Abstract
Enzymes used for passaging human pluripotent stem cells (hPSCs) digest cell surface proteins, resulting in cell damage. Moreover, cell dissociation using divalent cation-free solutions causes apoptosis. Here we report that Mg2+ and Ca2+ control cell-fibronectin and cell-cell binding of hPSCs, respectively, under feeder- and serum-free culture conditions without enzyme. The hPSCs were detached from fibronectin-, vitronectin- or laminin-coated dishes in low concentrations of Mg2+ and remained as large colonies in high concentrations of Ca2+. Using enzyme-free solutions containing Ca2+ without Mg2+, we successfully passaged hPSCs as large cell clumps that showed less damage than cells passaged using a divalent cation-free solution or dispase. Under the same conditions, the undifferentiated and early-differentiated cells could also be harvested as a cell sheet without being split off. Our enzyme-free passage of hPSCs under a serum- and feeder-free culture condition reduces cell damage and facilitates easier and safer cultures of hPSCs.
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent cells (hiPSCs), have increased the possible applications of stem cell research in biology and medicine1,2,3. Since dissociating hPSCs into single cells using divalent cation-free solution causes cell damage and death by apoptosis4,5,6,7,8, hPSC passaging usually entails dissociating the cell colonies into large cell clumps using enzymes in a divalent cation-containing solution (Table 1). However, these enzymes may also induce cell damage by digesting cell-surface proteins5,8.
Table 1. Passaging protocols for hPSC culture.
| Passage Protocols | Culture Conditions | References | ||||
|---|---|---|---|---|---|---|
| Divalent cations | Enzymes | Manipulation | Cell clump size | Serum replacement | Coat** & feeder | |
| Ca2+, Mg2+ * | Collagenase | Cutting, Scraping, Glass beads | Large | KSR, | MEF | 1,27 |
| Ca2+, Mg2+ * | Free | Cutting | Large | KSR | MEF | 5 |
| Ca2+ *** | Trypsin & Collagenase | Pipetting | Large | KSR | MEF | 2,6,23 |
| Free (EDTA) | Trypsin TrypLE | Pipetting | Single cells, Large | KSR | MEF | 4,5,6,19,28 |
| Free (EDTA) | Free (CDB#) | Pipetting | Single cells | KSR | MEF | 5 |
| Ca2+, Mg2+ * | Dispase, Collagenase TrypLE | Scraping, Pipetting | Large | Free | Mg, Gx, Lm, Vn, Fb, Cg, pVn, pBSP | 8,14,15,20,29 |
| Ca2+, Mg2+ * | Free | Cutting | Large | Free | Vn | 12 |
| Free (EDTA) | Free (CDB#) | Pipetting | Small | Free | Mg, HBP | 8,30 |
| Ca2+ | Free | Pipetting | Large (Small) | Free | Fb | Present study |
*DEME/F12 or ESF solution16 containing Ca2+, Mg2+.
**MEF: mouse embryonic fibroblast feeder cells on gelatin-coated dishes, Mg: Matrigel, Gx: geltrex, Lm: laminin, Vn: vitronectin, Cg: collagen, pVn: vitronectin-derived peptide, pBSP: bone sialoprotein-derived peptide, HBP: heparin-binding peptide.
***Dissociation solution named CTK containing CaCl2, trypsin, collagenase IV, and KSR23. KSR contains many divalent cations.
# CDB: Cell dissociation Buffer (Life Technologies).
To achieve enzyme-free and less damaging passage of hPSCs, we focused on the roles of Ca2+ and Mg2+ in cell-cell and cell-fibronectin binding. Physiological concentrations of Ca2+ regulate cell-cell binding of hPSCs mediated by E-cadherin2,7,9,10. On the other hand, physiological concentrations of Mg2+ are required for optimal, tight binding between cells and fibronectin, part of the extracellular coating matrix of hPSCs11,12,13,14,15. We therefore hypothesized that solution containing physiological concentration of Ca2+, but no Mg2+, could be used to passage hPSCs cultured on fibronectin-coated dishes as large cell clumps without the need for enzyme-based cell dissociation. We tested this hypothesis using our serum- and feeder-free culture medium (ESF9a) in fibronectin-coated dishes14,16,17, allowing us to examine hPSC attachments without masking by undefined adherent factors derived from the serum and feeder cells.
Results
Dose-dependent effects of Mg2+ and Ca2+ on cell-fibronectin and cell-cell binding
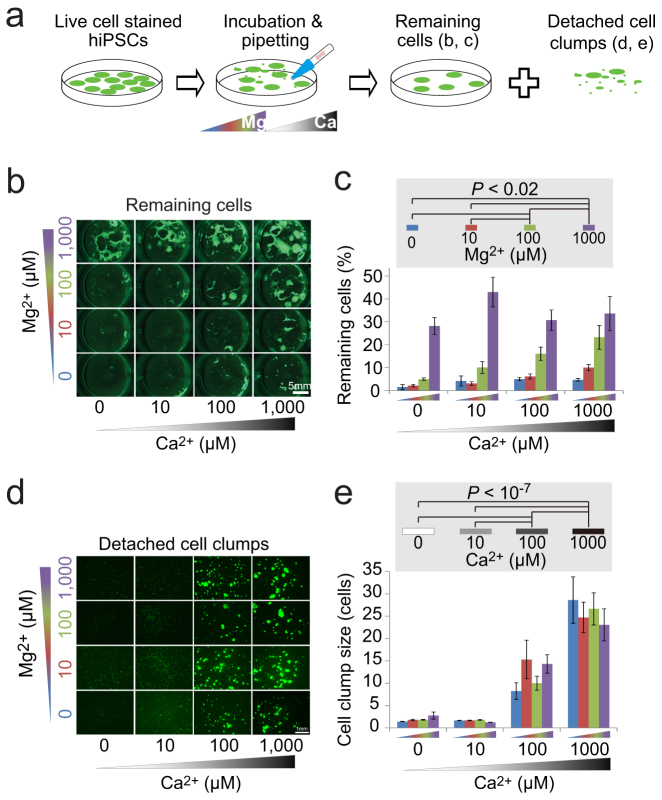
The hiPSCs 253G118 and 201B72 were incubated in phosphate-buffered saline (PBS) containing various concentrations (0, 10, 100, 1000 μM) of Mg2+ and Ca2+ and then triturated to detach cells from the fibronectin-coated plates and dissociate them into cell clumps (Fig. 1a). The number of cells remaining on the dishes decreased with decreasing Mg2+ concentration, although the sizes of the detached hPSCs clumps increased with increasing Ca2+ concentration (253G1: Fig. 1b–e, 201B7: Supplementary Fig. 1ab). These results suggest that the cell-fibronectin binding depended on Mg2+ concentration whereas cell-cell binding of hPSCs was dependent on Ca2+ concentration, and that these bindings could be independently controlled without enzyme.
Figure 1. Dose-dependent effects of Mg2+ and Ca2+ on cell-fibronectin and cell-cell binding.
(a): Schematics of experiments. The illustrations were drawn by KO. (b–e): Fluorescent imaging (live-cell dye, Calcein-AM) of the hiPSCs (253G1) remaining on the fibronectin-coated 24-well plate (b) and the cells detached from the plate (d) after incubation and then trituration using a 1-ml pipette tip in PBS containing 0–1000 μM Ca2+ and Mg2+. (c): Remaining-cell ratios equal the area of remaining cells divided by the area of the cells before incubation and trituration. (d): Mean cell clump size detached from the plates. Two-way ANOVA revealed no interaction effect between Ca2+ and Mg2+ concentration ((c): P = 0.066, mean ± SE, n = 5, (e): P = 0.47, mean ± SE, n = 5 experiments × 200 cells). Post-hoc Tukey's multiple comparison revealed significant differences in remaining-cell ratio between different Mg2+ concentrations (the same Ca2+ data were put together to derive the numbers and bars in (c)) and in cell clump size between different Ca2+ concentrations (the same Ca2+ data were put together to derive the numbers and bars in (e)). Scale bars are 5 mm (b), 1 mm (d).
Passage of hPSCs with enzyme-free solution containing a physiological concentration of Ca2+ without Mg2+
We tested whether the buffer solutions with Ca2+ and without Mg2+ could be used to passage hPSCs. Large cell colonies of hESCs, HUES819, and H91 were detached from fibronectin-coated dishes and dissociated into large cell clumps by incubation in PBS containing 1 mM Ca2+ without Mg2+ (PBSca/−) followed by pipetting (Supplementary Fig. 2a–g). These detached cell clumps were then plated into fibronectin-coated dishes and reattached as typical hPSC flat colonies on the next day (Supplementary Fig. 2h–m). In addition, hiPSCs cultured on vitronectin and laminin, which are also used as a coating matrix for culturing hPSCs (Table 1), can be detached from the culture dishes by PBSca/− (Supplementary Fig. 3). These results suggested that enzyme-free solution containing physiological concentration of Ca2+, but no Mg2+, could be useful for passaging hPSCs as large cell clumps.
Effects of dissociation and enzymatic digestion
We compared our enzyme-free passage method to both dissociation passaging in a divalent cation-free solution and enzymatic digestion passaging.
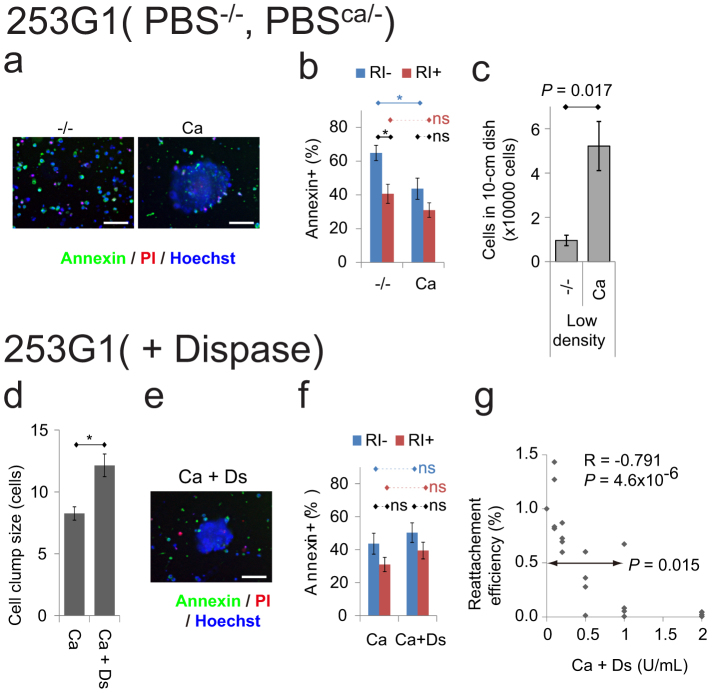
Dissociating hPSCs into single cells and replating at low density causes cell damage and death by apoptosis4,5,6,7,8. Thus, we hypothesized that detaching and dissociating hPSCs into larger clumps using PBSca/− could suppress apoptosis and support higher survival rates than detaching and dissociating these cells into smaller clumps using PBS without Ca2+ and without Mg2+ (PBS−/−). To test this hypothesis, apoptosis was detected in the hiPSCs 253G1 and 201B7 using annexin V-FITC, which stains cell surface phosphatidylserine, four hours after detachment and dissociation in PBS−/− and PBSca/− followed by floating culture in ESF9a solution. Fluorescence microscopy showed many annexin V-FITC-positive single cells dissociated by PBS−/−, whereas annexin V-FITC-negative cells predominated in the large cell clumps dissociated by PBS−/ca (253G1: Fig. 2a, 201B7: Supplementary Fig. 4a). Quantitative analysis using flow cytometry (FCM) revealed that fewer annexin V-FITC-positive cells were detached and dissociated by PBSca/− than by PBS−/−, and that addition of a ROCK inhibitor (RI) abolished these differences (253G1: Fig. 2b, 201B7: Supplementary Fig. 4b). RI blocks the dissociation-induced apoptosis of hPSCs6,7. To measure cell survival, hPSCs were detached and dissociated in PBSca/− or PBS−/−, plated at low density (2 × 103 cells/cm2), and then cultured for 3 days. The numbers of hiPSCs passaged in PBSca/− were higher than those passaged in PBS−/− (253G1: Fig. 2c, 201B7 & Tic: Supplementary Fig. 4c), suggesting that adding physiological concentration of Ca2+ to the dissociation solution increases cell survival rates by decreasing dissociation-induced apoptosis.
Figure 2. Effects of dissociation without divalent cation and with dispase.
(a–c): Comparison between the hiPSCs (253G1) detached and dissociated by PBS−/− (−/−) and those by PBSca/− (Ca). (ab): Micrograph (a) and FCM analysis (b) of apoptosis marker annexin V-FITC after four hours floating culture (RI: with 5 μM ROCK inhibitor) following detachment and dissociation. The cells were stained by annexin V-FITC (green), propidium iodide (red: late apoptosis or necrosis marker), and Hoechst 33342 (blue: nuclei marker). (c): The number of cells remaining after 3 days culture in ESF9a on fibronectin-coated 10-cm dishes (the initial cell number was 1.2 × 105 cells/10-cm dish, mean ± SE, n = 5, t-test). (d–g): Effects of dispase in PBSca/− for detaching and dissociating cells. (d): Mean cell clump size detached from the plates using PBSca/− (Ca) and 1 U/ml dispase in PBSca/− (Ca + Ds). (ef): Micrograph (e) and FCM analysis (f) of annexin V-FITC after four hours floating culture following detachment and dissociation by PBSca/− or 1 U/ml dispase in PBSca/−. The staining were the same as (a). (g): Reattachment efficiency. The cells were digested with 0–2 U/ml dispase in PBSca/− and were plated with ESF9a medium including 5 μM ROCK inhibitor. The numbers of cells were estimated using calcein-AM 1 day after plating and normalized against the PBSca/− results (0 U/ml dispase). The mean value at 1 U/ml dispase was smaller than 1 (P < 0.015, n = 4, t-test). Pearson's correlation coefficient = −0.791, P = 4.6 × 10−6 t-test, from 4 independent experiments. Scale bars are 100 μm. (bdf): * P = 0.05, ns: not significant, n = 6, mean ± SE (bf) and * P = 0.01, ns: not significant, n = 4 experiments × 200 cells, mean ± SE (d), Holm's multiple comparison tests (cf. Supplementary Fig 5).
It is also known that enzymatic digestion damages hPSCs5,8. We first used dispase, an enzyme often used to passage hPSCs under serum- and feeder-free conditions (Table 1)20. Because we routinely use 0.025–0.6 U/ml dispase (0.05–300 mg/ml), depending on the enzyme activity and on storage conditions14, excess dispase (1 U/ml) was used to evaluate its damaging effect with the expectation that dispase dissociation of cell-cell binding would decrease the size of cell clumps, resulting in apoptosis. However, addition of 1 U/ml dispase in PBS−/ca did not decrease hPSC clump size (253G1: Fig. 2d, 201B7: Supplementary Fig. 4d). Indeed, large clumps of annexin V-FITC-negative cells were also found when dispase was added to the PBSca/− (253G1: Fig. 2e, 201B7: Supplementary Fig. 4e), and quantitative analysis by FCM revealed that the relative percentages of annexin V-FITC-positive apoptotic cells were not changed by addition of dispase (253G1: Fig. 2f, 201B7: Supplementary Fig. 4f). The results were the same when 0.25% trypsin was added to PBSca/−, despite trypsin having more potent protease activity than dispase (Supplementary Fig. 5a–c, e–g). These findings together suggested that adding proteolytic enzyme to the PBSca/− dose not decrease the cell clump size and thus does not increase dissociation-induced apoptosis. Our results are consistent with a previous report that Ca2+ protects against trypsinization of cell-cell binding10. Because Ca2+ did not affect the cell-fibronectin binding during dissociation (Fig. 1bc, Supplementary Fig 1a), we next measured the re-attachment ability of hiPSCs to fibronectin-coated surfaces. To do this, hPSCs were incubated with dispase in PBSca/−, and replated in ESF9a medium with RI for counting the next day. The reattachment efficiency decreased with increasing concentrations of dispase, and the mean efficiency values at 1 U/ml dispase were smaller than those for PBSca/− alone (253G1: Fig. 2g, 201B7: Supplementary Fig. 4g); a similar result was attained when trypsin was added to PBSca/− (Supplementary Fig. 5dh). These results suggested that addition of enzyme damages cells by suppressing cell-fibronectin rebinding rather than by increasing apoptosis.
These results showed that enzyme-free solution containing a physiological concentration of Ca2+, without Mg2+, enables passaging of hPSCs with less cell damage than found using either divalent-free solution or Ca2+-containing solution with enzyme (dispase or trypsin).
Long-term culture of hPSCs with enzyme-free passaging
Next, we tried long-term culturing of hPSCs under enzyme-, serum-, and feeder-free culture conditions. Two hiPSC lines, 253G1 and 201B7, were successfully cultured for more than 10 passages in ESF9a medium on fibronectin-coated dishes by using the solution with Ca2+ and without Mg2+ (ESF-Fb-EzF condition), with both cell types expressing self-renewal markers (Supplementary Fig. 6a–c, j–l). Immunocytochemistry of embryoid bodies derived from the two cell lines indicated that pluripotency was maintained (Supplementary Fig. 6dm). Unexpectedly, karyotype abnormalities were found not only under the ESF-Fb-EzF condition (Supplementary Fig. 6ir) but also in the sister cultures under the other conditions (Supplementary Fig. 6hopq), suggesting that the abnormalities were induced before enzyme-, serum-, and feeder-free culture.
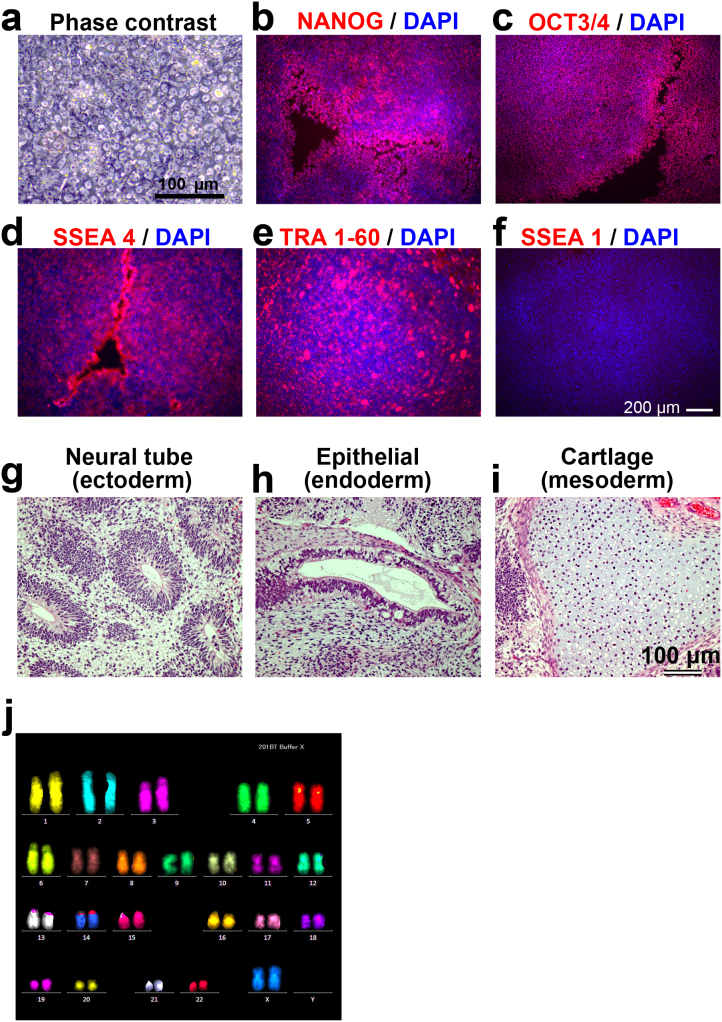
To confirm the karyotype normality, we newly performed long-term cultures using hiPSC 201B7 and Tic lines. The 201B7 cell line was pre-validated to ensure a normal karyotype, and then cultured under the ESE-Fb-EzF condition for more than 10 passages. The cells formed normal hiPSC colonies, which were tightly packed, and flat colonies consisting of cells with large nuclei and scant cytoplasm (Fig. 3a)1,2,3 and expressed four self-renewal markers, NANOG, OCT3/4, SSEA4 and TRA 1–60 but not an early differentiation marker, SSEA1 (Fig. 3b–f). The cells differentiated into derivatives of all three primary germ layers in vivo using teratoma formation (Fig. 3g–i). The cells showed a normal karyotype (Fig. 3j). Karyotype after long-term culture was also normal in another hiPSC line, Tic (Supplementary Fig. 7), confirming that karyotype remained stable during the enzyme-, serum-, and feeder-free culture. These results suggested that enzyme-free culture is a useful method for routine culturing of hPSCs.
Figure 3. Passage solution with Ca2+ and without Mg2+ supports long-term culture and pluripotency of hPSCs.
The hiPSC 201B7 were cultured for 15 passages under the ESF-Fb-EzF condition. (a): Phsecontrast micrograph. (b–f): Immunocytochemistry showed that the cells expressed self-renewal markers, NANOG ((b): red), OCT3/4 ((c): red), SSEA4 ((c): red) and TRA 1–60 ((e): red), but not an early differentiation marker, SSEA1 ((f): red). The nuclei were stained with DAPI (blue). (g–h): Histological analysis with HE staining demonstrated that hiPSC-derived teratoma contained derivatives of all three germ layers: neural tube ((g): ectoderm), epitherial ((h): endoderm), and cartridge ((i): mesoderm). (j): FISH karyotype analysis showed a normal karyotype (46XX). Scale bars are 100 μm (a, g–i) or 200 μm (b–f).
Cell sheet harvesting
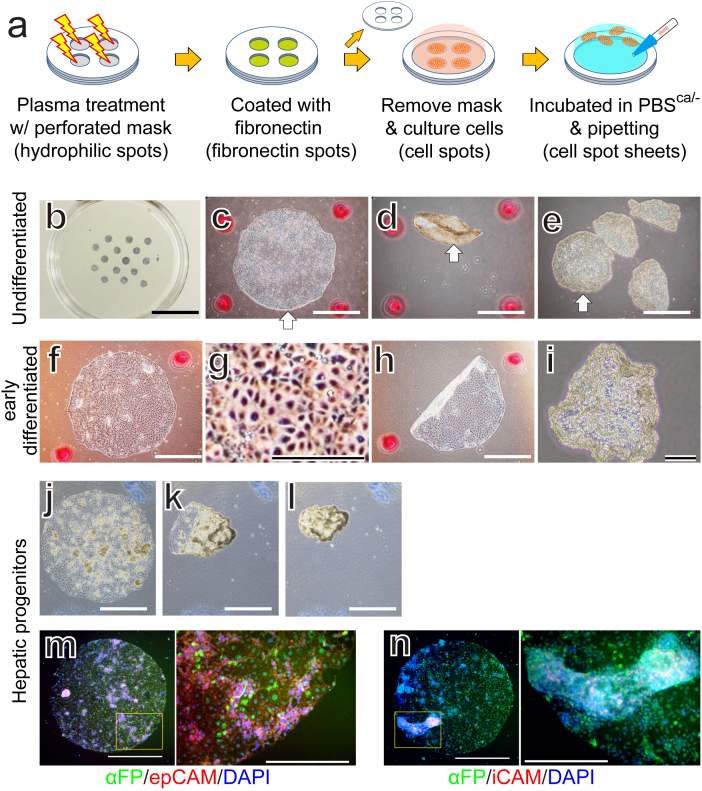
Finally, we tried cell sheet harvesting using our enzyme-free solution. Cell sheet harvesting using special equipment such as a temperature-responsive surface and magnet followed by transplantation is one of the most promising approaches for applying hPSCs to regenerative medicine21,22. However, in this study, simple incubation in PBS with Ca2+ followed by gentle pipetting enabled us to harvest the cells as 2-mm-diameter sheets without cells splitting off (Fig. 4a–e) and without specialized equipment. Similar results were obtained for early-differentiated cells induced by bone morphogenetic protein 4 (BMP4) (Fig. 4f–i) and for hiPSC-derived hepatic progenitors (Fig 4j–n), suggesting that the PBS with Ca2+ could be used to routinely and simply harvest cells as a large sheet without special equipment.
Figure 4. Cell sheet harvesting.
(a): Schematics of spot sheet formation. The hiPSCs (253G1) were cultured in ESF9a medium (undifferentiated: (b–e)), ESF6 medium supplemented with BMP4 for two days (early differentiated: (f–i)). The hiPSC (Tic)-derived hepatic progenitors (j–n). The illustrations were drawn by KO. (b): ALP staining of the hiPSCs plated on 2-mm-diameter fibronectin spots. Phase-contrast micrographs before (c, f, g, j), during (h, k), and after (d, e, i, l) 15 min in PBSca/− followed by pipetting. (g) is a magnified image of part of (f). The white arrows indicate the same cell spot sheet (c, d, e). The red spots in (c–e, f, h, i) and blue irregular marks in (j–l) are position makers. (mn): Immunohistochemistry of hepatic progenitors using the early stages of liver development marker, α-fetoprotein (αFP: green), EpCAM (red), and iCAM (red). Nuclei were stained with DAPI (blue). The right panels are magnified images of the boxed areas in the left panels. Scale bars are 1 cm (b), 1 mm ((c–f, h, j–l), left of (mn)), and 400 μm ((g, i), right of (m,n)).
Discussion
The present study showed that cell-fibronectin and cell-cell binding are controlled separately by Mg2+ and Ca2+, respectively, in hPSC cultures. Using enzyme-free solutions containing Ca2+ without Mg2+, we successfully passaged hPSCs cultured under serum- and feeder-free conditions as large cell clumps that showed less damage than those passaged in divalent cation-free solution or with dispase or trypsin. The cells were also harvested as a cell sheet without the need for splitting off.
The cell clumps dissociated by PBSca/− (1 mM Ca2+ and 0 mM Mg2+) and represented in Fig 2d were smaller than those represented in Fig 1e. The decreased cell clump size might be caused by the DNase added in all enzyme-related experiments (Fig 2d–g, and Supplementary Fig 4d–g and 5) to reduce the abundance of free-floating DNA fragments derived from damaged cells. Such addition of DNase might reduce cell-cell attachments arising from the free DNA fragments, and thereby also reduce cell clump size. However, even in the presence of DNase, the cell clumps dissociated by PBSCa/− without enzyme were significantly larger than those dissociated by PBS−/− without enzyme (Supplementary Fig 5ae).
Addition of enzyme increased the sizes of cell clumps in three of the four conditions in the presence of calcium (Fig 2d, Supplementary Fig 4d and 5ae). A possible reason for this size increase tendency is enzymatic digestion of some cell-fibronectin attachment that enabled cell colonies to detach more easily from the dish. Consequently, large colonies may be harvested intact with less splitting of cell-cell binding by pipetting.
Commonly, hPSCs are passaged with enzyme and in medium containing physiological concentrations of Mg2+ and Ca2+ (Fig. 5 upper right)1,2. Single-cell culture methods such as clonal isolation are achieved by dissociating cells in solutions containing low Mg2+ and Ca2+ concentrations (Fig. 5 lower left)4,6,8. In the present study we showed that hPSC cell-cell binding can be disrupted with less cell detachment from the dish surface in a solution containing high Mg2+, but low Ca2+ concentrations (Fig. 5 upper left), and that large cell clumps and sheets can then be harvested by dissociating in low Mg2+ and high Ca2+ solution (Fig. 5 lower right).
Figure 5. Schematics of the effects of Mg2+ and Ca2+ on hPSCs culture.
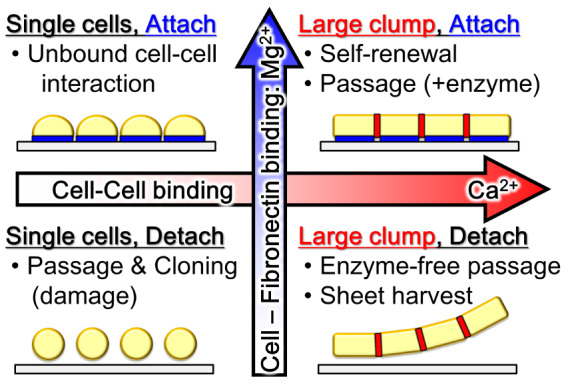
The serum-, feeder-, and enzyme-free composition described herein could provide practical culture methods for controlling hiPSCs physical interactions and thus enable further studies into the effects of such interactions and of endogenous and exogenous factors on cells, with the added benefit of eliminating instability caused by lot differences in enzyme. Moreover, such defined culture conditions could facilitate a stable and safe source of hiPSCs for potential clinical applications.
Methods
hPSCs culture
The hESC HUES819, H9 (WA09)1, KhES1, and KhES323 lines were obtained from Harvard University (Cambridge, MA, USA), from WiCell Research Institute (Madison, WI, USA), or from Kyoto University (Kyoto, Japan). The hiPSC 201B72 and 253G118 lines were obtained from RIKEN BRC Cell Bank (Tsukuba, Ibaraki, Japan) through the National Bio-Resource Project for MEXT, Japan and the hiPSCs Tic24 line (JCRB13331), which was derived from fetal lung fibroblasts (MRC-5), was obtained from JCRB Cell Bank (Osaka, Japan). hPSCs were maintained in a KSR-based medium on mouse embryonic fibroblast (MEF) feeder cells, and subcultured using CTK medium (KSR-MEF-CTK condition), as described in Supplementary Methods. In all experiments, hPSCs maintained in KSR-based medium on MEFs were transferred into serum-free medium, hESF9a on fibronectin-coated dishes, and passaged at least once before assaying (Supplementary Methods). The culture dishes were coated with 2 μg/cm2 fibronectin from human plasma (063-05591; Wako) or from bovine plasma (F-1141; Sigma, St. Louis, MO, USA) in PBS for at least 30 min at 37°C, and then excess solution was removed. For subculturing, the cells were detached from the culture dish using 0.2–0.5 U/ml dispase (17105-041; Life Technologies, Grand island, NY, USA) in hESF9a medium and replated in hESF9a medium with 5 μM ROCK inhibitor (RI, Y-27632; Wako). The hESF9a medium (ESF-Fb-Dsp condition) was changed daily.
For long-term culture under enzyme-, serum- and feeder-free condition (ESF-Fb-EzF condition), the cells were passaged with enzyme-free passage solution containing divalent cation-free DMEM-F12 medium (Supplementary table 1) supplemented with the same factors found in ESF9a medium. For subculturing, the cells were rinsed twice with PBS−/− and once with the enzyme-free passage solution, before being incubated in the same solution for more than 15 min at 37°C, and then triturated with a 1-ml micropipette tip. The cells were finally harvested by gentle centrifugation (1 min at 10 G) or stood for a few minutes before replating in hESF9a medium with 5 μM RI. Medium was changed daily.
Embryoid bodies formation
In vitro differentiation was induced by the formation of embryoid bodies as described previously14. Undifferentiated hiPSCs were cultured by floating in DMEM-F12 medium supplemented with 20% KSR, 0.1 mM 2-mercaptoethanol, and MEM non-essential amino acids (Life Technologies). The floating embryoid bodies were then replated onto 1 mg/ml gelatin-coated dishes in DMEM with 10% FBS. The medium was changed every other day with the same floating culture solution.
Karyotype analysis
Metaphase spreads were prepared from cells treated with colcemid (KaryoMAX Colcemid, Gibco 15212-012, final concentration of 40 ng/ml, overnight treatment) or metaphase arresting solution (Genial Genetic Solutions Ltd., Cheshire, UK). We performed a standard G-banding or multicolour fluorescence in situ hybridization (FISH) karyotypic analysis on at least 20 metaphase spreads for each population. For FISH karyotype analysis, 24XCyte Human Multicolor FISH Probe Kit (MetaSystems GmbH Altlussheim, Germany) were used.
Cell detachment and dissociation assay
The dose response size and removal ratio of hPSCs cultured on divalent cations using 24-well plates were measured as follows. The cells cultured in ESF9a on fibronectin-coated dishes were detached and dissociated into cells clump using 0.2–0.5 U/ml dispase, and then were plated in ESF9a medium on 24-well plates coated with 2 μg/cm2 fibronectin (Wako) at 37°C for more than 1 hour. At 4 or 5 days after plating, the attached cells were stained with 1 μM calcein-AM (Dojindo, Kamimashiki, Kumamoto, Japan), a fluorescent living cell dye, for 20 min at 37°C, and imaged as the control state. Then the cells were rinsed once with PBS−/−, rinsed again with PBS containing various concentration of Ca2+ and Mg2+, incubated in the same PBS for a further 15 min at 37°C, and then triturated 5 times with a 1-ml micropipette tip. For enzymatic digestion experiments, dispase or trypsin was added after 12 min incubation in PBS and left for 3 min. The cells were then triturated 5 times with a 1-ml micropipette tip in the presence of 1 mg/ml DNase I (Roche, Basel, Switzerland), 250 μg/ml trypsin inhibitor (Life Technologies), and 1 mg/ml BSA (sigma), and then 10X volumes of PBSca/− were added before spinning down the cells. The detached cells were then transferred to another plate, and the remaining cells were imaged for green fluorescence to estimate detachment efficiency. The detached cell clumps were placed between cover slips and cell clump size was estimated based on fluorescent signal using Image J software (NIH, Bethesda, MD, USA). To estimate the cell clump sizes, randomly picked cell clumps for each condition in a test were analyzed with Excel software (Microsoft, Redmond, WA, USA). The cell clump size was converted from area (μm2) into the number of cells by using the area of single cells, which was estimated to be 240 ± 86 μm2 (mean ± SD, n = 107) in a separate experiment.
Teratoma formation
Teratomas were generated in severe combined immunodeficient (SCID) mice from 201B7 hiPSCs grown under ESF-Fb-EzF conditions for more than 10 passages. The cells harvested by dispase were resuspended in DMEM supplemented with RI (10 μM). The cells from a confluent single well in a 6-well plate were injected into the thigh muscle of a SCID (C.B-17/lcr-scid/scidJcl) mouse (CLEA Japan, Tokyo, Japan). Nine weeks after injection, tumors were dissected, weighed, and then fixed with 10% formaldehyde Neutral Buffer Solution (Nacalai Tesque, Kyoto, Japan). Paraffin-embedded tissue was sectioned and stained with hematoxylin and eosin (HE). All animal experiments were conducted in accordance with the guidelines for animal experiments of the National Institute of Biomedical Innovation, Osaka, Japan.
Alkaline phosphatase (ALP) staining, immunocytochemistry
The hPSCs were stained with an Alkaline Phosphatase Staining Kit II according to the manual (StemGen, Cambridge, MA, USA). Briefly, the cells were rinsed twice with PBS+/+ and fixed with Fix Solution from the kit at room temperature for 4 minutes. The fixed cells were rinsed with PBS containing with 0.05% (v/v) Tween20 and incubated in AP Substrate Solution at room temperature for 20 to 30 minutes. Then the cells were rinsed with PBS+/+ and photographed.
Immunocytochemistry was performed as described previously14,17. Briefly, hiPSCs were fixed in 4% formaldehyde with 0.5 mM MgCl2 and 0.5 mM CaCl2. Then the cells were permeabilized and blocked with PBS containing 0.1–0.2% Triton X-100, 10 mg/ml BSA, 0.5 mM MgCl2, and 0.5 mM CaCl2, and then reacted with primary antibodies in the solution. The primary antibody binding was visualized using secondary antibodies. Antibody information is listed in Supplementary Table 2. Nuclei were stained with 1 μM DAPI (Wako). Micrographs were taken using a BZ-8100 fluorescence microscope (Keyence, Osaka, Japan).
Flow cytometry (FCM)
FCM analysis was performed as described previously14,17. All cells were removed from culture dishes using 0.02% (w/v) EDTA-4Na in PBS−/− and then fixed in 4% formaldehyde. The fixed cells were permeabilized and blocked with PBS−/− containing 0.1–0.2% Triton X-100 and 10 mg/ml BSA, and then reacted with primary antibodies in the solution. The primary antibody binding was visualized with secondary antibodies. Antibody information is listed in Supplementary Table 2. A cell sorter (JSAN, Bay Bioscience Co., Ltd., Hogo, Japan) was used for data acquisition.
Apoptosis
An annexin V-FITC apoptosis detection Kit was used to detect cell surface phosphatidylserine (BioVision, Milpitas, CA, USA). Cells were floating cultured for four hours in ESF9a solution following detachment and dissociation. For FCM analysis, the cells were re-dissociated by incubating in 0.02% EDTA solution followed by trituration using a 1-mL pipette tip. The living cells were then stained with FITC conjugate annexin V (1:100) and 50 μg/ml propidium iodide in binding buffer. The living cells nuclear were stained with 1 μg/ml Hoechst 33342 (Dojindo, Osaka, Japan).
Spot sheet formation
Silicone rubber masks made of polydimethylsiloxane (PDMS, Sylgard 184, 10:1 mix; Dow Corning) were perforated with 2-mm-diameter holes using a hole-punch. The bacterial culture dish (non-cell-attachment-treated dishes, Iwaki) with PDMS masking were treated for 60 seconds by air plasma to make hydrophilic spots (YHS-R, SAKIGAKE-Semiconductor Co., Ltd), then coated with 2 μm/cm2 fibronectin for more than 1 hour at 37°C to make fibronectin spots. After rinsing twice with PBS−/−, the PDMS mask was removed and the dish was sterilized under a UV lamp. hPSCs (253G1) cultured under the ESF-Fb-Dsp condition or hiPSC Tic-derived hepatic progenitors were dissociated in calcium- and magnesium-free solution, and then plated in ESF9a solution with 5 μg/ml RI or in CDM medium with 50 ng/ml FGF10 with RI.
Early differentiation was induced by 2 days cultivation in the ESF6 medium with 2 ng/ml recombinant human BMP4 (314-BP, R&D Systems, Inc, Minneapolis, MN, USA) as described previously17. Hepatic progenitors were differentiated based on the previously reported protocol25. Briefly, the hiPSC Tic line was passaged and grown for 2 days in CDM medium26. hiPSCs were then grown for 3 days in CDM/PVA medium26 with 100 ng/ml activin, 20 ng/ml basic FGF, 10 ng/ml BMP4, and 10 μM LY294003 (9901, Cell Signaling Technology, Beverly, MA, USA), followed by 3 days differentiation in CDM/PVA medium with 50 ng/ml recombinant human FGF10 (345-FG-025/CF, R&D Systems).
Data analysis
Image analyses were performed with Image J software (NIH). Statistical analyses were performed with R software (http://www.r-project.org).
Author Contributions
K.O. prepared all figures and supplementary figures. S.T. prepared figure 1, 2 and 4 and supplementary figure 1, 3, 4, 5 and 6. A.F., K.Y. and M.K.F. prepared Figure 3, 4 and Supplementary Figure 7. Y.I., Y.O. and M.A. prepared supplementary figure 6. Y.H., T.C., T.M. and M.A. prepared Supplementary Figure 2. K.O., Y.H. and M.K.F. wrote the manuscript text. All authors reviewed the manuscript.
Supplementary Material
Supplementary Infomation
Acknowledgments
The authors would like to thank Reiko Terada at The University of Tokyo for the culturing of hPSCs, and Kumiko Higuchi at AIST for karyotype analysis. This work was supported in part by Grants-in-Aid for Scientific Research and the Project for Realization of Regenerative Medicine from the Ministry of Education, Science, Sports, Culture and Technology (MEXT) of Japan to KO and TM, by Promotion of Independent Research Environment for Young Researchers funding to KO, by an Adaptable & Seamless Technology Transfer Program through Target-driven R&D (A-Step) of the Japan Science and Technology agency (JST) to KO, and by grants from the Ministry of Health, Labour, and Welfare of Japan to KO and MKF. The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Thomson J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). [DOI] [PubMed] [Google Scholar]
- Amit M. et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 227, 271–278 (2000). [DOI] [PubMed] [Google Scholar]
- Mitalipova M. M. et al. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol 23, 19–20 (2005). [DOI] [PubMed] [Google Scholar]
- Watanabe K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25, 681–686 (2007). [DOI] [PubMed] [Google Scholar]
- Ohgushi M. et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 7, 225–239 (2010). [DOI] [PubMed] [Google Scholar]
- Beers J. et al. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protocols 7, 2029–2040 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Okada T. S. & Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell 35, 631 (1983). [DOI] [PubMed] [Google Scholar]
- Pertz O. et al. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J 18, 1738–1747 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A. P., Akiyama S. K. & Humphries M. J. Regulation of integrin α5β1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+. J Biol Chem 270, 26270–26277 (1995). [DOI] [PubMed] [Google Scholar]
- Braam S. R. et al. Recombinant Vitronectin Is a Functionally Defined Substrate That Supports Human Embryonic Stem Cell Self-Renewal via αVβ5 Integrin. Stem Cells 26, 2257–2265 (2008). [DOI] [PubMed] [Google Scholar]
- Rowland T. J. et al. Roles of Integrins in Human Induced Pluripotent Stem Cell Growth on Matrigel and Vitronectin. Stem Cells Dev 19, 1231–1240 (2009). [DOI] [PubMed] [Google Scholar]
- Hayashi Y. et al. Reduction of N-Glycolylneuraminic Acid in Human Induced Pluripotent Stem Cells Generated or Cultured under Feeder- and Serum-Free Defined Conditions. PLoS ONE 5, e14099 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeev A. G. & Melkoumian Z. Synthetic Surfaces for Human Embryonic Stem Cell Culture. Embryonic Stem Cells - Basic Biology to Bioengineering. Kallos, M. S.(ed.) 89–104 (Intech, rijeka, 2011). [Google Scholar]
- Furue M. K. et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci U S A 105, 13409–13414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimitsu R. Microfluidic Perfusion Culture of Human Induced Pluripotent Stem Cells under Fully Defined Culture Conditions. Biotechnol Bioeng (In press). [DOI] [PubMed] [Google Scholar]
- Nakagawa M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnol 26, 101–106 (2007). [DOI] [PubMed] [Google Scholar]
- Cowan C. A. et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med 350, 1353–1356 (2004). [DOI] [PubMed] [Google Scholar]
- International Stem Cell Initiative Consortium. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim 46, 247–258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K. et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. New Eng J Med 351, 1187–1196 (2004). [DOI] [PubMed] [Google Scholar]
- Kito T. et al. iPS cell sheets created by a novel magnetite tissue engineering method for reparative angiogenesis. Sci Rep 3, 1418 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemori H. et al. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun 345, 926–932 (2006). [DOI] [PubMed] [Google Scholar]
- Nishino K. et al. DNA Methylation Dynamics in Human Induced Pluripotent Stem Cells over Time. PLoS Genetics 7, e1002085 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touboul T. et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754–1765 (2010). [DOI] [PubMed] [Google Scholar]
- Vallier L. et al. Signaling Pathways Controlling Pluripotency and Early Cell Fate Decisions of Human Induced Pluripotent Stem Cells. Stem Cells 27, 2655–2666 (2009). [DOI] [PubMed] [Google Scholar]
- Draper J. S. et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 22, 53–54 (2004). [DOI] [PubMed] [Google Scholar]
- Ellerström C., Strehl R., Noaksson K., Hyllner J. & Semb H. Facilitated Expansion of Human Embryonic Stem Cells by Single-Cell Enzymatic Dissociation. Stem Cells 25, 1690–1696 (2007). [DOI] [PubMed] [Google Scholar]
- Ludwig T. E. et al. Feeder-independent culture of human embryonic stem cells. Nat Methods 3, 637–646 (2006). [DOI] [PubMed] [Google Scholar]
- Klim J. R., Li L., Wrighton P. J., Piekarczyk M. S. & Kiessling L. L. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods 7, 989–994 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Infomation