Abstract
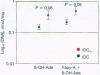
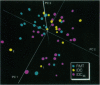
Hydroxyl radical damage in metastatic tumor DNA was elucidated in women with breast cancer, and a comparison was made with nonmetastatic tumor DNA. The damage was identified by using statistical models of modified base and Fourier transform-infrared spectral data. The modified base models revealed a greater than 2-fold increase in hydroxyl radical damage in the metastatic tumor DNA compared with the nonmetastatic tumor DNA. The metastatic tumor DNA also exhibited substantially greater base diversity than the nonmetastatic DNA, and a progression of radical-induced base damage was found to be associated with the growth of metastatic tumors. A three-dimensional plot of principal components from factor analysis, derived from infrared spectral data, also showed that the metastatic tumor DNA was substantially more diverse than the tightly grouped nonmetastatic tumor DNA. These cohesive, independently derived findings suggest that the hydroxyl radical generates DNA phenotypes with various metastatic potentials that likely contribute to the diverse physiological properties and heterogeneity characteristic of metastatic cell populations.
Full text
PDF
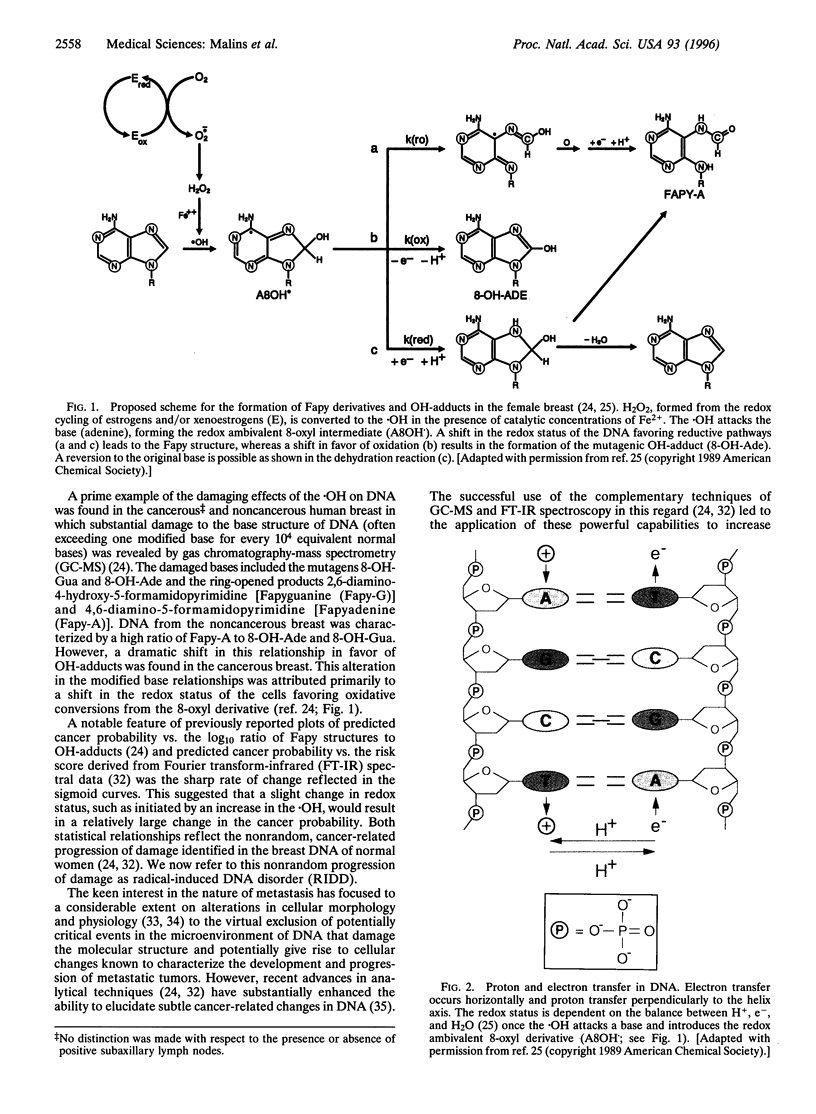
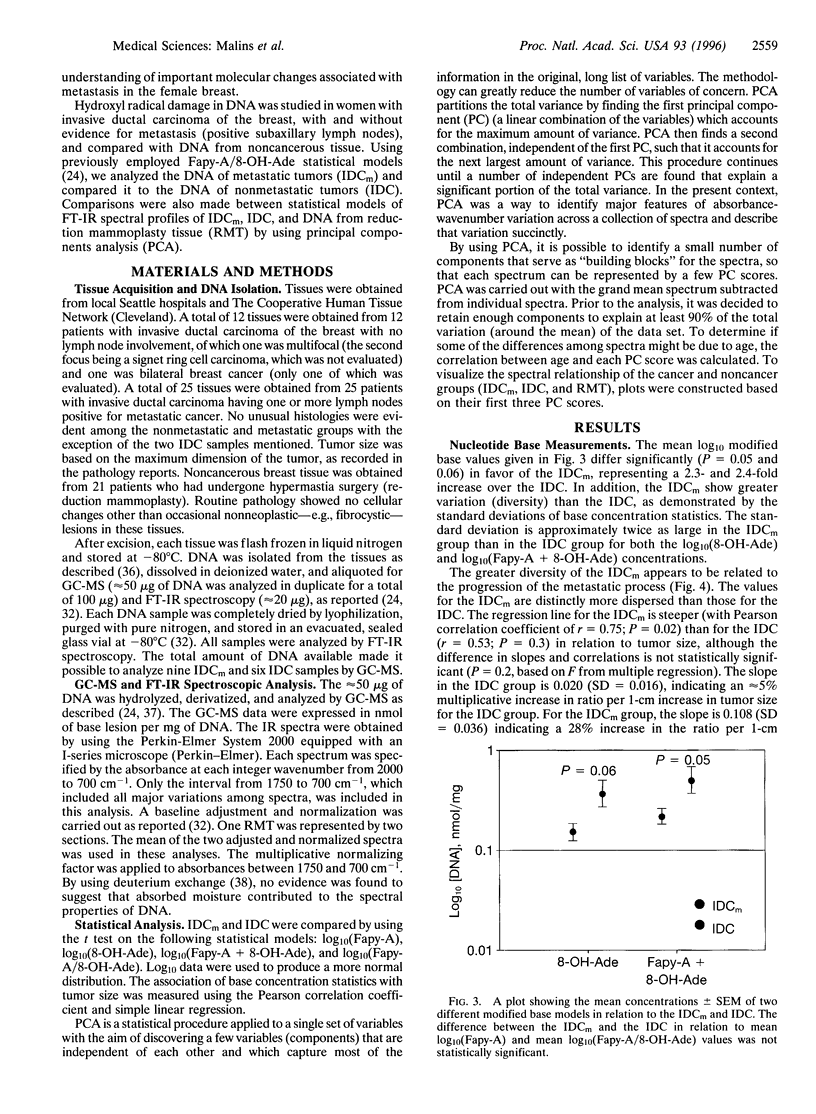
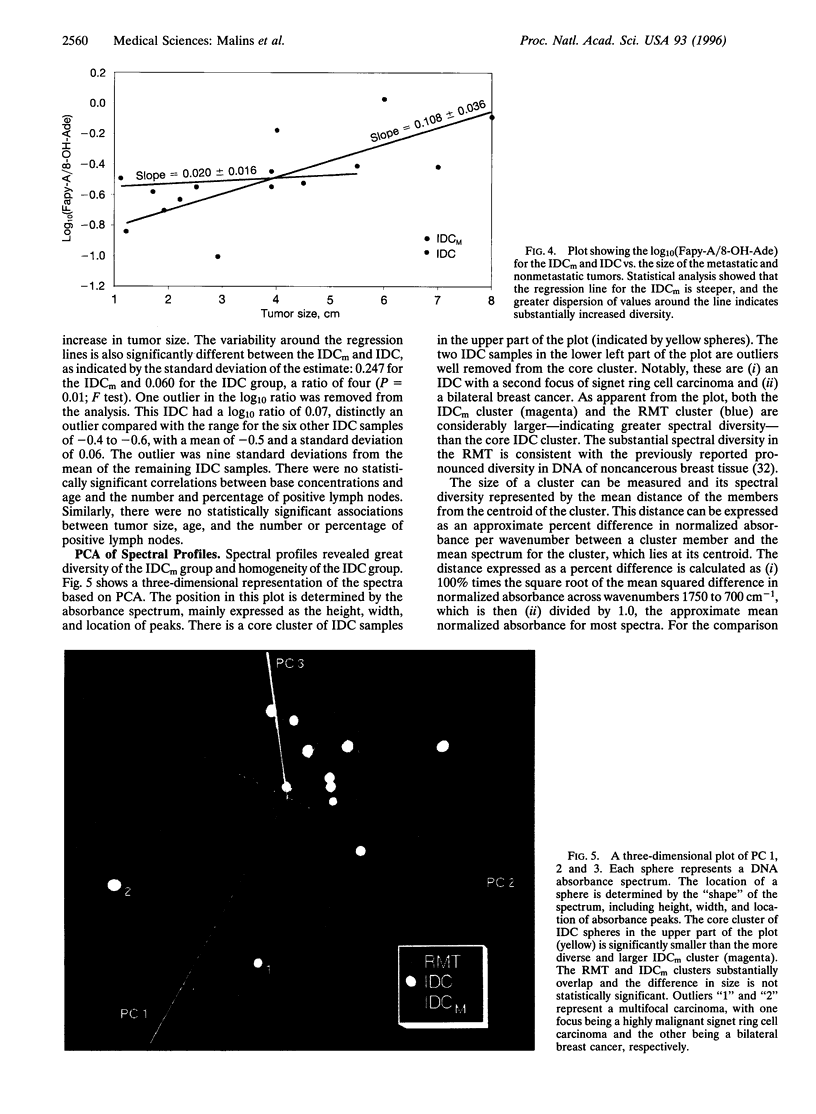


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida M., Nishimura S. An ab initio molecular orbital study on the characteristics of 8-hydroxyguanine. Mutat Res. 1987 Oct;192(2):83–89. doi: 10.1016/0165-7992(87)90101-1. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Lederer F., Gouyette A., Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990 Mar 5;265(7):3916–3922. [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Chetsanga C. J., Grigorian C. In situ enzymatic reclosure of opened imidazole rings of purines in DNA damaged by gamma-irradiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):633–637. doi: 10.1073/pnas.82.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetsanga C. J., Lozon M., Makaroff C., Savage L. Purification and characterization of Escherichia coli formamidopyrimidine-DNA glycosylase that excises damaged 7-methylguanine from deoxyribonucleic acid. Biochemistry. 1981 Sep 1;20(18):5201–5207. doi: 10.1021/bi00521a016. [DOI] [PubMed] [Google Scholar]
- Coles C., Condie A., Chetty U., Steel C. M., Evans H. J., Prosser J. p53 mutations in breast cancer. Cancer Res. 1992 Oct 1;52(19):5291–5298. [PubMed] [Google Scholar]
- Dizdaroglu M., Gajewski E. Selected-ion mass spectrometry: assays of oxidative DNA damage. Methods Enzymol. 1990;186:530–544. doi: 10.1016/0076-6879(90)86147-n. [DOI] [PubMed] [Google Scholar]
- Dunn B. P., Black J. J., Maccubbin A. 32P-postlabeling analysis of aromatic DNA adducts in fish from polluted areas. Cancer Res. 1987 Dec 15;47(24 Pt 1):6543–6548. [PubMed] [Google Scholar]
- Eskenazi A. E., Pinkas J., Whitin J. C., Arguello F., Cohen H. J., Frantz C. N. Role of antioxidant enzymes in the induction of increased experimental metastasis by hydroxyurea. J Natl Cancer Inst. 1993 May 5;85(9):711–721. doi: 10.1093/jnci/85.9.711. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53(1):127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K. Hydrogen peroxide formation and DNA base modification by tumor promoter-activated polymorphonuclear leukocytes. Carcinogenesis. 1987 Mar;8(3):455–460. doi: 10.1093/carcin/8.3.455. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Quinlan G. J. Antioxidant protection against organic and inorganic oxygen radicals by normal human plasma: the important primary role for iron-binding and iron-oxidising proteins. Biochim Biophys Acta. 1993 Feb 13;1156(2):144–150. doi: 10.1016/0304-4165(93)90129-v. [DOI] [PubMed] [Google Scholar]
- Han X., Liehr J. G. 8-Hydroxylation of guanine bases in kidney and liver DNA of hamsters treated with estradiol: role of free radicals in estrogen-induced carcinogenesis. Cancer Res. 1994 Nov 1;54(21):5515–5517. [PubMed] [Google Scholar]
- Han X., Liehr J. G. Microsome-mediated 8-hydroxylation of guanine bases of DNA by steroid estrogens: correlation of DNA damage by free radicals with metabolic activation to quinones. Carcinogenesis. 1995 Oct;16(10):2571–2574. doi: 10.1093/carcin/16.10.2571. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., Schraufstatter I. U., Hyslop P. A., Vosbeck K., Sauerheber R., Weitzman S. A., Cochrane C. G. Role of oxidants in DNA damage. Hydroxyl radical mediates the synergistic DNA damaging effects of asbestos and cigarette smoke. J Clin Invest. 1987 Oct;80(4):1090–1095. doi: 10.1172/JCI113165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H., Miura H., Murata-Kamiya N., Ishikawa H., Sakaguchi T., Inoue H., Sasaki T., Masutani C., Hanaoka F., Nishimura S. 8-Hydroxyadenine (7,8-dihydro-8-oxoadenine) induces misincorporation in in vitro DNA synthesis and mutations in NIH 3T3 cells. Nucleic Acids Res. 1995 Aug 11;23(15):2893–2899. doi: 10.1093/nar/23.15.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. C., Bleeker M. J., Saris C. P., Roelen H. C., Brugghe H. F., van den Elst H., van der Marel G. A., van Boom J. H., Westra J. G., Kriek E. Repair and replication of plasmids with site-specific 8-oxodG and 8-AAFdG residues in normal and repair-deficient human cells. Nucleic Acids Res. 1992 Sep 11;20(17):4437–4443. doi: 10.1093/nar/20.17.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. S., Fletcher R. G., Grond M. P., Inyang A. I., Lu X. P., Brenner D. A., Leffert H. L. Inactivation of plasmid reporter gene expression by one benzo(a)pyrene diol-epoxide DNA adduct in adult rat hepatocytes. Cancer Res. 1993 May 15;53(10 Suppl):2279–2286. [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995 May 1;55(9):1856–1862. [PubMed] [Google Scholar]
- Kundu N., Zhang S., Fulton A. M. Sublethal oxidative stress inhibits tumor cell adhesion and enhances experimental metastasis of murine mammary carcinoma. Clin Exp Metastasis. 1995 Jan;13(1):16–22. doi: 10.1007/BF00144014. [DOI] [PubMed] [Google Scholar]
- Liehr J. G., Wheeler W. J. Inhibition of estrogen-induced renal carcinoma in Syrian hamsters by vitamin C. Cancer Res. 1983 Oct;43(10):4638–4642. [PubMed] [Google Scholar]
- Malins D. C., Holmes E. H., Polissar N. L., Gunselman S. J. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer. 1993 May 15;71(10):3036–3043. doi: 10.1002/1097-0142(19930515)71:10<3036::aid-cncr2820711025>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Malins D. C. Identification of hydroxyl radical-induced lesions in DNA base structure: biomarkers with a putative link to cancer development. J Toxicol Environ Health. 1993 Oct-Nov;40(2-3):247–261. doi: 10.1080/15287399309531792. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Arrick B. A., Murray H. W., DeSantis N. M., Cohn Z. A. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. doi: 10.1084/jem.153.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- O'Donnell-Tormey J., DeBoer C. J., Nathan C. F. Resistance of human tumor cells in vitro to oxidative cytolysis. J Clin Invest. 1985 Jul;76(1):80–86. doi: 10.1172/JCI111981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinski R., Zastawny T., Budzbon J., Skokowski J., Zegarski W., Dizdaroglu M. DNA base modifications in chromatin of human cancerous tissues. FEBS Lett. 1992 Sep 7;309(2):193–198. doi: 10.1016/0014-5793(92)81093-2. [DOI] [PubMed] [Google Scholar]
- Peller S., Halevy A., Slutzki S., Kopilova Y., Rotter V. p53 mutations in matched primary and metastatic human tumors. Mol Carcinog. 1995 Jul;13(3):166–172. doi: 10.1002/mc.2940130306. [DOI] [PubMed] [Google Scholar]
- Pero R. W., Roush G. C., Markowitz M. M., Miller D. G. Oxidative stress, DNA repair, and cancer susceptibility. Cancer Detect Prev. 1990;14(5):555–561. [PubMed] [Google Scholar]
- Roy D., Floyd R. A., Liehr J. G. Elevated 8-hydroxydeoxyguanosine levels in DNA of diethylstilbestrol-treated Syrian hamsters: covalent DNA damage by free radicals generated by redox cycling of diethylstilbestrol. Cancer Res. 1991 Aug 1;51(15):3882–3885. [PubMed] [Google Scholar]
- Saran M., Bors W. Radical reactions in vivo--an overview. Radiat Environ Biophys. 1990;29(4):249–262. doi: 10.1007/BF01210406. [DOI] [PubMed] [Google Scholar]
- Sipe H. J., Jr, Jordan S. J., Hanna P. M., Mason R. P. The metabolism of 17 beta-estradiol by lactoperoxidase: a possible source of oxidative stress in breast cancer. Carcinogenesis. 1994 Nov;15(11):2637–2643. doi: 10.1093/carcin/15.11.2637. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Sobel M. E., Liotta L. A. Identification and characterization of differentially expressed genes in tumor metastasis: the nm23 gene. Basic Life Sci. 1991;57:355–361. doi: 10.1007/978-1-4684-5994-4_29. [DOI] [PubMed] [Google Scholar]
- Symmans W. F., Liu J., Knowles D. M., Inghirami G. Breast cancer heterogeneity: evaluation of clonality in primary and metastatic lesions. Hum Pathol. 1995 Feb;26(2):210–216. doi: 10.1016/0046-8177(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Szatrowski T. P., Nathan C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991 Feb 1;51(3):794–798. [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. M., Anderson T. J., Condie A., Prosser J., Chetty U., Carter D. C., Evans H. J., Steel C. M. p53 allele losses, mutations and expression in breast cancer and their relationship to clinico-pathological parameters. Int J Cancer. 1992 Feb 20;50(4):528–532. doi: 10.1002/ijc.2910500405. [DOI] [PubMed] [Google Scholar]
- Zaho M. J., Jung L., Tanielian C., Mechin R. Kinetics of the competitive degradation of deoxyribose and other biomolecules by hydroxyl radicals produced by the Fenton reaction. Free Radic Res. 1994 Jun;20(6):345–363. doi: 10.3109/10715769409145635. [DOI] [PubMed] [Google Scholar]