark photoperiod. All behavioral testing occurred during the dark phase of the L/D cycle. Food and water were provided ad libitum, except for the days that the buried cookie finding protocol was carried out in a subset of males (see below for more details). All procedures were approved by the Boston University Charles River Campus Animal Care and Use Committee. Male mice were sexually naive and were housed in colony racks with an air circulation system that prevented unwanted exposure to female body odors during these experiments. Female mice (6–8 weeks of age) were also purchased to serve as urine and bedding donors (details, below).
Placement of lesions in the AOB and MOE and confirmation of their location and efficacy
Males were given either complete bilateral electrolytic or sham lesions of the accessory olfactory bulbs (AOBx or AOB sham), as previously described (Jakupovic et al. 2008; Martel and Baum, 2009) 7 days prior to the onset of behavioral testing (see time line in Fig 1). Other males received authentic or sham MOE lesions by flushing the nares with 7 µl of a 10% ZnSO4 solution or saline (Martel and Baum, 2007) under ketamine (120 mg/kg) and xylazine (12mg/kg) anesthesia one day after the pre-conditioning assessment of males’ chamber preferences (Fig. 1). A final group of males received AOB lesions combined with MOE lesions. To assess lesion damage, AOBx and AOB sham-operated mice were perfused with 4% paraformaldehyde and the brains were processed histologically to verify the accurate placement of lesions. The right and left olfactory bulbs (including MOB and AOB) were blocked from the rest of the brain and cut sagittally on a cryostat. The resulting sections (30 µm thick) were collected sequentially from the medial to the lateral border of the olfactory bulb, mounted on glass slides, and later stained with Cresyl Violet to visualize the cell layers. The slides were coded to conceal subjects’ group identities, and an observer used power point software to trace the outline of each lesion onto a common template. Fig. 2 shows a schematic representation of the overlap of all lesions (black) along with the maximal extent (gray) of any lesion (left and right hemispheres shown separately) in males included in the groups that only received bilateral AOB lesions or that received both bilateral AOB lesions and zinc sulfate lesions of the MOE. Of 18 males given bilateral AOB lesions, data from seven males judged to have incomplete AOB lesions were omitted, leaving 11 males in the AOB lesioned group. Of the 12 males given combined AOB and MOE lesions, 11 were judged to have complete AOB plus MOE lesions and included in the present study. Note that the size and extent of lesions that essentially eliminated the AOB while causing minimal damage to the abutting main olfactory bulb appeared to be very similar in the two groups of males that received them.
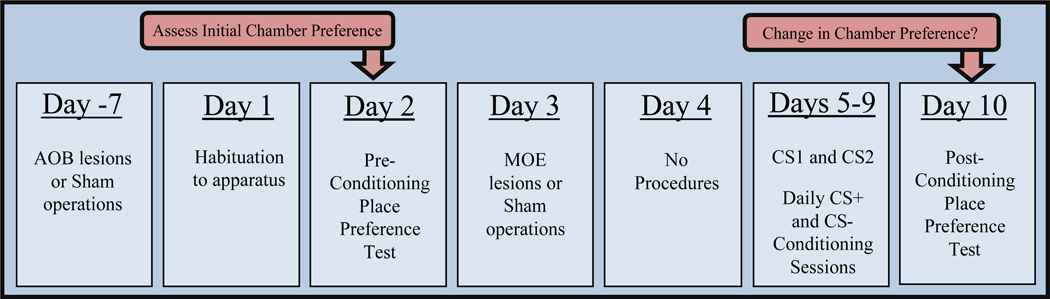
Figure 1.
Time line showing the sequence of surgical and behavioral testing procedures in the present study. CS+ conditioning sessions: male mice received estrous female urine on the nose prior to being confined to the previously non-preferred chamber with soiled estrous female bedding on the floor. CS− conditioning sessions: male mice received sterile saline on the nose prior to being confined to the previously preferred chamber with clean bedding on the floor. Each male mouse received both a CS+ and a CS− conditioning session (order counterbalanced in each treatment group; separated by approximately 2h) on test days 5–9.
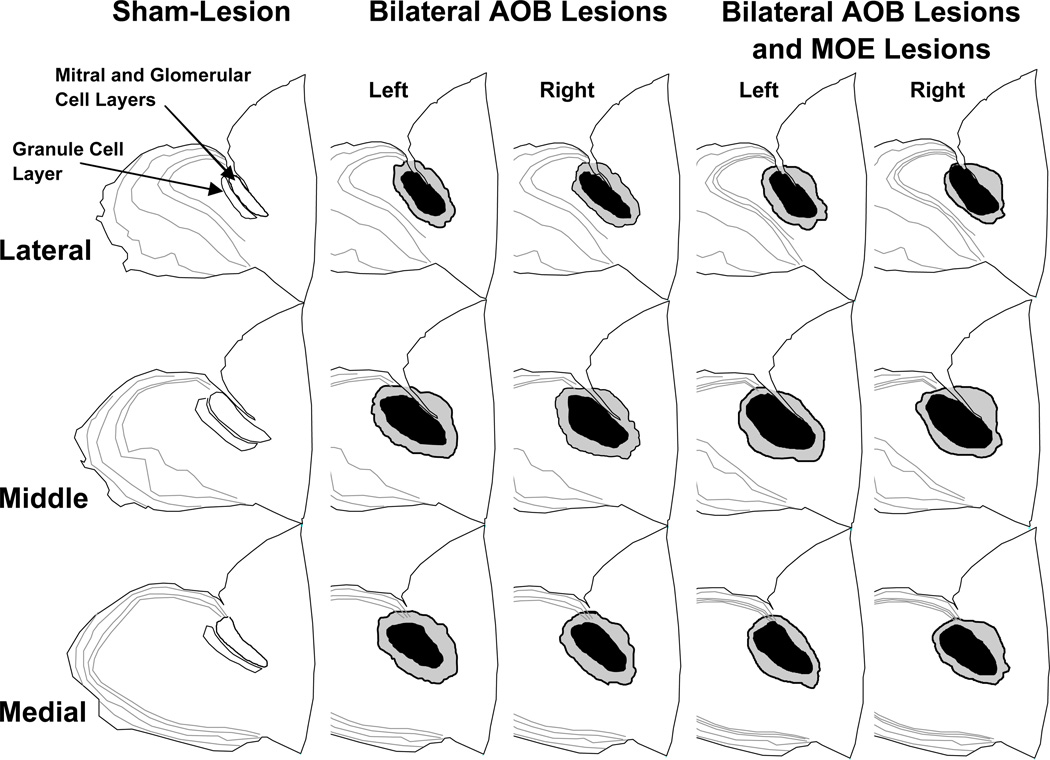
Figure 2.
Schematic representations of bilateral (left and right hemispheres shown separately) lesions of the accessory olfactory bulb (AOB) in groups of male mice that either received only AOB lesions (n = 11) or AOB lesions plus lesions of the main olfactory epithelium (MOE) (n = 11). Drawings of the glomerular, mitral, and granule cell layers of the AOB from a sham-operated control male are shown in sagittal sections taken from the lateral, middle, and medial aspects of the entire olfactory bulb from one hemisphere. The black shading represents the area of overlap of AOB lesions in all members of each group; the gray shading represents the area of greatest extent of AOB lesions in any member of each group.
Immediately following the completion of CPP testing (Day 10 in Fig. 1), each male that received either an authentic MOE lesion or a sham MOE lesion received two tests of main olfactory function: 1) habituation–dishabituation tests to determine whether males could discriminate between male and estrous female volatile urinary odors (Baum & Keverne, 2002), and 2) a latency to find a hidden cookie test (Martel and Baum, 2007). All MOE sham males successfully discriminated male vs. estrous female urinary volatiles and located the buried cookie within an average of 50 sec. By contrast, 8 out of 9 males that were given only MOE zinc sulfate lesions and 11 out of 12 males that were given combined MOE and AOB lesions were unable to discriminate male vs. estrous female urinary volatiles and failed to locate the buried cookie within 5 min (results not shown). MOE lesions were judged to be successful in the males that failed both olfactory tests, and only these males were retained in the study.
Collection of urine and soiled bedding
Estrous female urine and bedding were collected from female mice following ovariectomy and treatment with ovarian hormones: females were implanted subcutaneously with a Silastic capsule (inner diameter 1.57 mm; outer diameter 2.41 mm; 5 mm long) containing a 1:1 mixture of 17β-estradiol and cholesterol at the time of ovariectomy. Beginning 7 days later and again at 5–7 day intervals these estradiol-primed females received a s.c. injection of 500 µg of progesterone dissolved in sesame oil 4 hours before being placed either in a metabolic chamber for urine collection or in a colony cage on clean bedding. Male urine was collected from 8 testes-intact, group-housed males in separate metabolic chambers. To avoid the loss of ephemeral (volatile) estrous urinary chemosignals (Sipos et al. 1995), we collected and froze urine voided by estrous females every hour. Soiled bedding was collected after 4 hours. Urine and/or soiled bedding from 8 mice of the same sex/endocrine status were pooled and aliquots were stored at −80° C in 1.5 ml eppendorf tubes (urine) and plastic zip-lock freezer bags (soiled bedding), respectively.
Conditioned place preference training procedure
Group-housed males were transferred from the animal care facility to a quiet holding suite one hour before each testing session began. On the first day of testing, prior to administration of MOE lesion/sham operations to 4 groups of males (see time line in Fig. 1), food was removed in the morning to ensure that subjects were motivated to consume a cookie when it was presented 8 h later. After completion of CPP testing, the mice were again food deprived during the assessment of olfactory function using discrimination tests for mouse urinary odors for 8 h prior to the cookie finding test.
Conditioned place preference tests were conducted in two-chambered Plexiglas boxes (57× 14× 19 cm) inside an isolation cubicle (61× 65× 51cm; Coulbourn Instruments, Allentown, PA). One chamber had black walls and smooth plastic flooring while the other chamber had white walls with gray dots and textured plastic flooring. During testing, chambers were sealed with a clear Plexiglas ceiling with ventilation holes. Subjects’ moment-to-moment positions were video-tracked by a digital camera and Any-Maze software (Stoelting Co., Wood Dale, Il, USA). Chambers were cleaned with 10% Alconox (Alconox Inc., White Plains, NY, USA) in water and thoroughly dried after every session. On day 1 (habituation) subjects were allowed to move freely between the two adjacent chambers for 20 min (Fig. 1). On day 2 mice were again given free access to the adjacent chambers for 20 min and the time spent in each chamber was measured and used to specify a non-preferred chamber for each subject. No bedding was placed on the chamber floors during these tests. Over days 5–9, half of the mice in each treatment group were given a conditioning session in which they were confined behind a Plexiglas barrier in subjects’ initially non-preferred chamber for 20 minutes on bedding that contained soiled estrous female bedding (SEFB). Because we suspected that mice with MOE lesions might not investigate urinary odors, and therefore prevent the accessory olfactory system from being exposed to non-volatiles, 10 µl estrous female urine (EFU) was pipetted directly onto subjects’ noses prior to confinement in the non-preferred chamber containing SEFB to insure a minimal level of exposure to urinary non-volatiles. After the conditioning session, mice were returned to their home cages for two hours before being placed back in the CPP apparatus for confinement in the initially preferred chamber for 20 min. Clean bedding was placed on the floor of this chamber, and mice received 10 µl saline pipetted on the nose prior to this control session. Mice were then returned to the animal facility until testing the next day. The remaining males in each treatment group received the conditioning and control sessions in reverse order. On Day 10 mice were given free access to the apparatus for 20 min as was done on day 2, and the times spent in each chamber were measured. Time that subjects in each of the 8 groups spent in the initially non-preferred chamber as well as preference ratios (number of seconds spent in the initially non-preferred chamber divided by the time spent in both chambers) were compiled for each subject for the pre- and post-conditioning tests (test days 2 and 10, respectively) (Horseman and Paredes, 2004). Paired 2-tailed t tests were used to determine whether, for each treatment group, time spent in the initially non-preferred chamber along with preference ratios differed before vs. after conditioning.
Results
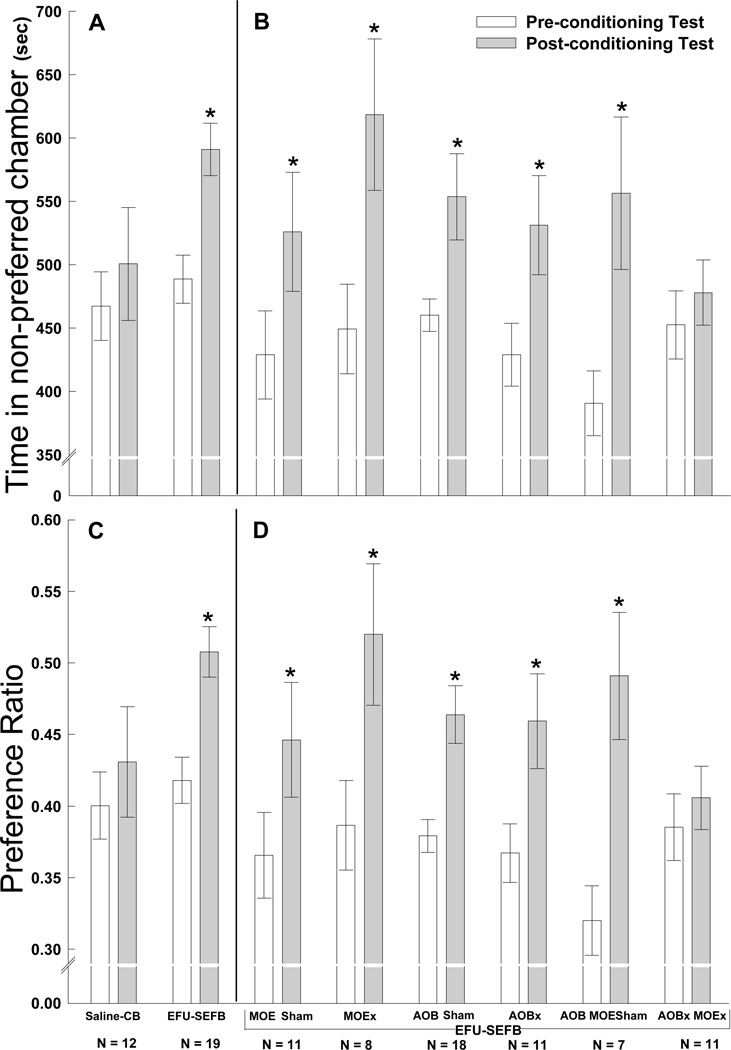
In the absence of any surgical manipulation of the olfactory nervous system, sexually naïve male mice spent significantly more time in the initially non-preferred chamber after receiving conditioning sessions in which characteristics of that chamber were paired with EFU-SEFB (Fig. 3A, C). Significant (2-tailed, paired t test comparisons) pre- vs post-conditioning values for time spent in the non-preferred side (t (df = 18) = 4.828, p < 0.0001) and for preference ratios (t (df = 18) = 4.98, p < 0.0001) were obtained, demonstrating that estrous odors established a CPP in male subjects. By contrast, CPP was not acquired in other males that were given saline on the nose and placed on clean bedding during conditioning sessions. Males given lesions of the MOE alone still showed significant CPP learning (Fig. 3B, D). Significant differences between pre-vs post-conditioning values for time spent in the non-preferred chamber (t (df = 7) = 2.967, p < 0.02) and for preference ratios (t (df = 7) = 2.73, p = 0.029) were obtained. Likewise, males given bilateral lesions of the AOB alone still showed significant CPP learning for estrous odors. Significant differences between pre- vs post-conditioning values for time spent in the non-preferred side (t (df = 10) = 2.352, p < 0.04) and for preference ratios (t (df = 10) = 2.59, p = 0.027) were obtained. The acquisition of a CPP to odor stimuli from estrous females was also seen in groups of males that received sham surgical manipulations of the MOE and/or the AOB (P < 0.05 by paired t tests in each case). By contrast, combined lesions of the AOB and MOE eliminated the ability of repeated exposure to EFU and SEFB to establish a CPP in a final group of males (Fig. 3B, D).
Figure 3.
(panels A and C). Acquisition by male mice of a conditioned place preference (CPP) for a chamber in which they previously received estrous female urine (EFU) on the nose and were exposed to soiled estrous female bedding (SEFB). Control males given saline on the nose (saline) and exposed to clean bedding (CB) acquired no CPP. (panels B and D) Separate lesions of the main olfactory epithelium (MOEx) or of the accessory olfactory bulb (AOBx), like sham surgical manipulations of these structures (MOE Sham; AOB Sham; AOB MOE Sham), failed to block the ability of male mice to acquire a CPP to EFU-SEFB. Combined lesions of the AOB and MOE (AOBx MOEx) blocked males’ acquisition of a CPP to EFU-SEFB. Data in the top panels (A, B) are expressed as time (in seconds) spent in the initially non-preferred chamber of the apparatus whereas data in lower panels (C, D) are expressed as preference ratios, defined as the number of seconds spent in the initially non-preferred chamber divided by the time spent in both chambers (mean +/− SEM). For each treatment group of males, time and preference ratio values are shown for the pre-conditioning and for the post-conditioning tests. *p < 0.05, 2-tailed, within-groups t test comparisons of values in the post- vs. pre-conditioning tests. The number (N) of subjects in each group is shown beneath each pair of bars.
Discussion
Combined lesions of the AOB and MOE blocked acquisition of a CPP for estrous female chemosignals in sexually naive male mice whereas CPP readily occurred in males given either MOE or AOB lesions alone. This suggests that the reward circuitry mediating CPP learning is activated by redundant chemosignal input from either the main or the accessory olfactory systems and provides comparative support for the suggestion that human social odors could be detected by circuitry within the main olfactory system. Previously, we identified a sub-population of MOB mitral cells that send direct projections to the medial amygdala (both anterior and posterior-dorsal subdivisions) in both male and female mice (Kang et al., 2009; Kang et al., 2011). Whether this pathway is involved in processing the rewarding aspects of social odors that were examined in the present study will require more work. Nevertheless, such a pathway, if conserved in humans, would be well positioned to transmit signals to hypothalamic targets that exhibit sexually dimorphic responses following exposure of human subjects to a putative pheromone, androstadienone (Savic et al., 2001). It is worth noting that existence of a direct MOB to medial amygdala projection in non-human primates has been known for many years (Carmichael et al, 1994; Turner et al, 1978.)
Our observation that robust acquisition of a CPP occurred in sexually naïve males after AOB lesions argues against the view that main olfactory system inputs only assume the ability to mediate rewarding effects of estrous female chemosignals after they are paired with accessory olfactory inputs that occur during mating in male mice (Swaney et al., 2008). It is well established that pairing socially irrelevant odor cues (e.g. methyl salicylate vapors) with mating experience in male rodents allows those odors to assume the capacity later to facilitate LH and testosterone secretion (Graham and DesJardins, 1980) and to promote mating partner preference (Kippin & Pfaus, 2001). However, our results suggest that estrous female chemosignals (unlike non-social odorants) detected by the main olfactory system readily gain access to brain reward circuits even in males that lack prior mating experience. In a previous study (Pankevich 2006) we observed significant CPP learning for access to odors from an estrous female in mating-experienced male mice given VNO lesions. Our initial interpretation of that result was that the main olfactory system was able to mediate that CPP learning in the absence of accessory olfactory inputs because we used sexually experienced males in our experiment. The present results suggest, however, that even in sexually naïve male mice both the main and the accessory olfactory systems are hard-wired to convey social odor inputs from estrous females into the circuitry that mediates reward. Our current results in male mice are at apparent odds with a previous study (Martinez-Ricos et al., 2007) using ovary-intact adult female mice that lacked mating experience and which had never been previously exposed to either volatile or non-volatile male chemosignals. Those females, which were studied at unspecified stages of the estrous cycle, acquired a CPP after nasal contact with soiled bedding from testes-intact males, but not after nasal contact with bedding from other females or from castrated males nor after exposure to volatiles emitted from the bedding of testes-intact males. The authors concluded that VNO-accessory olfactory system inputs were required for the CPP learning observed in their females. They suggested that only females with prior mating experience, or which had experienced the simultaneous activation of the main and the accessory olfactory systems in response to volatile and non-volatile male chemosignals, would be able to acquire a CPP to volatile male chemosignals. Conclusive evidence that VNO-accessory olfactory processing of male chemosignals caused the CPP observed by Martinos-Ricos awaits a study showing that VNO or AOB lesions block such learning in female mice.
The activation of mesolimbic dopamine (DA) neurons that project from the ventral tegmental area to the n. accumbens has been linked to the motivation to engage in a range of natural goal-directed behaviors (e.g., feeding, mating, parenting) as well as the ingestion of several drugs of abuse (Wise, 2004). These DA neurons were previously implicated in responses to female chemosignals by our (Pankevich et al., 2006b) observation that exposure of sexually naïve male mice to soiled estrous bedding augmented Fos expression in the n. accumbens core. Tract tracing studies (Pardo-Bellver et al., 2012; Usunoff et al., 2009) demonstrated robust labeling of the n. accumbens following injection of an anterograde tracer into the posterior-dorsal medial amygdala, raising the possibility that social odor inputs to the MeA from either the MOB or AOB (Kang et al., 2011) can be passed on to the n. accumbens via this pathway. In confirmation of this hypothesis, Malkesman et al. (2010) reported an increase in DA concentrations in the n. accumbens of sexually naïve male mice following nasal contact with estrous female urine. In apparent contradiction, however, neurotoxic destruction of DA neurons projecting to the n. accumbens failed to disrupt the preference of female mice to seek out a container of soiled male bedding (Martinez-Hernandez et al., 2012). Those investigators (Novejarque et al., 2011) suggested that projections from the medial amygdala to other targets in the ventral forebrain, including the olfactory tubercle or the islands of Calleja, may underlie the rewarding effects of chemosignals. However, a simple preference to approach and investigate urinary odors may not be a predictor of the ability of such odors to act as reinforcers in a CPP paradigm. In pilot studies for the present study, we found that urinary odors from castrated male mice also served to establish a CPP in gonadally intact male subjects, indicating that non-sexual, social odors can be reinforcing. Also, a single component of testes-intact male urine (darcin) can serve as an incentive stimulus for CPP learning in both female and male mice (Roberts et al., 2012). It seems likely that several conspecific social odors can be incentives for CPP learning, although there is no reason to expect that biologically “irrelevant” odors (e.g. peppermint) can serve this purpose. Importantly, our conditioning procedure, by itself, did not cause a change in chamber preference: male mice that were only exposed to saline on the nose plus clean bedding showed no change in compartment preference. More research using CPP training in mice of both sexes is needed to rigorously assess the reward salience of different social odors with the ultimate aim of identifying the reward circuits that are activated by inputs from the accessory and the main olfactory systems.
It is noteworthy that neither disruption of accessory olfactory function by surgical removal of the VNO nor disruption of main olfactory function by zinc sulfate irrigation of the nares blocked the ability of estrous female urinary chemosignals to elicit ultrasonic “courtship” vocalizations in sexually naïve male mice (Sipos et al., 1995). This outcome resembles our observation that CPP for estrous odors continued to be acquired in mice given either AOB or MOE lesions. Sipos et al. (1995) also reported that combined lesioning of the VNO and the MOE eliminated the ability of male mice to emit ultrasonics in response to female urinary volatiles just as we observed that combined AOB and MOE lesions eliminated the ability of males to acquire a CPP to estrous odors.
In conclusion, the ability of an incentive stimulus to establish a CPP has become the gold standard for assessing brain mechanisms controlling reward (e.g. Lammel et al., 2012). We used this procedure to show that the main olfactory system suffices to convey estrous female chemosignal inputs needed to activate reward circuits in the male mouse brain, even in the absence of prior mating experience/simultaneous activation of the VNO-accessory system that was previously thought to enable the main olfactory system to assume this function. Future studies are needed to identify the specific input pathways from the two olfactory systems to reward circuits in the forebrain, along with the behavioral contexts under which each system is activated in ways that contribute to reproductive success in the two sexes.
Acknowledgements
This work was supported by a National Institutes of Health grant (DC008962) to JAC.
References
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41(2):213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444(7117):308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71(4):1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346(3):403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Lundstrom JN, Boyle JA, Katsarkas A, Jones-Gotman M. The vomeronasal organ is not involved in the perception of endogenous odors. Hum Brain Mapp. 2011;32(3):450–460. doi: 10.1002/hbm.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelstein S, Yeshurun Y, Rozenkrantz L, Shushan S, Frumin I, Roth Y, et al. Human tears contain a chemosignal. Science. 2011;331(6014):226–230. doi: 10.1126/science.1198331. [DOI] [PubMed] [Google Scholar]
- Graham JM, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210(4473):1039–1041. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- Hasen NS, Gammie SC. Trpc2 gene impacts on maternal aggression, accessory olfactory bulb anatomy and brain activity. Genes Brain Behav. 2009;8(7):639–649. doi: 10.1111/j.1601-183X.2009.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman PG, Paredes RG. Dopamine Antagonists Do Not Block Conditioned Place Preference Induced by Paced Mating Behavior in Female Rats. Behavioral Neuroscience. 2004;118(2):356–364. doi: 10.1037/0735-7044.118.2.356. [DOI] [PubMed] [Google Scholar]
- Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol Behav. 2008;93(3):467–473. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the 'vomeronasal' amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29(3):624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem Senses. 2011;36(3):251–260. doi: 10.1093/chemse/bjq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, McCarthy EA, Cherry JA, Baum MJ. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. Neuroscience. 2011;172:196–204. doi: 10.1016/j.neuroscience.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Spehr M, Li XH, Zufall F, Leinders-Zufall T. Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur J Neurosci. 2006;23(12):3385–3390. doi: 10.1111/j.1460-9568.2006.04866.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Ma L, Jensen KL, Kim MM, Bond CT, Adelman JP, et al. Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat Neurosci. 15(9):1236–1244. doi: 10.1038/nn.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Pfaus JG. The development of olfactory conditioned ejaculatory preferences in the male rat. I. Nature of the unconditioned stimulus. Physiol Behav. 2001;73(4):457–469. doi: 10.1016/s0031-9384(01)00484-x. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240(1):27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Licht G, Meredith M. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp Brain Res. 1987;69(1):7–18. doi: 10.1007/BF00247024. [DOI] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24(14):3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104(7):2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom JN, Olsson MJ. Subthreshold amounts of social odorant affect mood, but not behavior, in heterosexual women when tested by a male, but not a female, experimenter. Biol Psychol. 2005;70(3):197–204. doi: 10.1016/j.biopsycho.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry. 2010;67(9):864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26(2):463–475. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009;29(24):7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez J, Lanuza E, Martinez-Garcia F. Lesions of the dopaminergic innervation of the nucleus accumbens medial shell delay the generation of preference for sucrose, but not of sexual pheromones. Behav Brain Res. 2012;226(2):538–547. doi: 10.1016/j.bbr.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem Senses. 2007;32(2):139–148. doi: 10.1093/chemse/bjl039. [DOI] [PubMed] [Google Scholar]
- Novejarque A, Gutierrez-Castellanos N, Lanuza E, Martinez-Garcia F. Amygdaloid projections to the ventral striatum in mice: direct and indirect chemosensory inputs to the brain reward system. Front Neuroanat. 2011;5:54. doi: 10.3389/fnana.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico R, Dewan A, Cawley D, Guo C, Bozza T. An olfactory subsystem that mediates high-sensitivity detection of volatile amines. Cell Rep. 2(1):76–88. doi: 10.1016/j.celrep.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiol Behav. 2006a;87(4):781–788. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006b;120(4):925–936. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cadiz-Moretti B, Novejarque A, Martinez-Garcia F, Lanuza E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat. 6:33. doi: 10.3389/fnana.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Berglund H, Gulyas B, Roland P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron. 2001;31(4):661–668. doi: 10.1016/s0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- Sipos ML, Wysocki CJ, Nyby JG, Wysocki L, Nemura TA. An ephemeral pheromone of female house mice: perception via the main and accessory olfactory systems. Physiol Behav. 1995;58(3):529–534. doi: 10.1016/0031-9384(95)00089-2. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295(5559):1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Swaney WT, Curley JP, Champagne FA, Keverne EB. The paternally expressed gene Peg3 regulates sexual experience-dependent preferences for estrous odors. Behav Neurosci. 2008;122(5):963–973. doi: 10.1037/a0012706. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 520(8):1819–1830. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotier D. Vomeronasal organ and human pheromones. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(4):184–190. doi: 10.1016/j.anorl.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Turner BH, Gupta KC, Mishkin M. The locus and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta. J Comp Neurol. 1978;177(3):381–396. doi: 10.1002/cne.901770303. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Schmitt O, Itzev DE, Haas SJ, Lazarov NE, Rolfs A, et al. Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse. Cells Tissues Organs. 2009;190(5):256–285. doi: 10.1159/000209233. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wyart C, Webster WW, Chen JH, Wilson SR, McClary A, Khan RM, et al. Smelling a single component of male sweat alters levels of cortisol in women. J Neurosci. 2007;27(6):1261–1265. doi: 10.1523/JNEUROSCI.4430-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Delay R. JCalcium-activated chloride current amplifies the response to urine in mouse vomeronasal sensory neurons. J Gen Physiol. 135(1):3–13. doi: 10.1085/jgp.200910265. [DOI] [PMC free article] [PubMed] [Google Scholar]