Figure 6.
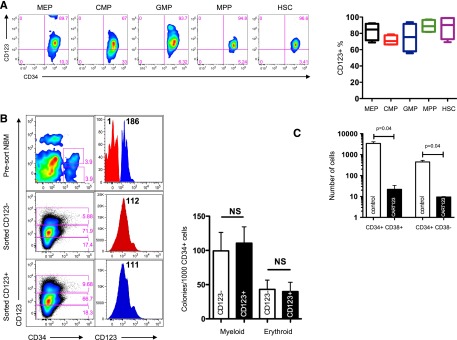
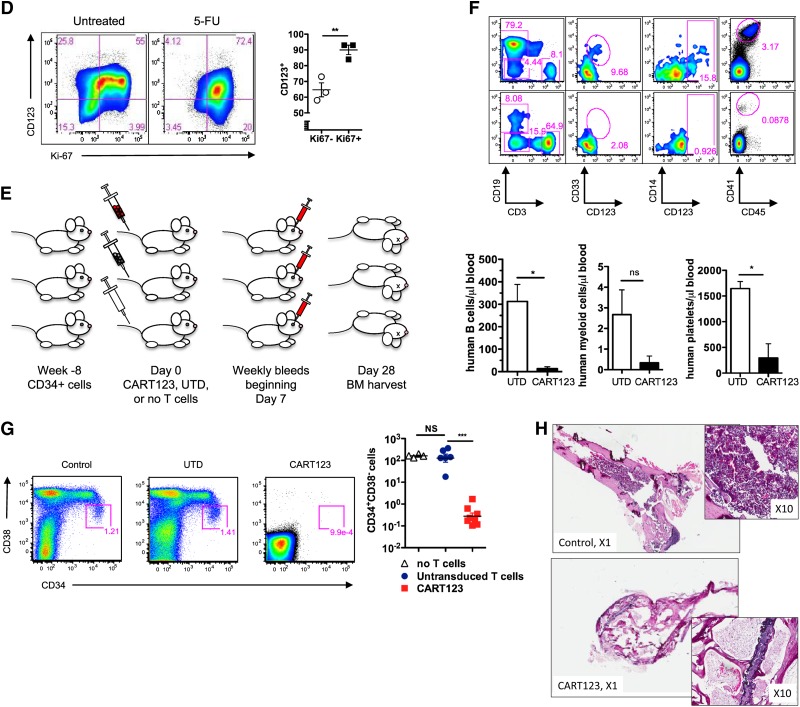
Eradication of normal hematopoiesis by CART123 in a xenograft model. (A) Healthy bone marrow progenitor populations exhibit moderate to bright expression of CD123. Bone marrow from 4 normal donors was stained for CD123 after gating on live singlet lineage-negative CD45dim cells, and the indicated progenitor subpopulations were identified using CD34, CD38, CD45RA, and CD90 (gating strategy is shown in supplemental Figure 8). CD123 gating was based on normal lymphocytes and confirmed with fluorescence-minus-one controls. MEP, megakaryocyte-erythroid progenitors; CMP, common myeloid progenitors; GMP, granulocyte-monocyte progenitors; MPP, multipotent progenitors; HSC, hematopoietic stem cells. (B) CD123dim/− bone marrow progenitors differentiate to CD123+ in semisolid culture. CD123dim (top panel, red histogram) or CD123intermediate/+ (top panel, blue histogram) CD34+ cells were sorted from normal bone marrow (NBM) and cultured in MethoCult Optimum medium for 14 days. The middle and lower panels show the phenotype of colonies that developed from sorted CD123dim/− and CD123intermediate/+ populations, respectively. The sorted cultured populations exhibited similar CD123 expression and an indistinguishable ability to form myeloid or erythroid colonies. MFI of CD123 is shown at top right. (C) CART123 cells markedly impair hematopoietic function. CD34+ cells selected from normal human cord blood were incubated at a 1:10 target-to-effector ratio with CART123 or control UTD T cells for 4 hours, followed by a 14-day culture in Methocult Optimum. Coculture with UTD T cells was used to control for the allogeneic effect. Hematopoietic function was assessed by manual colony counts (not shown) or quantified by flow cytometry for the indicated cell populations using Countbright beads. (D) Cycling bone marrow cells upregulate CD123. Mice previously engrafted with human CD34+ cells were treated with 5-fluorouracil (5-FU) or vehicle. Fourteen days later, bone marrow was harvested from these mice and analyzed for the intracellular proliferation marker Ki67 and for CD123 after gating on live lineage-negative human cells; P < .01 (Student t test). (E) Schematic of xenograft model to evaluate potential CART123-mediated myeloablation. NSG mice were engrafted with human fetal liver CD34+ cells (HIS mice) and bled for confirmation of engraftment after 6 to 8 weeks. On day 0, mice received CART123, control UTD T cells, or saline vehicle. Flow cytometric quantification of human hematopoietic cells in peripheral blood (days 7, 14, 21) and in bone marrow (day 28) was performed. (F) Specific decline in circulating human B cells, myeloid cells, monocytes, and platelets is seen after treatment with CART123. Representative plots are shown after control (top) or CART123 (bottom) infusion and quantified in the lower panel (control T cells, open column; CART123, solid column). (G) Specific myeloablation of human bone marrow in CART123 mice. On day 28 after T-cell injection, bone marrow was harvested and analyzed for human progenitor cell populations after gating on live singlet human lineage-negative cells. (H) Sections of femur taken from HIS mice 1 month after treatment with control (top) or CART123 (bottom). Hematoxylin and eosin staining; Zeiss microscope original magnification ×1 and ×10 shown. Results are representative of at least 2 independent experiments. NS, nonsignificant.