Abstract
Amyloid beta (Aβ), a key component in the pathophysiology of Alzheimer’s disease, is thought to target excitatory synapses early in the disease. However, the mechanism by which Aβ weakens synapses is not well understood. Here we showed that the PDZ domain protein, protein interacting with C kinase 1 (PICK1), was required for Aβ to weaken synapses. In mice lacking PICK1, elevations of Aβ failed to depress synaptic transmission in cultured brain slices. In dissociated cultured neurons, Aβ failed to reduce surface GluA2, a subunit of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors that binds with PICK1 through a PDZ ligand–domain interaction. Lastly, a novel small molecule (BIO922) discovered through structure-based drug design that targets the specific interactions between GluA2 and PICK1 blocked the effects of Aβ on synapses and surface receptors. We concluded that GluA2–PICK1 interactions are a key component of the effects of Aβ on synapses.
Keywords: Alzheimer’s disease, mouse, rat, synapse
Introduction
The amyloid hypothesis (Hardy & Selkoe, 2002), proposing that an excessive amount of amyloid beta (Aβ) is responsible for the cognitive impairment in Alzheimer’s disease, is the most widely accepted pathophysiological model for the disease. Despite its proposed prominent role, little is known regarding how Aβ produces deleterious effects that lead to Alzheimer’s disease (Hardy & Selkoe, 2002). There has been considerable interest in the effects of Aβ on synapses (Shankar et al., 2007; Freir et al., 2011), as synapses appear to be an early target in the disease (DeKosky & Scheff, 1990; Terry et al., 1991; Masliah et al., 2001). A number of studies indicate that elevated levels of Aβ lead to the loss of postsynaptic receptors on excitatory synapses (Kamenetz et al., 2003; Cirrito et al., 2005; Snyder et al., 2005; Hsieh et al., 2006).
Excitatory synapses transmit much information by releasing glutamate onto the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR). AMPARs are tetrameric receptors comprised of combinations of four subunits, GluA1–4 (Wisden & Seeburg, 1993; Hollmann & Heinemann, 1994; Rosenmund et al., 1998). In the hippocampus, most of the AMPARs are composed of GluA1/GluA2 and GluA2/GluA3 (Wenthold et al., 1996). Elevated Aβ appears to produce synaptic depression by enhancing the endocytosis of AMPARs through a GluA2-mediated process (Hsieh et al., 2006).
We sought to examine this process more carefully. We focused our studies on the GluA2-interacting protein [protein interacting with C kinase 1 (PICK1)] (Xia et al., 1999; Xu & Xia, 2006). Recent studies have indicated that PICK1 is required for the endocytosis of AMPARs that occurs in long-term depression (Terashima et al., 2004; Citri et al., 2010), a physiological process that may be hijacked by Aβ to produce synaptic depression (Snyder et al., 2005; Hsieh et al., 2006). Here we test whether PICK1 is required for the effects of Aβ on synapses. We find that, in tissue from animals lacking PICK1, Aβ fails to depress AMPAR-mediated synaptic transmission and fails to reduce surface AMPARs. Furthermore, a small synthetic molecule (BIO922) that blocks the PDZ domain-mediated interaction between GluA2 and PICK1 blocks the effects of Aβ on synaptic transmission and surface receptors. We conclude that a PDZ domain-mediated PICK1 interaction with GluA2 is required for the effects of Aβ on synapses.
Materials and methods
Tissue preparation
Experiments were conducted in accordance with and received approval from the Institutional Animal Care and Use Committees at University of California at San Diego and Biogen Idec Inc. The experiments were carried out in accordance with guidelines laid down by the NIH regarding the care and use of animals for experimental procedures.
Hippocampal slice cultures and Sindbis virus infection
Organotypic hippocampal slice cultures were made from postnatal day 6 or 7 rat pups as described previously (Stoppini et al., 1991). Slice cultures were maintained in culture for 6–8 days and then infected using a Sindbis virus (pSinRep5 dp APP-CT100 + tdTomato). Cells were recorded at 16–30 h after Sindbis virus infection.
Dissociated primary neuron cultures
Primary hippocampal cultures were prepared from embryonic day 18 rodent brains as described previously (Goslin, 1991). Cells were plated on coverslips coated with poly-D-lysine (30 μg/mL) and laminin (2 μg/mL) at a density of 70 000 per well in a 12-well plate. Hippocampal neurons were grown in Neurobasal medium (Invitrogen) and supplemented with B27 (Invitrogen), 0.5 mM glutamine, and 12.5 μM glutamate, and used for the described studies at 21 days in vitro. Embryonic neuronal cultures were prepared from a minimum of three independent pregnant rats or mice on separate days in all of the studies described, and at least 13 neurons were analyzed for each experimental group.
Electrophysiology and pharmacological treatments
Slices were maintained in a solution of artificial cerebrospinal fluid containing (in mM): 119 NaCl, 26 NaHCO3, 1 NaH2PO4, 11 D-glucose, 2.5 KCl, 4 CaCl2, 4 MgCl2, and 1.25 NaHPO4, and gassed with 95% O2. In addition, the following drugs were added: 4 μM 2-chloroadenosine (to prevent stimulus-induced bursting), and 100 μM picrotoxin or 10 μM or gabazine (to block inhibitory transmission). Simultaneous whole-cell recordings were obtained from pairs of neighboring (<50 μm) control and infected CA1 pyramidal neurons using 3–5 MΩ glass pipettes with an internal solution containing the following (in mM): 115 cesium methanesulfonate, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 Na2ATP, 0.4 Na3GTP, 10 sodium phosphocreatine, 0.6 EGTA, and 0.1 spermine, at pH 7.25. All recordings were performed by stimulating two independent synaptic inputs. Excitatory postsynaptic currents (EPSCs) were recorded while holding the cells at −60 mV, alternating pathways every 8.4 s. The stimulus strength was set so that responses from both cells were greater than ~30 pA. The results from each pathway were averaged and counted as n = 1. For pharmacological experiments, slices were incubated for 2 h prior to recordings with 10 μM BIO922. This compound was also added to the recording chamber at the same concentration. To measure rectification, paired recordings were performed (as described above) and cells were held at −60, +40, and 0 mV. In addition, 100 μM APV (to block the N-methyl-D-aspartate response) was added to the perfusion chamber. The following equation was used to compute the rectification index: (EPSC−60mV - EPSC0mV)/(EPSC+40mV - EPSC0mV). The mean rectification of infected cells was normalized by the mean control cell rectification. All data are reported as mean ± SEM. Statistical analysis for paired recordings used the paired t-test, with p < 0.05 considered significant.
Biochemical assays
Competition and binding fluorescence polarization assays were used to determine PDZ binding selectivity. For all assays, a fixed concentration (5 nM) of FITC-labeled peptides composed of the C-terminal amino acids of GluA2 (875–883) and GluN2B (1474–1482) was used. Binding fluorescence polarization assays were performed by using increasing concentrations of recombinant full-length PICK1, PSD-95 PDZ 1–2 and GRIP1 PDZ 4–6 in order to determine sufficient binding for subsequent competition assays. The binding assays were normalized by subtracting the tracer-only background. Competition fluorescence polarization assays were performed with both fixed FITC-labeled peptides (5 nM) and non-saturating protein concentrations (PICK1, 600 nM; PSD-95 PDZ 1–2, 8 μM; GRIP1, 10 μM) while changing the concentrations of unlabeled peptides and the BIO922 compound from 3 to 10 µM (data point for 10 μM shown in Fig. 2), and normalized by standardizing to both binding and tracer-only controls. All fluorescence polarization assay components were diluted in a buffer system containing: 50 mM Tris, pH 7.4, 200 mM NaCl, 2 mM DTT, 0.05% PF68 and 5% glycerol. We note that BIO922 will be made available to other investigators for studies upon request.
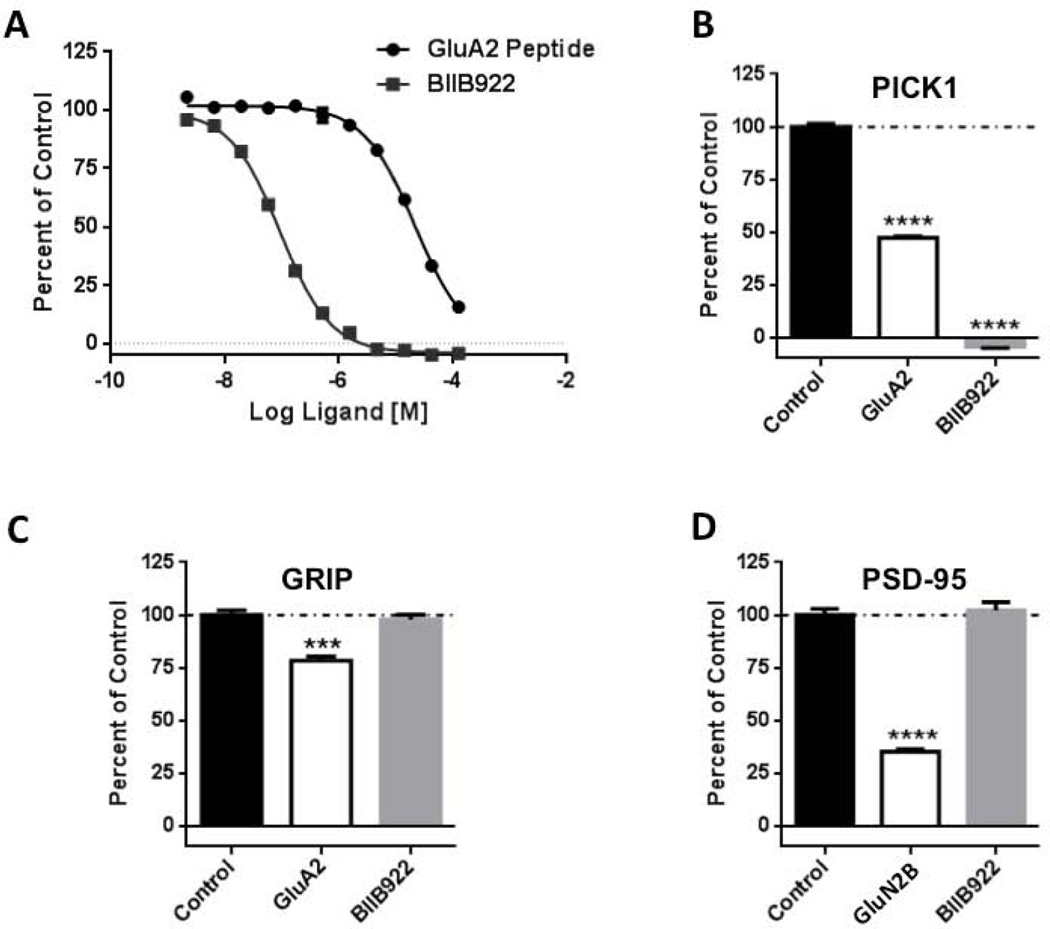
Fig. 2.
Small molecule inhibitor of PICK1–GluA2 interaction specifically blocks the binding of GluA2 to PICK1 relative to other PDZ domain-containing proteins. (A) Fluorescence polarization competition binding of PICK1 inhibitor (BIO922) with FITC-labeled BIO424 tracer. Fluorescence polarization competition assay of FITC-labeled GluA2 peptide with increasing concentrations of unlabeled GluA2 and BIO922 using either recombinant full-length PICK1 (B) or purified GRIP PDZ 4–6 proteins (C). (D) Fluorescence polarization binding of FITC-labeled GluN2B peptide at increasing concentrations of unlabeled GluN2B and BIO922 using purified PSD-95 PDZ 1–2 protein.
Surface staining of hippocampal neurons
Live staining of endogenous AMPARs and GABA receptors was performed as previously described (Wyszynski et al., 2002) using antibodies recognizing extracellular regions of GluA1, GluA2 and GABA2/3 subunits. Briefly, hippocampal neurons were incubated with antibody for 15 min at 37 °C to decorate surface receptors, fixed under non-permeabilizing conditions in phosphate buffer containing 2% formaldehyde/4% sucrose at room temperature, washed in phosphate buffer, and visualized with Alexa488-conjugated secondary antibody.
Total staining of hippocampal neurons
Neurons were fixed with 4% paraformaldehyde and 4% sucrose in phosphate buffer, permeabilized with 0.25% Triton X-100, and immunolabeled using anti-GluA1, anti-GluA2 and anti-GABA2/3 primary antibodies. Staining was visualized with Alexa488-conjugated secondary antibodies.
Image analysis and quantification
Confocal images of immunostained neurons were obtained using a confocal microscope objective (LSM 710, Zeiss) with sequential acquisition settings at a resolution of 1024 × 1024 pixels. Each image was a z-series of eight to 10 spaced at intervals of about 0.5 µm, and the resultant stack was ‘flattened’ into a single image using a maximum projection. The confocal microscope settings were kept the same for all scans. All analysis and quantifications were performed using MetaMorph image analysis software (Universal Imaging Corporation). Dendrites from experimental groups were randomly selected and carefully traced, and the average intensity of fluorescence staining was determined for the traced regions. Intensity measurements are expressed in arbitrary units of fluorescence per square area. Blind conditions were used for the acquisition and quantification of images.
Surface biotinylation assay
High-density cortical neurons at 21 days in vitro were used for surface biotinylation as described previously (Lin et al., 2000). Briefly, cortical neurons were cultured from wild-type mice (n=2) and PICK1 knockout mice (n=2), and neuronal surface proteins were biotinylated with 600 μg/mL Sulfo-NHS-SS-Biotin (Pierce, Rockford, IL, USA) in artificial cerebrospinal fluid buffer for 20 min at 4 °C. Unreacted Sulfo-NHS-SS-Biotin was removed by washing with ice-cold 50 mM glycine in phosphate buffer. Cells were lysed with ice-cold lysis buffer (20 mM sodium phosphate, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 0.5% NP-40, and 0.5% sodium deoxycholate) containing protease inhibitors (1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mm PMSF). Biotinylated surface proteins were isolated using immobilized NeurtrAvidine beads (Pierce), subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis and the resulting blots were probed using anti-GluA2 and anti-N-cadherin antibodies.
Preparation of amyloid beta oligomers
Synthetic Aβ42 hexafluoroisopropanol peptide was prepared to the stock concentration of 200 µM by adding 10 µL dimethylsulfoxide to 100 µg of Aβ42 hexafluoroisopropanol pellet and incubating for 30 min at room temperature with occasional mixing. Phosphate buffer was added to a final concentration of 1 mg/mL and mixed with a pipette. The solution was kept at room temperature for 2 h and then used for experiments.
Results
We used a previously established method to raise Aβ levels in neurons to examine the role of PICK1 in Aβ-induced synaptic depression (Kamenetz et al., 2003; Hsieh et al., 2006; Wei et al., 2010; Kessels et al., 2013). We virally expressed, in organotypic hippocampal slices for 16–30 h, CT100, the beta-secretase product of APP and precursor to Aβ [the same construct is called β-CTF in Kamenetz et al. (2003)]. We compared the evoked synaptic AMPAR-mediated transmission between neighboring infected and uninfected CA1 neurons by paired whole-cell recordings. In brain slices prepared from wild-type mice, neurons expressing CT100 displayed significantly depressed excitatory transmission (Fig. 1). In contrast, in brain slices prepared from animals lacking PICK1 (Gardner et al., 2005), neurons expressing CT100 showed no significant synaptic depression (Fig. 1). In 19 out of 21 paired recordings the cell expressing CT100 displayed depression in control slices, whereas in only 11 out of 20 paired recordings did the cell expressing CT100 display depression in slices from animals lacking PICK1 (comparing depression in control and PICK1−/− tissue; p< 0.05, χ2 test). These results support the view that PICK1 is required for Aβ to produce synaptic depression.
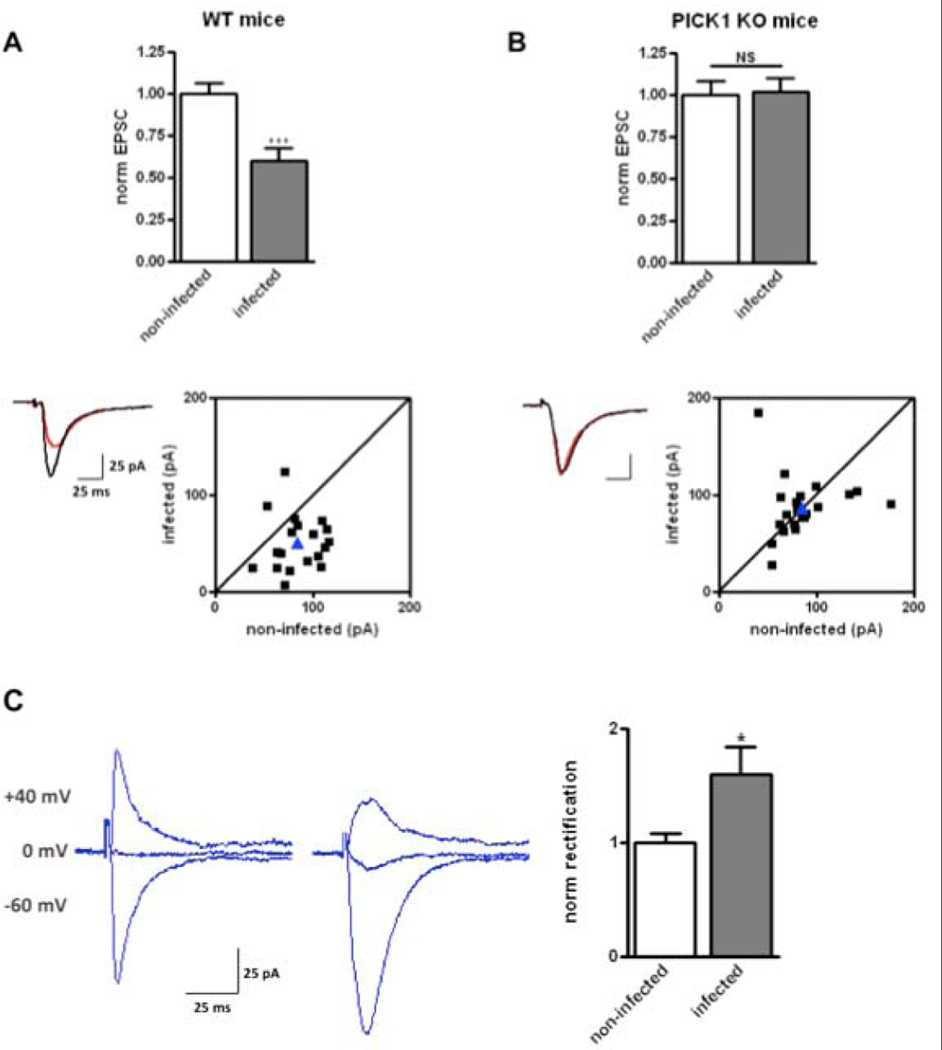
Fig. 1.
PICK1 knockout (KO) mice do not show Aβ-induced synaptic depression. Organotypic hippocampal slices prepared from wild-type (WT) (A) and PICK1 KO (B) mice were infected with CT100 virus to elevate Aβ. EPSCs were recorded from infected and non-infected cell pairs (WT, n = 19 pairs, p < 0.001; PICK1 KO, n= 21 pairs, p = 0.8). Top: graph of normalized average EPSC amplitudes for infected and non-infected neurons. Lower left: sample traces from infected (red) and non-infected (black) cell pairs. Lower right: dot plot of EPSC amplitude of infected vs. non-infected neuron. Each black square represents the responses from one cell pair; blue triangle indicates the average of all responses. (C) Aβ elevation increases the rectification of synaptic transmission. Left: sample traces from paired recordings at indicated holding potentials from non-infected (left) and infected (right) cells. Right: graph of normalized rectification index (n = 7 pairs; p = 0.01).
As PICK1 is known to bind GluA2 (Xia et al., 1999), we sought to examine whether Aβ preferentially acts on GluA2-containing receptors. We measured the rectification index of transmission in neurons expressing CT100. Receptors lacking GluA2 transmit more poorly at positive potentials, and thus display a greater rectification index (see Materials and methods). Synaptic transmission onto neurons expressing CT100 showed a larger rectification index (1.6 ± 0.1 in control neurons; 2.6 ± 0.4 in neurons expressing CT100; Fig. 1). These results support the view that Aβ preferentially removes synaptic receptors containing GluA2; the remaining transmission thus contains more GluA2-lacking receptors that can explain the increase in rectification index. Although we cannot rule out an effect of Aβ on the AMPAR interaction with TARPS, which can affect rectification (Soto et al., 2007), such an effect of Aβ has not previously been reported.
To test if an interaction between PICK1 and GluA2 is required for the synaptic effects of Aβ, we used a small molecule (BIO922) that blocks this interaction. BIO922 is an inhibitor (Ki = 98 nM, Fig. 2) of the interaction between full-length recombinant PICK1 and the GluA2 cytoplasmic domain (Ki = 24 µM, Fig. 2). A co-crystal structure of the series of BIO922 compound shows that this class of molecules binds to the PICK1 PDZ domain at the same site as the C-terminus of GluA2 (data not shown).
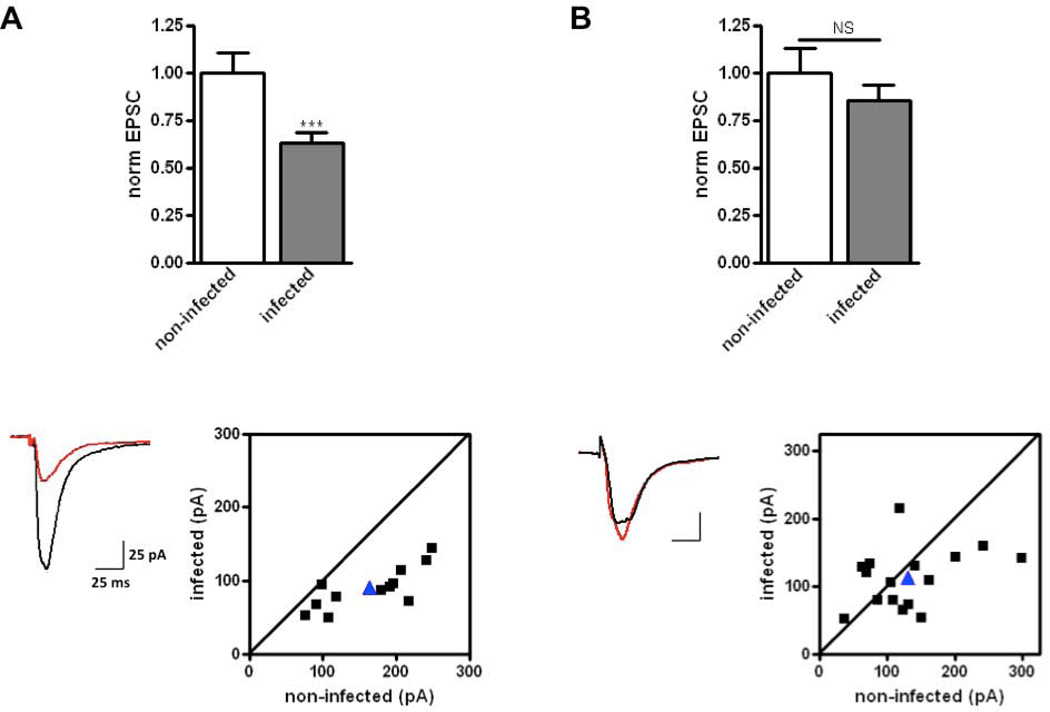
BIO922 shows greater than 100-fold selectivity over other related PDZ domain-containing proteins, namely PSD-95 and GRIP (Fig. 2). BIO922 was discovered by structure-based drug design targeted to the PICK1 PDZ domain (the complete discovery of BIO922 will be described elsewhere, manuscript in preparation). Brain slices from wild-type animals were infected with a virus producing CT100. After ~16–18 h, slices were exposed to media containing 10 μM BIO922 or normal media as a control for 2 h. We obtained paired whole-cell recordings from infected and non-infected neurons. Whereas slices exposed to normal media displayed the normal synaptic depression in CT100-infected neurons, slices exposed to BIO992 showed no significant synaptic depression in CT100-infected neurons (Fig. 3). In 12 out of 12 paired recordings the cell expressing CT100 displayed depression in control slices, whereas in only 10 out of 16 paired recordings did the cell expressing CT100 display depression in BIO992-exposed slices (comparison between with and without BIO992, p< 0.05, χ2 test), indicating a significant block of BIO992 on Aβ-induced synaptic depression. Incubation of slices with BIO992 for 2–4 h produced no significant change in the amplitude (no compound: 11 ± 0.6 pA, N=15; compound: 12 ± 0.6 pA, N=15; p > 0.05) or frequency (no compound: 0.5 ± 0.08/s, N=15; compound: 0.7 ± 0.1/s, N=15; p > 0.05) of spontaneous miniature synaptic responses. These results with PICK1−/− tissue and BIO992 support the view that a PDZ domain interaction between PICK1 and GluA2 is required for Aβ to produce synaptic depression. As BIO922 was added after synaptic depression occurred, the results indicate that BIO992 blocks depression (see also Supplementary Fig. 1), rescues synapses from a depressed state and that Aβ-induced synaptic depression observed at 16–18 h is not irreversible.
Fig. 3.
Blocking the interaction between GluA2 and PICK1 PDZ domain rescues Aβ-induced synaptic depression. (A) Same as Fig. 1A for a separate group of cell pairs (n= 12 pairs; p < 0.001). (B) Same as A, but in the presence of a PICK1 inhibitor (BIO922, 10 μM; n = 16 pairs; p = 0.3).
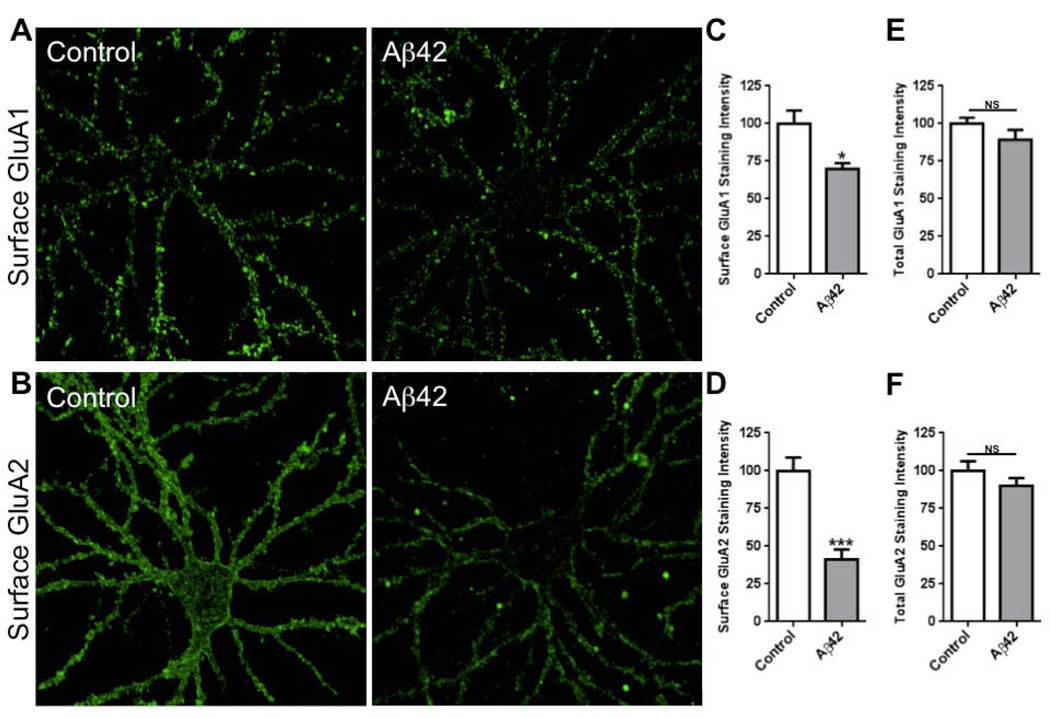
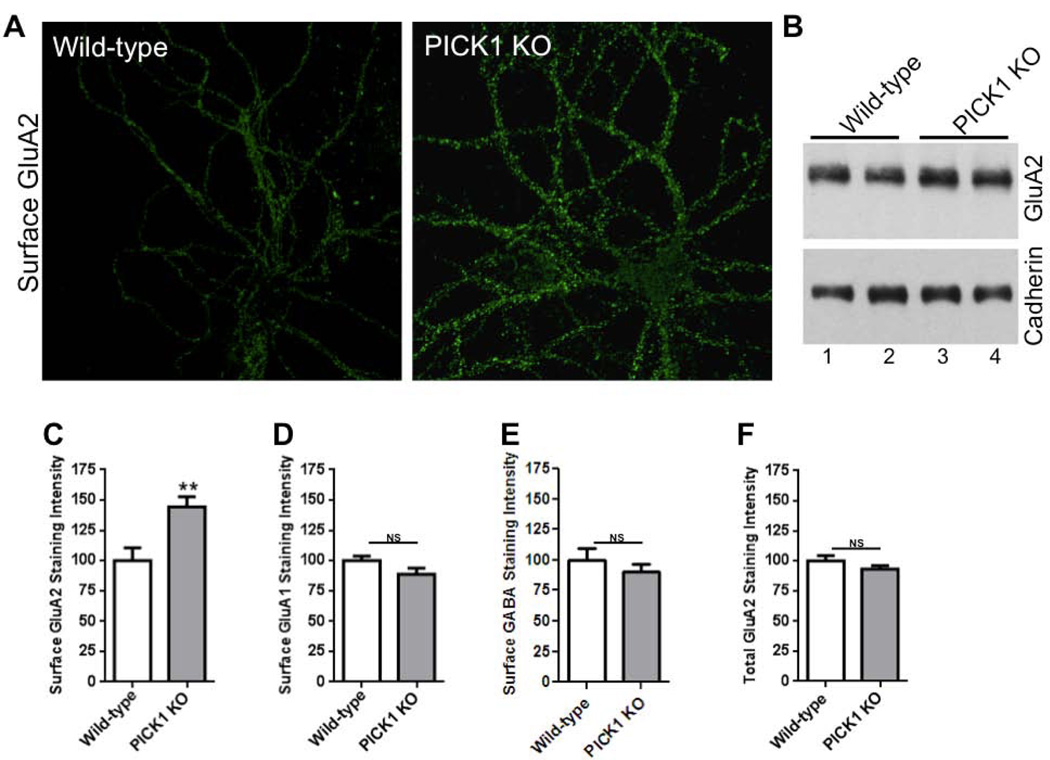
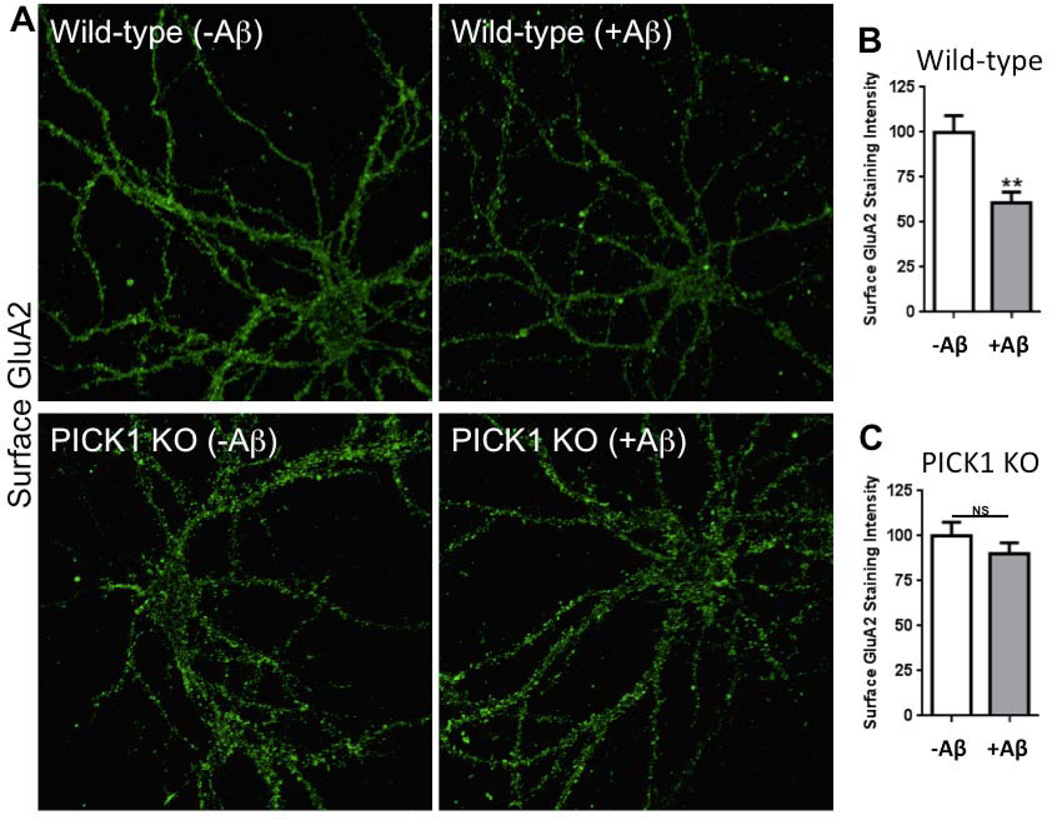
To examine the role of PICK1 on Aβ-induced AMPAR endocytosis we measured surface AMPARs in dissociated cultured neurons (see Materials and methods). Following exposure of dissociated cultured neurons to Aβ for 24 h, we noted a reduction in surface AMPAR staining with no effect on total AMPAR staining, consistent with the view that Aβ drives AMPAR endocytosis and/or stabilizes intracellular recycling of AMPARs (Fig. 4). In particular, the effect was more prominent on surface GluA2 compared with surface GluA1 (Fig. 4) (GluA1 reduction, 70 ± 4%; GluA2 reduction, 41 ± 7%; p < 0.05 test). We next examined surface AMPARs in tissue prepared from mice lacking PICK1. We noted that surface staining for GluA2, but not surface GluA1, was elevated in neurons lacking PICK1 compared with wild-type neurons (Fig. 5). Total GluA2 staining and surface GABA receptor staining were not changed in neurons lacking PICK1. The elevated surface GluA2 was confirmed using a surface biotinylation-based assay (see Materials and methods) (Fig. 5). When exposed to Aβ, surface GluA2 staining on wild-type neurons was reduced; however, Aβ application on neurons lacking PICK1 did not reduce surface GluA2 staining (Fig. 6). These findings are consistent with the view that PICK1 normally participates in maintaining a significant fraction of GluA2-containing AMPARs in an intracellular pool. In the absence of PICK1, these GluA2-containing intracellular receptors are released onto the surface. Upon addition of Aβ, PICK1 is required for the movement of receptors from the surface to an intracellular pool, or for an increased intracellular lifetime of recycling GluA2-containing receptors.
Fig. 4.
Soluble oligomeric Aβ42 decreases surface AMPARs in neurons. Cultured rat hippocampal neurons were treated with soluble Aβ42 (5 μM) and labeled for surface GluA1 (A) and GluA2 (B). (C-F) Histograms show quantification of immunofluorescence intensities of surface and total GluA1 and GluA2 subunits, normalized to control group. (C) n = 17 control, 17 Aβ42-treated (p = 0.01); (D) n = 18 control, 18 Aβ42-treated (p < 0.001); (E) n = 15 control, 15 Aβ42-treated (p = 0.19); (F) n = 16 control, 16 Aβ42-treated (p = 0.24). Scale bars, 20 µm.
Fig. 5.
PICK1 deletion increases surface AMPARs. (A) Cultured hippocampal neurons from wild-type and PICK1 knockout (KO) mice were immunostained for surface GluA2. (B) Surface biotinylation analysis of GluA2 in cortical neurons cultured from wild-type (n=2) and PICK1 KO (n=2) mice. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis was performed as described in Materials and methods. GluA2 signal intensity normalized to cadherin for wild-type mice was 1.2-fold in PICK1 KO mice. Histograms show quantification of staining intensities of surface GluA1 (C), surface GluA2 (D), surface GABA (E) and total GluA2 (F) receptors in wild-type and PICK1 KO mice neurons normalized to the wild-type group. (C) n = 18 wild-type, 18 PICK1 KO (p = 0.01); (D) n = 16 wild-type, 16 PICK1 KO (p = 0.13); (E) n = 15 wild-type, 15 PICK1 KO (p = 0.41); (F) n = 16 wild-type, 16 PICK1 KO (p = 0.23). Scale bars, 20 µm.
Fig. 6.
PICK1 deletion blocks Aβ-induced reduction in surface GluA2. Hippocampal neurons cultured from wild-type (A) and PICK1 knockout (KO) (B) mice were treated with Aβ and labeled for surface GluA2. Histograms show quantification of surface GluA2 immunofluorescence intensities from wild-type and PICK1 KO mouse Aβ42-treated neurons analyzed as follows: wild-type Aβ42-treated normalized to untreated wild-type neurons (B); PICK1 KO Aβ42-treated normalized to untreated PICK1 KO neurons (C); and wild-type Aβ42-treated, PICK1 KO Aβ42-treated and PICK1 KO Aβ42-untreated normalized to untreated wild-type neurons (D). (B) n = 17 wild-type (-Aβ42), n = 17 wild-type (+Aβ42) (p < 0.01); (C) n = 18 PICK1 KO (-Aβ42), 18 PICK1 KO (+Aβ42) (p = 0.34). Scale bars, 20 µm.
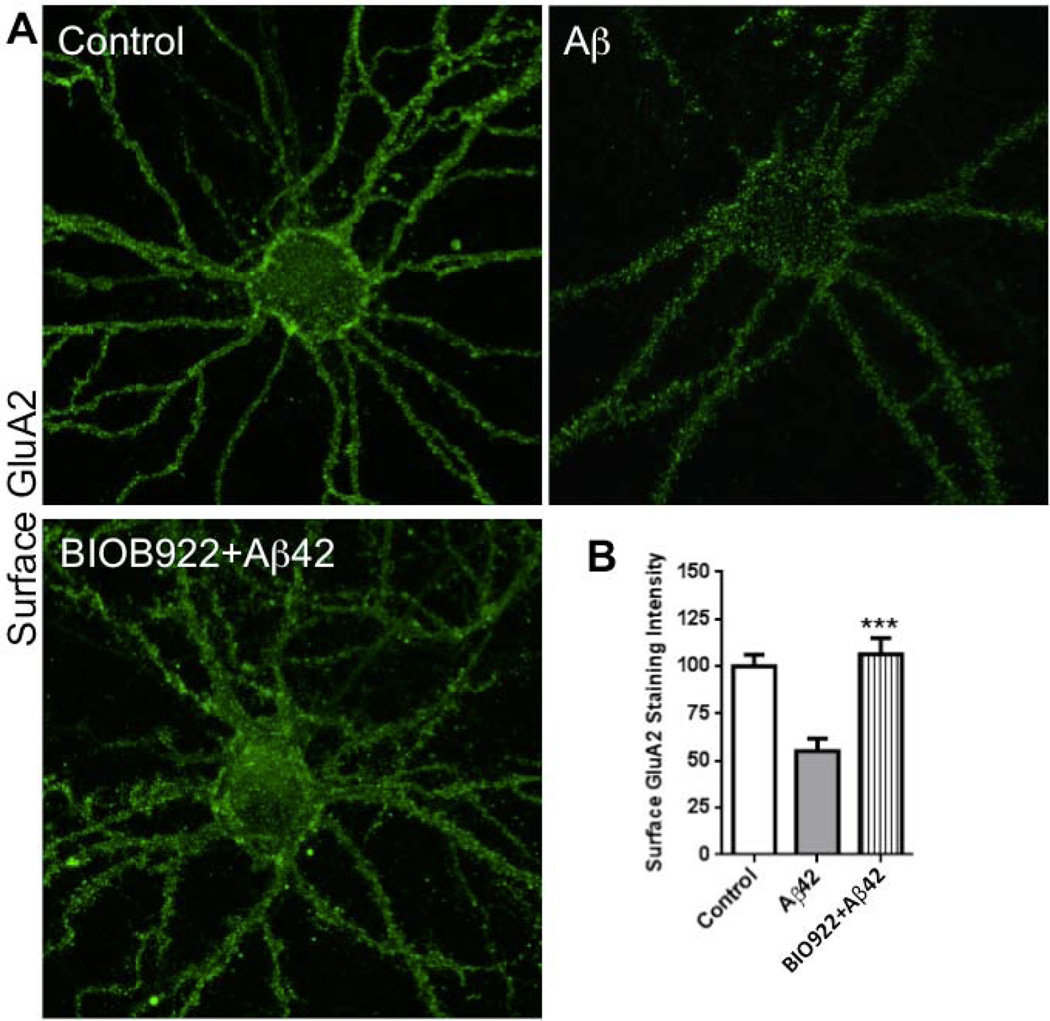
We used BIO922 to test whether a PDZ domain interaction between PICK1 and GluA2 is necessary for the actions of Aβ on surface AMPARs. Dissociated cultured neurons were exposed to Aβ for 24 h in the presence or absence of 3 μM BIO922. Whereas neurons exposed to Aβ in the absence of BIO922 showed a significant reduction in surface GluA2 staining, neurons exposed to Aβ in the presence of BIO922 showed no reduction in surface GluA2 staining (Fig. 7). These findings support the view that an interaction between PICK1 and GluA2 is required for Aβ to drive surface AMPAR endocytosis.
Fig. 7.
PICK1 inhibitor blocks Aβ-mediated reduction in surface AMPARs. (A) Hippocampal neurons were treated with no drug (upper left), or Aβ42 in the presence (lower left) or absence (upper right) of PICK1 inhibitor (BIO922, 3 μM) for 24 h, and immunostained for surface GluA2. (B) Histograms show quantification of surface GluA2 immunofluorescence intensity normalized to control (non-treated) values. n = 17 control, n = 17 Aβ42 treated, n = 17 Aβ42 treated + BIO922 (p < 0.001) comparing Aβ42-treated with Aβ42-treated + BIO922. Scale bars, 20 µm.
Discussion
In this study we have examined the mechanism by which Aβ affects synapses. We used two different assays to monitor AMPARs, i.e. synaptic transmission and surface labeling of AMPARs. We confirm that the virally-driven elevation of Aβ leads to synaptic depression in organotypic hippocampal slices (Hsieh et al., 2006). We now find that synaptic transmission remaining after exposure to Aβ displays greater rectification, consistent with the view that Aβ preferentially drives the synaptic removal of AMPARs containing GluA2. We also find that exposure of dissociated cultured neurons to synthetic Aβ drives the removal of surface AMPARs, with a greater effect on GluA2 compared with GluA1. As a significant proportion of AMPARs are thought to contain GluA1 and GluA2 (Wenthold et al., 1996), it is possible that the surface loss of GluA1 is a consequence of the GluA2-mediated loss of GluA1/GluA2 heteromers. A significant loss of surface GluA2/GluA3 heteromers could account for the greater effect seen on GluA2 compared with GluA1. Thus, in both assays, GluA2-containing receptors are preferentially targeted for surface and synaptic removal by Aβ.
We have examined the role of PICK1 in the effects of Aβ by using mice lacking PICK1 and BIO922, a compound that targets the interactions between the GluA2 PDZ ligand and the PICK1 PDZ domain. We find that, in organotypic slices prepared from mice lacking PICK1, the virally-driven elevation of Aβ fails to produce depression of synaptic transmission. We also find that, in dissociated cultured neurons prepared from mice lacking PICK1, Aβ fails to drive the removal of surface AMPARs. Furthermore, we find that exposure of slices to BIO922 reverses the synaptic depression produced by elevated Aβ, and that exposure of dissociated cultured neurons to BIO922 blocks the surface removal of AMPARs produced by Aβ. These findings support a model in which Aβ triggers signaling that increases the interaction between the GluA2 cytoplasmic tail and PICK1 PDZ domain, and that such an interaction promotes the endocytosis of surface AMPARs. Mechanistically, the increased GluA2–PICK1 interaction could stabilize an intracellular pool of AMPARs (Citri et al., 2010; Thorsen et al., 2010) if AMPARs are continually cycling between intracellular and surface locations (Luscher et al., 1999). The finding that BIO922 application to brain slices was able to rescue synaptic depression is consistent with such AMPAR dynamics. The signaling triggered by Aβ that produces these effects on AMPAR distribution remains to be elucidated, but could include activation of a protein kinase that phosphorylates the GluA2 cytoplasmic domain, which has been shown to reduce GluA2 interactions with the synaptic protein GRIP, while maintaining GluA2 interactions with PICK1 (Chung et al., 2000; Lin & Huganir, 2007; Thorsen et al., 2010). Our findings suggest that drugs targeting the interaction between GluA2 and PICK1 may be beneficial in offsetting the effects of elevated Aβ, and therefore may warrant consideration in the therapeutics of Alzheimer’s disease.
Supplementary Material
Acknowledgements
We thank I. Hunton for the preparation of organotypic brain slices. S.A. is supported by a Neuroplasticity of Aging Training Grant (AG000216). R.M is supported by NIH grant AG032132 and the Cure Alzheimer’s Foundation.
Abbreviations
- Aβ
amyloid beta
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- EPSC
excitatory postsynaptic current
- GluA1–3
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunits 1−3
- PICK1
protein interacting with C kinase 1
References
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J Neurosci. 2010;30:16437–16452. doi: 10.1523/JNEUROSCI.4478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Abeta oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiol Aging. 2011;32:2211–2218. doi: 10.1016/j.neurobiolaging.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Culturing nerve cells. Vol. 2. Cambridge, MA: MIT Press; 1991. Rat hippocampal neurons in low-density culture. [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Nabavi S, Malinow R. Metabotropic NMDA receptor function is required for beta-amyloid-induced synaptic depression. Proc Natl Acad Sci U.S.A. 2013;110:4033–4038. doi: 10.1073/pnas.1219605110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci. 2007;10:1260–1267. doi: 10.1038/nn1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thorsen TS, Madsen KL, Rebola N, Rathje M, Anggono V, Bach A, Moreira IS, Stuhr-Hansen N, Dyhring T, Peters D, Beuming T, Huganir R, Weinstein H, Mulle C, Stromgaard K, Ronn LC, Gether U. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc Natl Acad Sci U.S.A. 2010;107:413–418. doi: 10.1073/pnas.0902225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3:291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Xu J, Xia J. Structure and function of PICK1. Neurosignals. 2006;15:190–201. doi: 10.1159/000098482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.