Abstract
Millions of people are affected by visual impairment and blindness globally, and the prevalence of vision loss is likely to increase as we are living longer. However, many ocular diseases remain poorly controlled due to lack of proper understanding of the pathogenesis and the corresponding lack of effective therapies. Consequently, there is a major need for animal models that closely mirror the human eye pathology and at the same time allow higher-throughput drug screening approaches. In this context, zebrafish as an animal model organism not only address these needs but can in many respects reflect the human situation better than the current rodent models. Over the past decade, zebrafish have become an established model to study a variety of human diseases and are more recently becoming a valuable tool for the study of human ophthalmological disorders. Many human ocular diseases such as cataract, glaucoma, diabetic retinopathy, and age-related macular degeneration have already been modelled in zebrafish. In addition, zebrafish have become an attractive model for pre-clinical drug toxicity testing and are now increasingly used by scientists worldwide for the discovery of novel treatment approaches. This review presents the advantages and uses of zebrafish for ophthalmological research.
Keywords: zebrafish, vision, visual behaviour, ocular diseases, retina, drug development
Overview
Visual impairment is a major health problem affecting millions of people globally.1 Major causes of visual impairment and blindness include uncorrected refractive index, cataract, glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy (DR), with cataracts being the leading cause of blindness globally.1 The worldwide economic burden of vision loss is estimated to be about US $3 trillion in 2010 and is anticipated to increase significantly by 2020.2 Therefore, strategies to develop animal models that closely mirror human ocular diseases are necessary for a better understanding of the underlying causes and in parallel for the development of novel therapeutic approaches. Over the past decade, zebrafish have become an established model to study a variety of human diseases and are more recently becoming a valuable tool for the study of human ophthalmological disorders.3, 4, 5, 6 Due to many advantages inherent to this model organism, zebrafish are also highly suitable for screening approaches to identify novel therapeutics in the field of ophthalmological medicine.
Zebrafish as an animal model
Over the past 20 years zebrafish (Danio rerio) have been increasingly used as a vertebrate model for developmental and genetic studies.7 There are many reasons for their popularity. Zebrafish are easy to maintain and breed in large numbers at relatively low cost. They become sexually mature after 3–4 months and each pair can generate 200–300 offspring on a weekly basis. The eggs fertilize and develop into transparent embryos outside the mother, which allows real-time observation of organogenesis occurring inside the embryo. The embryos are small enough (<1 mm in diameter) to be easily distributed and maintained in 96-well plates, which facilitates drug-screening approaches using only small quantities of drug solution. In addition, embryos are permeable to many small molecules allowing easy administration of drugs.8 Embryonic development in zebrafish is rapid and takes only few days with the majority of organs such as brain, heart, liver, intestine, and eye developing within 24 h and becoming functional within a week. The free-swimming larvae hatch from around 72 h post fertilization (hpf) and soon start foraging for food. All of these attributes explain the steady increase in the use of zebrafish in pharmaceutical research. The large number of embryos produced per clutch makes zebrafish a prominent model for higher-throughput screening of small molecules,9 which is mandatory for preclinical drug development and toxicity assessment.10 So far, zebrafish have been used to model a wide variety of human diseases, including hereditary muscle diseases,11 neurological disorders,12 tuberculosis,13 cancer,14 cardiovascular diseases,15 and haematopoietic and infectious diseases.16 In addition, zebrafish are a valuable vertebrate model for studying vision-related disorders.17 However, there are few differences between the visual system of zebrafish and humans, which need to be taken into account.
The visual system of zebrafish
Eye structure
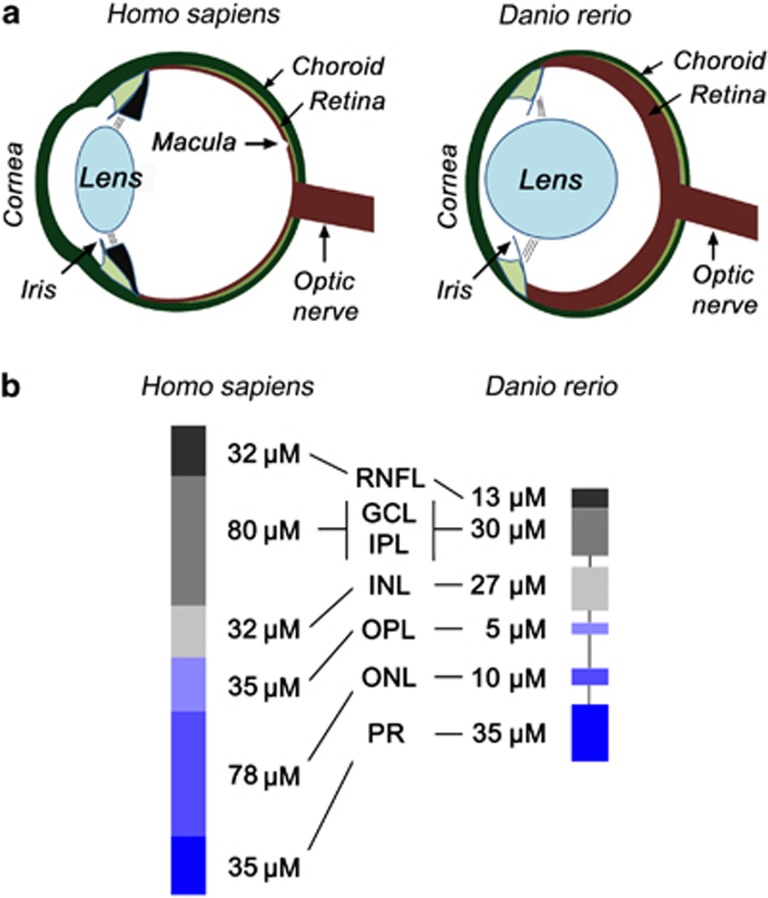
The visual system of zebrafish is fundamentally similar to human subjects but exhibits some notable structural differences (Figure 1, Table 1).17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The lens in zebrafish eyes is spheroid and not ellipsoid as compared with the human eye, and as a consequence, the zebrafish eye has a much lower volume of vitreous compared with the human eye (Figure 1a).
Figure 1.
Comparison of the human and zebrafish eye. (a) Comparison of the human and zebrafish eye: Human and zebrafish eyes mainly differ in lens shape and space between the lens and retina. (b) Comparison of human and zebrafish retinal structure: Schematic representation of the differences of the retinal layers between human and zebrafish retina. Information on human retinal thickness taken from Yousef and Finger, 25 and for zebrafish derived from http://zfatlas.psu.edu/view.php?atlas=18&s=207; zebrafish atlas, 12 months post-fertilized male transverse section. All quantifications are approximates only and may differ between individuals and areas within the retina. RNFL, retinal nerve fibre layer.
Table 1. Comparison of the human and zebrafish eye.
| Structure | Human | Zebrafish |
|---|---|---|
| Eye position24 | Frontal eyes with highly overlapped binocular vision | Lateral eyes with less overlapped binocular vision |
| Lens | Ellipsoidal | Completely spherical extending partially through iris providing wide-angle view |
| Retina (thickness)25 | ≍289 μm | ≍180 μm |
| Ganglion cells20 | Highly populated | Less densely populated |
| Presence of fovea24 | Yes | No |
| Vision23 | Cone dominant and trichromatic vision (lacks UV-sensitive colour vision | Cone dominant and tetrachromatic vision (contains UV-sensitive short single cones) |
| Retinotectal projection24 | Half of the optic fibres from each eye project on to the same side of the brain and other half crosses over at the optic chiasm and projects to the other side of the brain | All of the optic fibres coming from each eye crosses over at the optic chiasm and extends on to the opposite side of the brain |
| Myelination18, 26, 27 | The part of optic fibres before the lamina cribrosa are not myelinated, whereas fibres after protruding from lamina cribrosa are all myelinated | Whole optic fibres are myelinated. Loose, single-layer myelin sheath is present around the intra-retinal axon and optic nerve consists of compact myelin |
The zebrafish retina.
As zebrafish use vision to protect themselves against predators and depend on light to search their food, their visual system develops rapidly.23 The retinal structure starts to develop from 32 hpf and continues to develop extraordinarily fast within 5 days post fertilization (dpf).17 By then, the zebrafish retina becomes functional. Unlike the mammalian eye, that contains a specialized area within the retina that is responsible for high acuity vision,19 this so called macula is notably absent in zebrafish (Figure 1a). Comparable to the human eye, the retinal structure of zebrafish is comprised of five distinct layers (Figure 1b); three nuclear layers—the outer nuclear layer (ONL), the inner nuclear layer (INL), and the ganglion cell layer (GCL); and two plexiform layers (PL)—the inner (IPL) and the outer plexiform layer (OPL).19 The ONL consists of the cell bodies of photoreceptors (rods and cones), whereas the cell bodies of horizontal cells, bipolar cells and amacrine cells reside within the INL.19 The GCL contains the cell bodies of ganglion cells while their axons are located in the retinal nerve fibre layer before becoming bundled into the optic nerve to carry the visual information from the eye to the brain. Synapsis between different neurons takes place at PLs.19 The synapsis between photoreceptors and bipolar cells (and horizontal cells) occurs in the OPL, and the synapsis between bipolar and ganglion cells (and amacrine cells) occurs in the IPL.19 Horizontal and amacrine cells initiate sidewise interaction in the OPL and IPL, respectively.28 Horizontal cells are responsible for enhancing contrast while amacrine cells, in addition to detecting change in illumination, also process movement and direction of light on the retina.28 In contrast to human ganglion cells where the retinal part of the axon is unmyelinated and the axon in the optic nerve is myelinated, in zebrafish the whole axon is myelinated to different extents.26, 27
The photoreceptor outer segment (POS) of zebrafish consists of rods and cones, comparable to the human retina. Anatomically cones are arranged in a mosaic pattern that can be categorized into four types: short single cones (SSCs)—ultraviolet (UV)-sensitive cones, long single cones (LSCs)—blue-sensitive cones, and double cones (DCs) consisting of short (green sensitive) and long (red sensitive) cones.21, 29 They are also classified based on the peak sensitivities of the photopigment present in each cone as follows: UV (360–361 nm), short (S, 407–417 nm), medium (M, 473–480 nm), and long (L, 556–564 nm) cones.21 Based on the four cone types in zebrafish, their vision is tetrachromatic. In contrast, the human eye lacks the UV-sensitive cones and therefore has only trichromatic vision.23 Unlike cones that have four types of opsins as their photopigment, the rods consist of only a single type and contain rhodopsin as their photopigment.23 During the development of the zebrafish retina, the SSCs become distinct at 5–6 dpf while LSCs appear at 7–8 dpf and the DCs at 10–12 dpf. However, all of the visual pigments are expressed between 50 and 55 hpf, so the various cone types are presumably there at 50–55 hpf but not recognizable. The first rod cells appear at 5–6 dpf.17, 30 Although small rod responses can be detected at this stage, full rod responses are not evident until 15–21 dpf.17, 31, 32 Therefore, until 15 dpf zebrafish only have functional cones that are responsible for colour vision.
Visual processing
Light signals entering the eye are first refracted from the cornea to the lens and then projected onto the retina. The signal is then collected by the photopigment of the photoreceptor layer where the rod and cone cells convert the light signals into electrical signals33 that are transmitted via the horizontal, amacrine, and bipolar cells to the RGCs.28 RGCs give rise to the nerve fiber layer, which is responsible for carrying the signals from the eye, via the optic nerve to the optical centres in the brain.
Eye development
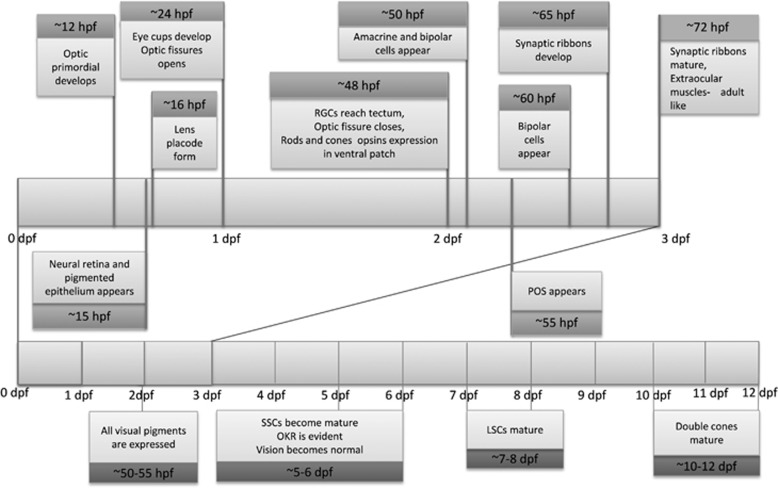
The initial development of the eye structures in zebrafish generally resembles those of other vertebrates34, 35 (Figure 2). Eye morphogenesis begins with the evagination of the optic vesicle from the forebrain at 11 hpf as a flat structure, which then extends laterally, forming an optic lobe.34, 36 The anterior portion of the optic lobe remains attached to the forebrain through the optic stalk.36 The optic vesicle finally gives rise to the neural retina and pigmented epithelium at 15 hpf. By 16–20 hpf, the eyecups are developed through a series of different stages from the optic lobe. Surface ectoderm cells underlying the eyecups thicken and form a lens placode at around 16 hpf. Delamination of the lens placode from the surface ectoderm occurs at approximately 24 hpf and then detaches fully by 26 hpf. By 30 hpf, the corneal epithelium is formed from the surface ectoderm, which overlies the lens. Further morphogenesis of the eyecups forms an optic fissure by 24 hpf and closes thereafter at 48 hpf, which signals that morphogenesis of the embryonic eye is largely terminated and that only retinal neurogenesis is in progress at that time.7, 34, 35 At around 32 hpf, the first ganglionic cells differentiate37 and soon after that their axons reach the optic tectum.19 The amacrine and horizontal cells within the INL first appear at 50 hpf, and by 55 hpf rod and cone outer segment in the ONL are developed.35 Rods cells and Muller glial cells are the last to differentiate.38 The presynaptic photoreceptor ribbon (Figure 3) develops at about 65 hpf.35 Visual information is processed once the ribbon synapses of the bipolar cells reach maturity at 74 hpf,35 which are responsible for passing the light-induced changes in photoreceptor cell membrane potential down the retinal circuit.39 This means that the retinal structure becomes functional at around 74 hpf.40 At the same time, the extra-ocular muscles become fully functional, which is a prerequisite for tracking of moving objects by eye movement.41 Consequently, visually mediated response improves thereafter with adult-like performance after 96 hpf, where full tracking of eye movement (optokinetic response (OKR)) is evident.
Figure 2.
Sequences of the eye development in zebrafish: dpf, days post fertilization; hpf, hours post fertilization; LSC, long single cone; OKR, optokinetic response; POS, photoreceptor outer segment; RGC, retinal ganglion cell; SSC, short single cone.
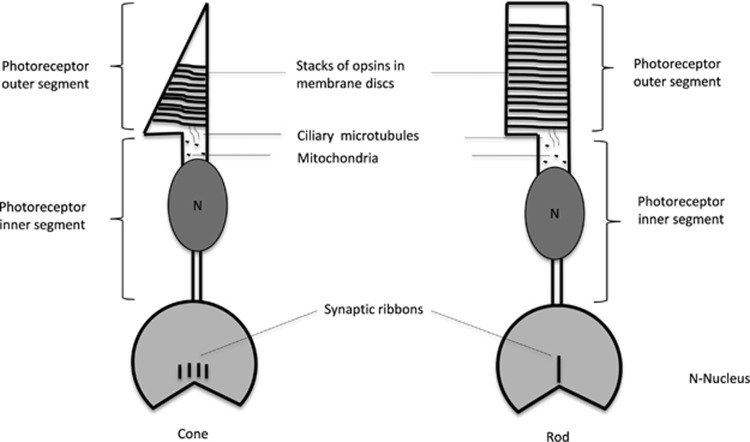
Figure 3.
Synaptic ribbons: Photoreceptors showing presynaptic ribbons, which are responsible for signal transmission from photoreceptor layer to bipolar and horizontal cells.
Why zebrafish are a good animal model for ophthalmological studies
Unlike mouse models, zebrafish are relatively easy to maintain at a fraction of the cost. Zebrafish are prolific breeders and produce as many as 100–200 eggs on every mating, while a pair of mice reliably produces a maximum 6–8 offspring. This large number of eggs per clutch in zebrafish allows higher-throughput screening for the identification of mutants and potential drug candidates.17 In many respects, the zebrafish's visual system mirrors the human situation better compared with other animal models. Contrary to mice that have rod-dominated vision, zebrafish have cone-dominant vision like humans,7 which is a prerequisite to study human disorders associated with cone degeneration, such as AMD.22 In addition, the eyes of zebrafish develop fast from only 12 hpf and display a functional visual system by 5 dpf. This is significantly faster compared with mice (around 15–20 days)42, 43 and allows to study visual function already in 5-day-old larvae. Furthermore, the significant amount of genetic information available from zebrafish mutants associated with defective visual development and function illustrates the power of this model for understanding human ophthalmological disorders.
Measurement of visual behaviour
Behavioural responses have been utilized as an important tool for screening genetic defects of the visual system as well as to study the development of the visual system in zebrafish. Several well-described behavioural responses with unique advantages and limitations have been used so far (Table 2).
Table 2. Summary of behavioural assays used in zebrafish.
| Assays | Suitability | Validity | Advantages | Disadvantages |
|---|---|---|---|---|
| SR40, 53 VMR55, 56 | ≥4dpf (embryo) | Yes | Suitable for analysing simple visual function, such as differentiating light from darkness Useful to study visual function development VMR complements OKR and is useful for visual mutants screening | Not suitable for larvae≥5 dpf as spontaneous movement is often difficult to distinguish from startle response Not suitable for screening visual defect mutants |
| OKR4, 6, 41, 46 | 5–7 dpf (embryo and adult) | Yes | Suitable for complex visual function test (visual acuity, contrast sensitivity, colour blindness, etc) Useful for mutant screens. Provides specificity in screening mutants | Time consuming assay (test one fish at a time) Possibility of missing the strong mutants.-randomly selected |
| OMR4, 6, 50 | >6 dpf (embryo and adult) | Yes | Fast assay for high-throughput analysis of visual mutants (large number of fish can be analyzed at once) Lower risk of missing mutants while screening | Lack of specificity in result (possibility of false result sometimes)-as it does not test each and every individual fish |
| PTB3 | >6 dpf (embryo and adult) | No | Suitable for simple light responses | Not suitable for mutants screening-due to unreliable result |
| ER57, 59 | >2 months (adult) | Yes | Simple, robust method Provides high-throughput analysis Useful in screening visual mutants, visual sensitivities as well as for drug identification and toxicity studies |
Abbreviations: dpf, days post fertilization; ER, escape response; OKR, optokinetic response; OMR, optomotor response; PTB, phototactic behaviour; SR, startle response; VMR, visual motor response.
OKR
The OKR is based on the eye movement reflex in response to a moving stimulus to help stabilize the image on the retina to maintain visual acuity and is evidence of a fully functional visual system in zebrafish.44, 45 The OKR is tracking eye movements, which consist of smooth pursuits (slow phase) and fast resetting saccades in the opposite direction.24 OKR begins at around 74 hpf when only 5% of the zebrafish larvae respond to the visual stimulus, which then increases steadily to 100% by 80 hpf.34, 40 The OKR improves in terms of time spent in tracking the stimulus and velocity of tracking eye movement by 96 hpf.3, 40, 41 For this method, zebrafish larvae are immobilized to restrain the body while maintaining the ability for eye movement and are then exposed to a visual stimulus that usually consists of alternate black and white stripes that move around the larvae.4, 23, 24, 46 The larvae respond to this stimulus by moving their eyes in the direction of the moving stripes.23 Experimental visual stimuli, such as spatial frequency, contrast, and angular velocity can be altered in this setting to gain a deeper understanding of a potential defect.46 With some experimental adaptations, such as constant supply of oxygen-rich water while body movement is restrained, this method can be applied to adult fish as well.47 This assay is rapid and responses of larvae or adult fish can be processed within only few minutes.44 OKR was used to identify and characterize many zebrafish mutants that are associated with vision system defects (Table 3), such as bumper mutant (defect in lens development), noir, dropje, lakritz mutants (defect in retinal structure), belladonna mutant (RGCs axon misrouting), and grumpy and sleepy mutants (optic nerve disorder).4 Other examples with visual function defects are the brass mutant with a phenotype similar to glaucoma.48 In addition, OKR was also useful in isolating a colour-blind mutant by changing the colour of visual stimulus.49 In summary, the OKR appears to result in more a robust, reliable, and quantifiable behavioural data compared with other methods described below, especially after automated commercial systems have become available.3, 49
Table 3. Summary of existing zebrafish model of some human ocular diseases.
| Affected eye structure | Zebrafish mutant/morpholino | Human ocular disease | Assays used |
|---|---|---|---|
| Lens65, 71, 75 | Mutation of CRYGC gene Cloche Mutation of PITX3 and FOXE3 | Congenital cataract | Histology |
| Anterior segment80, 83 | Mutation of FOXC1 Bugeye mutant | Glaucoma | Immunohistological OMR, electroretinograms, histology |
| Retina | Oval (Ovl), elipsa (elip) mutants | Human ciliopathies (oculo-renal diseases)—Bardet–Biedl syndrome, Senior–Loken syndrome | OKR, electroretinograms, histology |
| Photoreceptors105 | REP 1 gene mutation | Oculo-cutaneous albinism—human choroideremia | SR, transmission electron microscopy |
| RPE108, 111 | Fade out (fad) mutant | Hermansky Pudlak syndrome | Histology, transmission electron microscopy, immunohistochemistry |
| Retinal Vasculature88, 89, 90 | Fli-EGFP-Tg Transgenic gnn mutant | Diabetes retinopathy, AMD AMD | Confocal microscopy OKR, histology, immunocytochemistry, electron microscopy |
| Retinotectal projection4 | Belladonna (bel) mutant | Human congenital nystagmus | OKR, histology, electroretinograms |
Abbreviations: AMD, age-related macular degeneration; OKR, optokinetic response; OMR, optomotor response; RPE, retinal pigmented epithelium; SR, startle response.
Optomotor response (OMR)
The OMR tests the visual behaviour of zebrafish by their tendency to swim towards moving black and white stripes.6 As numerous fish with possible visual function defects can be tested at once, this OMR assay enables higher-throughput analysis compared with the OKR. However, it has to be noted that compared with the OKR there is a significantly higher risk to miss mutants when using OMR.6 The experimental setup to measure the OMR is similar to that of the OKR except that the embryos or adult fish are not constrained and are able to swim towards the perceived black–white stripes.50 This type of behavioural response shown by zebrafish is one of the innate behaviours known as taxis where fish orient themselves in the direction of the stimulus.51 This behaviour is important to maintain their orientation in water.51 In the assay, the wild-type fish swim towards the perceived motion and gather at one end of a chamber while zebrafish with visual defects swim in random patterns.50 OMR is suitable to test the visual acuity of both larvae and adult zebrafish and has been used to isolate at least 17 mutants from 411 previously identified loci in zebrafish that affect visual function so far.4, 17, 40, 50 Furthermore, the development of visual behaviour in zebrafish under different light conditions (ON and OFF)50 and the effects of ethanol exposure on embryonic eye development50, 52 have also been studied using OMR.
The startle response (SR)
The SR in zebrafish is based on a form of body movement within 2 s in response to a sudden exposure to a low intensity light (∼60 μW/m2) or an acoustic signal and is also useful to determine the development and maturation of the visual system in zebrafish.40, 53 The SR is typically first seen at 68 hpf where 17% of the fish show SRs, which then steadily increase to 100% at 79 hpf at a time when the POS appear abundantly together with a functional retina and fully developed synaptic ribbon.40 This assay has been successfully used to assess the visual development in zebrafish embryos as well as used to detect defects of visual development in response to exposure to toxins, such as methylmercury.54 Recently, a modification of the SR, called visual motor response (VMR), was described, which can be performed on 4 dpf zebrafish larvae to screen for visual mutants. VMR was developed to confirm the result of OKR and examines whether visual mutants that fail to detect motion under OKR can still detect changes in light intensities.55, 56
Phototactic behaviour (PTB)
The PTB is based on the tendency of zebrafish with normal vision to move towards an illuminated chamber.3 The experimental setup for this assay consists of a rectangular acrylic box with two chambers inside separated by a sliding bar. There are two methods in which PTB can be assessed.3 The first approach is based on two chambers, which are equally illuminated. The fish are allowed to distribute between both chambers, and the number of larvae in each chamber are counted after a set of time interval. Then the same procedure is repeated with only one chamber illuminated, while the other chamber is covered. In general, larvae between 7–14 dpf are suitable for the first method. For the second method, larvae aged between 7 and 19 days are kept in a darkened chamber for up to 2 min before the partition is removed, and the fish are free to enter the second illuminated chamber. The partition is replaced after few minutes, and the numbers of fish in both chambers are quantified. Typically, fish with normal vision will move towards the illuminated chamber, whereas fish with vision defects will not show a preference for the illuminated chamber. For this assay, it was noted that fish move towards the illuminated chamber in both methods, which is evidence of PTB in zebrafish. However, there appears to be no significant difference in the number of fish moving from one chamber to another in the light and dark condition approach, which has to be taken into account when interpreting the results. Because of this limitation, the PTB is less suitable for screening zebrafish for mutants with defective vision.
Escape response (ER)
Another method to assess vision in zebrafish is the ER, which is based on the natural tendency of zebrafish to evade an approaching predator. In a typical experimental setup, zebrafish are kept inside a white, circular rotating drum with a single black strip that mimics a threatening predator.57 In this assay, zebrafish show a tendency to keep on opposite site of the black stripe. The response of zebrafish either to move towards the visual cues or to move away from it is determined by the size of the visual cue.58 Zebrafish orient themselves towards a small moving visual stimulus as in the case of OMR. As the size of the visual stimulus increases, their behavioural response change into aversive turns, and they move away from it as in the case of ER. This is well explained as a transition from a prey capture-related orienting response to a predator-avoidance response.58 This test is robust and has been used before in several studies to characterize mutants that affect vision,57, 59 such as nba (night blindness a) and nbb (night blindness b), which are dominant mutants with adult retinal degeneration.57 Using ER, the role of the circadian clock in modulating visual sensitivity has also been studied in zebrafish.60, 61 When the threshold light intensity to initiate an ER was measured over a period of 24 h under light dark cycle, the threshold light intensity was at its highest before ‘lights on' early in the morning and at its lowest before ‘light off' in the late afternoon. Therefore, the authors concluded that zebrafish are more sensitive towards visual stimuli at dusk than at dawn.60
Limitations of visual behaviour-based assays
It has to be pointed out that the assessment of visual function in zebrafish by the assays described above is entirely based on their behavioural response to the visual stimulus. As a consequence, there is a potential that these assays could produce misleading results. In particular, it can be hypothesized that defects in tissues other than the eye that are nevertheless crucial for behaviour, such as neuronal circuits that affect muscle function, have the potential to produce misleading results. Genetic analysis of visual behaviour in zebrafish mutants (10 loci, 12 new alleles) did reveal defective visual behaviours (OMR, OKR), with no obvious morphological defect in retina and RGC projection.6 This study has highlighted the potential involvement of brain functions beyond retinotectal projection, which is not unexpected given our knowledge of drugs that can alter behaviour. For example, it has been reported that acute alcohol exposure reduces the fear responses and zebrafish become unresponsive towards visual cues.62 Nevertheless, some drugs can inhibit motility, thus ending up with false impression on visual behaviour assays that is examined by the fish movement such as OMR, therefore further assessment in such condition is necessary.63
Finally, defects in all processes and systems that are involved from the processing of visual information to the actual observed change of visual behaviour, such as motor function, have to be seen as possible sites that could influence results. As such, processing of the visual signal in the visual cortex (in higher vertebrates) or optic tectum (in case of zebrafish) only serves as a prerequisite for induced behaviour, therefore dysfunction not only in the visual cortex but also other brain areas involved in decision-making could potentially lead to unresponsiveness to the visual stimulation. Thus, confirmation of behavioural assay results with additional methods, such as detection of pathology in the retina or optic nerve or the use of electroretinography5, 57 will significantly increase the reliability of results.
Modelling human eye diseases in zebrafish
The strength of the zebrafish model lies in the ability to potentially screen thousands of compounds and drugs for their therapeutic potential in human diseases. Many human pathological conditions from Alzheimer's disease to metabolic syndrome have been successfully modelled in zebrafish. Over the past decade, zebrafish have also been used to model several human ocular diseases, such as cataract, glaucoma, DR, and AMD (Table 3).
Cataracts
Globally, cataracts are the leading cause of blindness1 caused by opacity of the lens.64 Cataracts are most prevalent in the aged population but also occur as congenital cataracts in children with genetic predisposition in the first year of life.64 Some studies have described zebrafish as a promising animal model to study dominant congenital cataracts.65, 66 Under normal conditions, the protein crystalline (βγ, γC types) assures lens and cornea transparency and is also necessary for refractive power.67, 68 Therefore, it is expected that mutations in the crystalline gene are linked to cataract formation in humans.69, 70 Recently, a cataract-causing gene has been identified using a zebrafish animal model. Mutation of the CRYBA2 gene, a member of βγ crystalline causes congenital cataracts.66 Furthermore, mutation in CRYGC gene (γC-crystalline) makes the lens less thermally stable and increases the risk of lens opacity when exposed to heat and UV radiation and ultimately also leads to cataract formation.65 Similarly the cloche zebrafish mutant displays cataracts associated with a defect in gamma crystalline induced by alpha A crystalline.71 Alpha A crystalline is essential for decreasing insolubility of gamma crystalline, which in turn increases lens transparency and initiates the differentiation of lens fibre cells. Therefore, this study has highlighted that cataract development could be preventable, provided that a sufficient alpha A crystalline expression can be achieved in the lens. Human congenital cataracts have also been modelled in zebrafish by gene knockdown experiments. For example, mutations in the PITX3 and FOXE3 genes, which are expressed in the lens epithelium, cause anterior segment defects, including cataracts.72, 73, 74, 75
Glaucoma
Glaucoma is the second leading cause of blindness in the world.1 Glaucoma is an array of adult onset retinal neuropathies characterized by progressive degeneration of RGCs and the optic nerve head.7 Despite the anatomical difference of trabecular meshwork76 and aqueous humour dynamics in human and zebrafish,77 the overall similarities in aqueous humour outflow tissue structure and average intraocular pressure (IOP)48 make zebrafish a potential model to study the complex genetics of glaucoma. Severe myopia, anterior segment defects (iris, trabecular meshwork, and cornea), and increased IOP, which precedes blockage of aqueous humour drainage, are the major risk factors associated with glaucoma.78 The disease-risk phenotype of glaucoma is well studied in zebrafish mutants and was successfully modelled in zebrafish, for example, by mutation of the FOXC1 (forkhead transcription factor) gene.79, 80, 81 FOXC1 is expressed in anterior segment and periocular mesenchymal cells82 and is essential for the development and maintenance of the anterior segment of the eye. Therefore, mutations in this transcription factor cause a glaucoma-like pathology, such as abnormalities in the iris, trabecular meshwork, and cornea. Furthermore, the mutation of the gene coding for low-density lipoprotein receptor-related protein 2 represented by the bugeye mutant of zebrafish has recently been analyzed with regards to glaucoma-linked risk factors using OMR, electroretinograms (ERGs) and histology analysis.83, 84 This bugeye mutant showed glaucoma-risk phenotypes such as enlarged eyes as a result of increased IOP, decrease in retinal ganglion cell numbers at 3 months, and outer retinal dysfunction by 5 months due to prolonged mechanical stress.
AMD
AMD is a degenerative disease of the central retina and the third leading cause of blindness worldwide mostly affecting people aged >50 years.1, 85 It is characterized by a presence of pale yellowish lesion in macula and peripheral retina as a result of deposition of acellular polymorphous debris called drusen. The pathology includes the degeneration of cone cells, RPE, and Bruch's membrane. Later stages show choroid neovascularization,86 which subsequently leads to a loss of colour vision and visual acuity. Zebrafish have been used to study retinopathy (seen in AMD and DR) induced by hypoxia.87 Hypoxia is induced in adult zebrafish by exposing them to hypoxic water for 3–10 days,88 which then initiates neovascularization and produces immature, leaky, and disorganized blood vessels.89 The use of orally active anti-vascular endothelial growth factor (anti-VEGF) demonstrated significant efficacy against the pathological symptoms in zebrafish.88, 89 These findings suggest that VEGF-induced hypoxia is an essential component of retinal neovascularization in AMD and DR.88 Similarly, the zebrafish mutant gnn displays a phenotype analogous to AMD in humans. The macroscopic and behavioural screening of gnn zebrafish mutant from 2 to 9 days revealed dystrophy of red cones at around 5 dpf, which later spread to all other cone types,90 a pathology that is reminiscent of AMD in human patients.
DR
Diabetes mellitus (DM) affected 366 million people globally in 2011, which is estimated to increase to 552 million in 2030.91 Around 80% of DM patients develop DR within 20 years of being diagnosed with DM.92 DR is a frequent pathology as a result of microvascular complications of DM that affect the retinal structure.93 The risk factors for DR include neovascularization, vitreous haemorrhage, loss of blood–brain barrier (BBB) integrity, and macular oedema.94 Retinal neovascularization in DR generally tends to be more fragile and leaky, which leads to vitreous haemorrhage that ultimately leads to vision loss.94 The current treatment options for DR are limited to symptomatic relief and are not able to reverse the structural abnormalities once the disease has progressed. Therefore, animal models have been used to provide insight into underlying pathogenesis of DR and to screen novel drugs entities. There is a growing interest in the use of zebrafish in DR due to the fact that they exhibit similar retinal vascular physiology and pathology compared with mammals.95 Hyperglycaemic zebrafish are shown to have similar retinal structure abnormalities as seen in diabetic patients.96 Hyperglycaemia can be induced in adult zebrafish by immersing alternately in glucose solution and water over 28 days followed by retinal examination. IPL and INL layers were shown to be significantly reduced in treated zebrafish, with the thickness of the IPL approximately 55% of the INL.96 This study provided some support to the idea that zebrafish can be utilized to model DR. This finding was confirmed by another study where visual impairment was evident after inducing hyperglycaemia in zebrafish.97 In this study, hyperglycaemia affected the cone photoreceptor neuron layer as shown by their structural degeneration and through the use of ERGs.97 This study suggested that neuro-protective agents in combination with the drugs to combat the vascular complications would provide clinical benefits in DR. Furthermore, hypoxia-induced retinal neovascularization or angiogenesis in the adult Fli-EGFP-Tg transgenic zebrafish model recapitulates DR in humans. Using these models, it is a realistic prospect to assess the therapeutic efficacy of anti-angiogenic agents.88, 89
Human congenital nystagmus (HCN)/infantile nystagmus syndrome (INS)
INS is a congenital occulomotor disorder and genetically heterogeneous condition having different modes of inheritance: X-linked, autosomal dominant, and autosomal recessive.98 HCN/INS is characterized by periodic alternation of involuntary oscillations of the eye consisting of slow movement of the eye called slow phase followed by quick phase to focus on the object of interest called saccades. The pathophysiology of the disease is poorly understood.99 Studies have shown that three autosomal (NYS2, NYS3, and NYS4) and X-chromosomal loci (NYS1, NYS5) are thought to have a key role in aetiology of congenital nystagmus. Recently, it was shown that mutation of the FRMD7 gene on loci NYS1, which is expressed in neuronal tissue of the developing retina, mid brain, and hindbrain, is associated with INS.98 INS has been modelled in zebrafish.4, 24 A large number of homozygous belladonna (bel) mutants were found to display a reversed OKR indicative of a projection defect. In contrast to wild-type animals, visual stimulation of one eye in bel mutants leads to movement of unstimulated eye, which is indicative of optic nerve misrouting owing to absence of optic chiasm. These findings in zebrafish are in good agreement with a recent study that concluded that optic nerve misrouting could be a primary cause of INS.100
Human ciliopathies
Human cilliopathies are caused by a diverse set of mutations that lead to defects in a range of cilia-containing tissues,101 and retinal dysfunction is frequently associated with ciliopathies. The photoreceptors consists of mainly two segments: the outer segment (POS) contains stacks of membranous discs in which photopigments are embedded and the inner segment contains the nuclei and the machinery necessary for synthesis of proteins and metabolites.102 These two segments are linked by a connecting cilium that contains microtubules.102 Photopigments are synthesized in the inner segment and transported to the POS by the ciliary microtubules (Figure 3), a mechanism that is known as intraflagellar transport (IFT).103 Around 10% of the POS is replaced every day. For this purpose, proteins are transported from the inner segment by IFT to ensure the maintenance of the POS.104 Consequently, mutations of IFT proteins (present in the flagella of photoreceptor cells) have been shown to lead to retinal degeneration and subsequent blindness such as in Bardet–Biedl syndrome and Senior–Loken syndrome that are characterized by photoreceptor dysfunction and renal dysplasia.103, 104 These human ciliopathies are mirrored in the zebrafish mutants oval (ovl) and elipsa (elip) that show outer retinal dystrophy and renal dysfunction.39, 105 Analysis of the visual defect in the ovl and elip mutants using OKR (high-contrast visual stimulation), electroretinography, and histology revealed involuntary eye movements as well as light-insensitive and progressive degeneration of the POS.39, 105 Mutations in ovl affect intraflagellar components such as IFT 88, IFT 52, and IFT 57 and, as a consequence, produces defective cilia that lead to POS degeneration and vision impairment.39 Recently, the Rer1p gene, which is highly expressed in the cilia of zebrafish and mammals has been implicated in maintaining cilliary length and sensory function.106 Therefore, but not unexpected, mutations in Rer1p caused cilliary shortening and subsequent loss of sensory function, including vision loss.106
Others
Being situated in close proximity to each other, genetic defects of the RPE often lead to subsequent dystrophy of photoreceptor cells.107 This situation is observed in human choroideremia (CHM) and Hermansky–Pudlak syndrome, which have been also well modelled in zebrafish mutants.
Human CHM.
Human CHM is an inherited human retinal blindness caused by mutations of the Rab escort protein 1 (REP 1) gene, which leads to a progressive degeneration of rod photoreceptors subsequent to pigmentation defects in RPE cells.108, 109 This disease is modelled in the zebrafish larvae mutant line ru848, in which partial retinal dystrophy has been observed.108 However, it has to be noted that in humans the related gene, rep2, can compensate for the loss of REP 1. As zebrafish lack rep2 gene expression,110 the relevance of the ru848 mutation for the study of human CHM remains to be evaluated.
Hermansky–Pudlak syndrome.
Hermansky–Pudlak syndrome is a group of disorders characterized by oculocutaneous albinism (decrease in pigmentation of the skin, eye, and pilar caused by defective melanin production pathway), bleeding, and lysosome-related organelle defect.107 This syndrome has been reported in the zebrafish fading vision mutant (fad) that shows depigmentation of the RPE followed by loss of photoreceptor cells.111
Ophthalmological drug discovery and development in zebrafish
Zebrafish have been utilized to study the cellular and molecular mechanism of pathogenesis of human eye diseases. At the same time, the benefit of large clutch size of this model provides an opportunity for screening drugs that have the potential to be used for treating human eye diseases. For example, aminoglycosides (gentamicin and paromycin) have shown remarkable therapeutic efficacy in the zebrafish mutants (lamb and pax 2).112 These mutants recapitulate coloboma formation, which can be characterized by a triangular eyelid defect as a result of unsuccessful closure of choroid fissure during early morphogenesis of the eye.64 These findings reveal the potential of aminoglycosides in the treatment of human coloboma.112 Furthermore, the zebrafish model has established itself as a prominent model system for hypoxia-induced retinopathy.87 These hypoxic zebrafish models have been utilized to test oral anti-angiogenesis inhibitors (anti-VEGF agents), such as sunitinib and zm323881.89 These drugs rescued neovascularization of the retina confirming that VEGF, triggered by hypoxia to compensate for the reduced oxygen supply by forming new blood vessels, has a key role in retinal angiogenesis.89
Drug-related ophthalmological toxicity assessment in zebrafish
More than 200 currently used drugs are associated with ophthalmic toxicity as a result of adverse drug reaction.113 Assessment of oculotoxicity associated with drugs at the early stage of their development is crucial. However, this process is partly hindered due to a lack of predictive, convenient methods as well as the high expense associated with testing drug candidates in mammals.63, 114 For many years, researchers have been using zebrafish assays for drug toxicity screening with a view to provide early drug safety assessments.115, 116 So far, zebrafish have been used to predict drug-related cardiotoxicity, ototoxicity, developmental toxicity, neurotoxicity, oculotoxicity, and many more.115, 116 Furthermore, zebrafish have efficiently predicted toxicity associated with the effects of systemic drugs in humans.116 More recently, a study described zebrafish as a potential animal model to predict drug-related oculotoxicity at the preclinical stage.114 In this study, 3 dpf zebrafish larvae were exposed to the known oculotoxic drugs (digoxin, gentamicin, ibuprofen, minoxidil, and quinine) for 48 h, and toxicity was assessed using visual behaviour. These drugs resulted in damage to visual function in zebrafish, thereby confirming the potential toxicity of these drugs to the human eye.
In addition, many studies have been carried out to validate zebrafish assays for drug-toxicity assessment. In one of those, 27 drugs, 19 of which with known and 8 with no record of oculotoxicity in humans, were assessed in zebrafish using OMR.63 Of the 19 compounds, 13 drug compounds, including chlorpromazine, quinine, digoxin, AZ compound 1, deferoxamine, flecainide, ganciclovir, ibuprofen, minoxidil, thioridazine, and vardenafil, were found to be oculotoxic in zebrafish. This finding is supported by another similar study that tested nine known drug compounds with oculotoxicity and found that 7 out of the 9 compounds had adverse effect on visual function of zebrafish as well.117 Furthermore, zebrafish have been used extensively as a screening tool for small molecules that modulate vertebrate development.118 For example, small-molecule screens have identified compounds that can affect vascular development in the zebrafish retina. In one study, around 2000 small molecules were tested in zebrafish embryos followed by monitoring changes in retinal vasculature, and out of those, 5 compounds were identified,119 which suggests that these drugs may also contribute to the development of pathological changes to the retinal vasculature in human patients. These findings suggest that zebrafish models are successful in mirroring known oculotoxic characteristics of drugs in humans and have the potential to predict oculotoxicity profiles of novel drugs.
Summary
Visual impairment is a major health problem worldwide, and our understanding of the pathological mechanisms associated with disease is crucial for the development of novel therapeutic approaches. Likewise, the availability of animal models that closely mirror eye pathology but at the same time allow medium-throughput drug screening is desirable. In this context, zebrafish with their short-generation time, large clutch, ease of mutagenesis and genetic analysis, rapid eye development, and comparable eye morphology to humans seem ideally suited to address these needs. Similar phenotypes related to many human vision disorders have been reproduced in zebrafish, and for many available strains, their visual capacity has already been characterized using assays that exploit natural fish behaviour. Thus, the zebrafish model has the potential to open the door for a deeper understanding of ophthalmological disorders, as well as for the development of novel therapeutic agents.
The authors declare no conflict of interest.
References
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96 (5:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Gordois A, Cutler H, Pezzullo L, Gordon K, Cruess A, Winyard S, et al. An estimation of the worldwide economic and health burden of visual impairment. Glob Public Health. 2012;7 (5:465–481. doi: 10.1080/17441692.2011.634815. [DOI] [PubMed] [Google Scholar]
- Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci USA. 1995;92 (23:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss SC, Biehlmaier O, Seeliger MW, Das T, Kohler K, Harris WA, et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci. 1999;19 (19:8603–8615. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss SC. Behavioral genetic approaches to visual system development and function in zebrafish. J Neurobiol. 2003;54 (1:148–160. doi: 10.1002/neu.10165. [DOI] [PubMed] [Google Scholar]
- Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS, et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1 (5:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibliowicz J, Tittle RK, Gross JM. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog Mol Biol Transl Sci. 2011;100:287–330. doi: 10.1016/B978-0-12-384878-9.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Saxena MT, Mumm JS. Advances in zebrafish chemical screening technologies. Future Med Chem. 2012;4 (14:1811–1822. doi: 10.4155/fmc.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10 (2:116–124. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82 (1:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- Lin YY. Muscle diseases in the zebrafish. Neuromuscul Disord. 2012;22 (8:673–684. doi: 10.1016/j.nmd.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Norton WH. Toward developmental models of psychiatric disorders in zebrafish. Front Neural Circuits. 2013;7:79. doi: 10.3389/fncir.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan L. Looking within the zebrafish to understand the tuberculous granuloma. Adv Exp Med Biol. 2013;783:251–266. doi: 10.1007/978-1-4614-6111-1_13. [DOI] [PubMed] [Google Scholar]
- Amatruda JF, Shepard JL, Stern HM, Zon LI. Zebrafish as a cancer model system. Cancer Cell. 2002;1 (3:229–231. doi: 10.1016/s1535-6108(02)00052-1. [DOI] [PubMed] [Google Scholar]
- Lien CL, Harrison MR, Tuan TL, Starnes VA. Heart repair and regeneration: recent insights from zebrafish studies. Wound Repair Regen. 2012;20 (5:638–646. doi: 10.1111/j.1524-475X.2012.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi O, Parikka M, Ramet M. The zebrafish as a model for paediatric diseases. Acta Paediatr. 2013;102 (2:104–110. doi: 10.1111/j.1651-2227.2012.02835.x. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Saszik S. The zebrafish as a model visual system. Int J Dev Neurosci. 2001;19 (7:621–629. doi: 10.1016/s0736-5748(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Villegas GM. Neurophysiologie und Psychophysik des Visuellen Systems/The Visual System: Neurophysiology and Psychophysics. Springer: Berlin, Germany; Heidelberg, Germany; 1961. Comparative ultrastructure of the retina in fish, monkey and man; pp. 3–13. [Google Scholar]
- Dowling J.The Retina: An Approachable Part of the BrainVol 18Belknap Press of Harvard University Press: Cambridge, MA, USA; 198712–32. [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300 (1:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Robinson J, Schmitt EA, Harosi FI, Reece RJ, Dowling JE. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci USA. 1993;90 (13:6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P, Harris WA. The zebrafish as a tool for understanding the biology of visual disorders. Semin Cell Dev Biol. 2003;14 (1:11–18. doi: 10.1016/s1084-9521(02)00167-2. [DOI] [PubMed] [Google Scholar]
- Fleisch VC, Neuhauss SC. Visual behavior in zebrafish. Zebrafish. 2006;3 (2:191–201. doi: 10.1089/zeb.2006.3.191. [DOI] [PubMed] [Google Scholar]
- Maurer CM, Huang YY, Neuhauss SC. Application of zebrafish oculomotor behavior to model human disorders. Rev Neurosci. 2011;22 (1:5–16. doi: 10.1515/RNS.2011.003. [DOI] [PubMed] [Google Scholar]
- Yousef YA, Finger PT. Optical coherence tomography of radiation optic neuropathy. Ophthalmic Surg Lasers Imaging. 2012;43 (1:6–12. doi: 10.3928/15428877-20111129-09. [DOI] [PubMed] [Google Scholar]
- Schweitzer J, Gimnopoulos D, Lieberoth BC, Pogoda HM, Feldner J, Ebert A, et al. Contactin1a expression is associated with oligodendrocyte differentiation and axonal regeneration in the central nervous system of zebrafish. Mol Cell Neurosci. 2007;35 (2:194–207. doi: 10.1016/j.mcn.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Zou S, Tian C, Ge S, Hu B. Neurogenesis of retinal ganglion cells is not essential to visual functional recovery after optic nerve injury in adult zebrafish. PLoS One. 2013;8 (2:e57280. doi: 10.1371/journal.pone.0057280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L. Retinal processing: amacrine cells keep it short and sweet. Curr Biol. 1998;8 (17:R598–R600. doi: 10.1016/s0960-9822(98)70385-9. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones. Neuron. 1993;10 (6:1161–1174. doi: 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224 (1:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Saszik S, Bilotta J, Givin CM. ERG assessment of zebrafish retinal development. Vis Neurosci. 1999;16 (5:881–888. doi: 10.1017/s0952523899165076. [DOI] [PubMed] [Google Scholar]
- Moyano M, Porteros A, Dowling JE. The effects of nicotine on cone and rod b-wave responses in larval zebrafish. Vis Neurosci. 2013;30 (4:141–145. doi: 10.1017/S0952523813000187. [DOI] [PubMed] [Google Scholar]
- Baylor DA. Photoreceptor signals and vision. Proctor lecture. Invest Ophthalmol Vis Sci. 1987;28 (1:34–49. [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early eye morphogenesis in the zebrafish, Brachydanio rerio. J Comp Neurol. 1994;344 (4:532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999;404 (4:515–536. [PubMed] [Google Scholar]
- Malicki J. Development of the retina. Methods Cell Biol. 1999;59:273–299. doi: 10.1016/s0091-679x(08)61830-0. [DOI] [PubMed] [Google Scholar]
- Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol. 1999;207 (2:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Prog Retin Eye Res. 2008;27 (1:89–110. doi: 10.1016/j.preteyeres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Genetics of photoreceptor development and function in zebrafish. Int J Dev Biol. 2004;48 (8-9:925–934. doi: 10.1387/ijdb.041890mt. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180 (2:646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Nicola GN. The development of eye movements in the zebrafish (Danio rerio) Dev Psychobiol. 1997;31 (4:267–276. doi: 10.1002/(sici)1098-2302(199712)31:4<267::aid-dev4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kitambi SS, Chandrasekar G, Addanki VK. Teleost fish—a powerful models for studying development, function and diseases of the human eye. Curr Sci. 2011;100 (12:1815. [Google Scholar]
- Renninger SL, Schonthaler HB, Neuhauss SC, Dahm R. Investigating the genetics of visual processing, function and behaviour in zebrafish. Neurogenetics. 2011;12 (2:97–116. doi: 10.1007/s10048-011-0273-x. [DOI] [PubMed] [Google Scholar]
- Brockerhoff SE. Measuring the optokinetic response of zebrafish larvae. Nat Protoc. 2006;1 (5:2448–2451. doi: 10.1038/nprot.2006.255. [DOI] [PubMed] [Google Scholar]
- Huang YY, Neuhauss SC. The optokinetic response in zebrafish and its applications. Front Biosci. 2008;13:1899–1916. doi: 10.2741/2810. [DOI] [PubMed] [Google Scholar]
- Huber-Reggi SP, Mueller KP, Neuhauss SC. Analysis of optokinetic response in zebrafish by computer-based eye tracking. Methods Mol Biol. 2013;935:139–160. doi: 10.1007/978-1-62703-080-9_10. [DOI] [PubMed] [Google Scholar]
- Mueller KP, Neuhauss SC. Quantitative measurements of the optokinetic response in adult fish. J Neurosci Methods. 2010;186 (1:29–34. doi: 10.1016/j.jneumeth.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Link BA, Gray MP, Smith RS, John SW. Intraocular pressure in zebrafish: comparison of inbred strains and identification of a reduced melanin mutant with raised IOP. Invest Ophthalmol Vis Sci. 2004;45 (12:4415–4422. doi: 10.1167/iovs.04-0557. [DOI] [PubMed] [Google Scholar]
- Brockerhoff SE, Hurley JB, Niemi GA, Dowling JE. A new form of inherited red-blindness identified in zebrafish. J Neurosci. 1997;17 (11:4236–4242. doi: 10.1523/JNEUROSCI.17-11-04236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta J. Effects of abnormal lighting on the development of zebrafish visual behavior. Behav Brain Res. 2000;116 (1:81–87. doi: 10.1016/s0166-4328(00)00264-3. [DOI] [PubMed] [Google Scholar]
- Quentin B, Moore RH.Behavior and Cognition Biology of Fishes3rd edn.Taylor & Francis Group: New York, USA; Abingdon, UK; 2007409–433. [Google Scholar]
- Bilotta J, Saszik S, Givin CM, Hardesty HR, Sutherland SE. Effects of embryonic exposure to ethanol on zebrafish wvisual function. Neurotoxicol Teratol. 2002;24 (6:759–766. doi: 10.1016/s0892-0362(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebra fish. Dev Psychobiol. 1974;7 (1:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- Weber DN, Connaughton VP, Dellinger JA, Klemer D, Udvadia A, Carvan MJ., 3rd Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Physiol Behav. 2008;93 (1-2:250–260. doi: 10.1016/j.physbeh.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Adolph AR, Wong KY, Kraves S, Dowling JE. OFF ganglion cells cannot drive the optokinetic reflex in zebrafish. Proc Natl Acad Sci USA. 2007;104 (48:19126–19131. doi: 10.1073/pnas.0709337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Dowling JE.A behavioral assay to measure responsiveness of zebrafish to changes in light intensities J Vis Exp 2008(20)pii. 923 doi: 10.3791/923 [DOI] [PMC free article] [PubMed]
- Li L, Dowling JE. A dominant form of inherited retinal degeneration caused by a non-photoreceptor cell-specific mutation. Proc Natl Acad Sci USA. 1997;94 (21:11645–11650. doi: 10.1073/pnas.94.21.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Kampff AR, Engert F. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front Syst Neurosci. 2011;5:101. doi: 10.3389/fnsys.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. Zebrafish mutants: behavioral genetic studies of visual system defects. Dev Dyn. 2001;221 (4:365–372. doi: 10.1002/dvdy.1159. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15 (5:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- Li L, Dowling JE. Disruption of the olfactoretinal centrifugal pathway may relate to the visual system defect in night blindness b mutant zebrafish. J Neurosci. 2000;20 (5:1883–1892. doi: 10.1523/JNEUROSCI.20-05-01883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca RM, Gerlai R. Animated bird silhouette above the tank: acute alcohol diminishes fear responses in zebrafish. Behav Brain Res. 2012;229 (1:194–201. doi: 10.1016/j.bbr.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards FM, Alderton WK, Kimber GM, Liu Z, Strang I, Redfern WS, et al. Validation of the use of zebrafish larvae in visual safety assessment. J Pharmacol Toxicol Methods. 2008;58 (1:50–58. doi: 10.1016/j.vascn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Lang GK.Ophthalmology A Pocket Textbook Atlas2nd ed.Thieme: New York, NY, USA; 2007 [Google Scholar]
- Li XQ, Cai HC, Zhou SY, Yang JH, Xi YB, Gao XB, et al. A novel mutation impairing the tertiary structure and stability of gammaC-crystallin (CRYGC) leads to cataract formation in humans and zebrafish lens. Hum Mutat. 2012;33 (2:391–401. doi: 10.1002/humu.21648. [DOI] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Muheisen S, Raggio V, Salviati L, Han DP, et al. Whole exome sequencing in dominant cataract identifies a new causative factor, CRYBA2, and a variety of novel alleles in known genes. Hum Genet. 2013;132 (7:761–770. doi: 10.1007/s00439-013-1289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26 (1:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jonasova K, Kozmik Z. Eye evolution: lens and cornea as an upgrade of animal visual system. Semin Cell Dev Biol. 2008;19 (2:71–81. doi: 10.1016/j.semcdb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Graw J. The crystallins: genes, proteins and diseases. Biol Chem. 1997;378 (11:1331–1348. [PubMed] [Google Scholar]
- Graw J. Genetics of crystallins: cataract and beyond. Exp Eye Res. 2009;88 (2:173–189. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Goishi K, Shimizu A, Najarro G, Watanabe S, Rogers R, Zon LI, et al. AlphaA-crystallin expression prevents gamma-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development. 2006;133 (13:2585–2593. doi: 10.1242/dev.02424. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, et al. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19 (2:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10 (3:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Aspock G, Burdine RD, Schier A, Westerfield M, et al. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132 (7:1579–1590. doi: 10.1242/dev.01723. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Zilinski CA, Hashimoto R, Shah R, Lane ME, Jamrich M. Regulation and function of foxe3 during early zebrafish development. Genesis. 2008;46 (3:177–183. doi: 10.1002/dvg.20380. [DOI] [PubMed] [Google Scholar]
- Chen CC, Yeh LK, Liu CY, Kao WW, Samples JR, Lin SJ, et al. Morphological differences between the trabecular meshworks of zebrafish and mammals. Curr Eye Res. 2008;33 (1:59–72. doi: 10.1080/02713680701795026. [DOI] [PubMed] [Google Scholar]
- Gray MP, Smith RS, Soules KA, John SW, Link BA. The aqueous humor outflow pathway of zebrafish. Invest Ophthalmol Vis Sci. 2009;50 (4:1515–1521. doi: 10.1167/iovs.08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein BE, Klein R, Knudtson M, Lee KE. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003;110 (1:211–217. doi: 10.1016/s0161-6420(02)01260-5. [DOI] [PubMed] [Google Scholar]
- Tamimi Y, Skarie JM, Footz T, Berry FB, Link BA, Walter MA. FGF19 is a target for FOXC1 regulation in ciliary body-derived cells. Hum Mol Genet. 2006;15 (21:3229–3240. doi: 10.1093/hmg/ddl400. [DOI] [PubMed] [Google Scholar]
- Berry FB, Skarie JM, Mirzayans F, Fortin Y, Hudson TJ, Raymond V, et al. FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Hum Mol Genet. 2008;17 (4:490–505. doi: 10.1093/hmg/ddm326. [DOI] [PubMed] [Google Scholar]
- Skarie JM, Link BA. FoxC1 is essential for vascular basement membrane integrity and hyaloid vessel morphogenesis. Invest Ophthalmol Vis Sci. 2009;50 (11:5026–5034. doi: 10.1167/iovs.09-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C, Semina EV, Link BA. Using zebrafish to study the complex genetics of glaucoma. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138 (3:343–350. doi: 10.1016/j.cca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stujenske JM, Dowling JE, Emran F. The bugeye mutant zebrafish exhibits visual deficits that arise with the onset of an enlarged eye phenotype. Invest Ophthalmol Vis Sci. 2011;52 (7:4200–4207. doi: 10.1167/iovs.10-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veth KN, Willer JR, Collery RF, Gray MP, Willer GB, Wagner DS, et al. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7 (2:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379 (9827:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358 (24:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Jensen LD, Rouhi P, Cao Z, Lanne T, Wahlberg E, Cao Y. Zebrafish models to study hypoxia-induced pathological angiogenesis in malignant and nonmalignant diseases. Birth Defects Res C Embryo Today. 2011;93 (2:182–193. doi: 10.1002/bdrc.20203. [DOI] [PubMed] [Google Scholar]
- Cao Z, Jensen LD, Rouhi P, Hosaka K, Lanne T, Steffensen JF, et al. Hypoxia-induced retinopathy model in adult zebrafish. Nat Protoc. 2010;5 (12:1903–1910. doi: 10.1038/nprot.2010.149. [DOI] [PubMed] [Google Scholar]
- Cao R, Jensen LD, Soll I, Hauptmann G, Cao Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS One. 2008;3 (7:e2748. doi: 10.1371/journal.pone.0002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehlmaier O, Neuhauss SC, Kohler K. Double cone dystrophy and RPE degeneration in the retina of the zebrafish gnn mutant. Invest Ophthalmol Vis Sci. 2003;44 (3:1287–1298. doi: 10.1167/iovs.02-0363. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94 (3:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27 (5:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci (Lond) 2013;125 (1:1–17. doi: 10.1042/CS20120588. [DOI] [PubMed] [Google Scholar]
- Bandello F, Lattanzio R, Zucchiatti I, Del Turco C. Pathophysiology and treatment of diabetic retinopathy. Acta Diabetol. 2013;50 (1:1–20. doi: 10.1007/s00592-012-0449-3. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Cederlund ML, Cottell DC, Bill BR, Ekker SC, Torres-Vazquez J, et al. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev Biol. 2007;7:114. doi: 10.1186/1471-213X-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Connaughton V, Arneson LS. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007;44 (3:157–163. doi: 10.1007/s00592-007-0257-3. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Chen K, Reynolds AL, Waghorne N, O'Connor JJ, Kennedy BN. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis Model Mech. 2010;3 (3-4:236–245. doi: 10.1242/dmm.003772. [DOI] [PubMed] [Google Scholar]
- Thomas MG, Crosier M, Lindsay S, Kumar A, Thomas S, Araki M, et al. The clinical and molecular genetic features of idiopathic infantile periodic alternating nystagmus. Brain. 2011;134 (Pt 3:892–902. doi: 10.1093/brain/awq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self J, Lotery A. A review of the molecular genetics of congenital idiopathic nystagmus (CIN) Ophthalmic Genet. 2007;28 (4:187–191. doi: 10.1080/13816810701651233. [DOI] [PubMed] [Google Scholar]
- Huber-Reggi SP, Chen CC, Grimm L, Straumann D, Neuhauss SC, Huang MY. Severity of infantile nystagmus syndrome-like ocular motor phenotype is linked to the extent of the underlying optic nerve projection defect in zebrafish belladonna mutant. J Neurosci. 2012;32 (50:18079–18086. doi: 10.1523/JNEUROSCI.4378-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia. 2012;1 (1:22. doi: 10.1186/2046-2530-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincensini L, Blisnick T, Bastin P. 1001 model organisms to study cilia and flagella. Biol Cell. 2011;103 (3:109–130. doi: 10.1042/BC20100104. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157 (1:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237 (8:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadori R, Huber M, Rinner O, Seeliger MW, Geiger-Rudolph S, Geisler R, et al. Retinal function and morphology in two zebrafish models of oculo-renal syndromes. Eur J Neurosci. 2003;18 (6:1377–1386. doi: 10.1046/j.1460-9568.2003.02863.x. [DOI] [PubMed] [Google Scholar]
- Jurisch-Yaksi N, Rose AJ, Lu H, Raemaekers T, Munck S, Baatsen P, et al. Rer1p maintains ciliary length and signaling by regulating gamma-secretase activity and Foxj1a levels. J Cell Biol. 2013;200 (6:709–720. doi: 10.1083/jcb.201208175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestri G, Link BA, Neuhauss SC. The visual system of zebrafish and its use to model human ocular diseases. Dev Neurobiol. 2012;72 (3:302–327. doi: 10.1002/dneu.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Kappler JA, Chan DK, Kollmar R, Hudspeth AJ. Mutation of the zebrafish choroideremia gene encoding Rab escort protein 1 devastates hair cells. Proc Natl Acad Sci USA. 2004;101 (8:2572–2577. doi: 10.1073/pnas.0308474100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Sumaroka A, Aleman TS, Schwartz SB, Windsor EA, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47 (9:4113–4120. doi: 10.1167/iovs.06-0424. [DOI] [PubMed] [Google Scholar]
- Moosajee M, Tulloch M, Baron RA, Gregory-Evans CY, Pereira-Leal JB, Seabra MC. Single choroideremia gene in nonmammalian vertebrates explains early embryonic lethality of the zebrafish model of choroideremia. Invest Ophthalmol Vis Sci. 2009;50 (6:3009–3016. doi: 10.1167/iovs.08-2755. [DOI] [PubMed] [Google Scholar]
- Bahadori R, Rinner O, Schonthaler HB, Biehlmaier O, Makhankov YV, Rao P, et al. The Zebrafish fade out mutant: a novel genetic model for Hermansky-Pudlak syndrome. Invest Ophthalmol Vis Sci. 2006;47 (10:4523–4531. doi: 10.1167/iovs.05-1596. [DOI] [PubMed] [Google Scholar]
- Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. J Clin Invest. 2012;122 (7:2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications: recognition and management. Drugs. 2007;67 (1:75–93. doi: 10.2165/00003495-200767010-00006. [DOI] [PubMed] [Google Scholar]
- Deeti S, O'Farrell S, Kennedy BN. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J Pharmacol Toxicol Methods. 2013;69 (1:1–8. doi: 10.1016/j.vascn.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL. Zebrafish assays for drug toxicity screening. Expert Opin Drug Metab Toxicol. 2006;2 (2:231–240. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Rubinstein AL. The use of in vivo zebrafish assays in drug toxicity screening. Expert Opin Drug Metab Toxicol. 2009;5 (4:393–401. doi: 10.1517/17425250902882128. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, et al. Zebrafish based assays for the assessment of cardiac, visual and gut function—potential safety screens for early drug discovery. J Pharmacol Toxicol Methods. 2008;58 (1:59–68. doi: 10.1016/j.vascn.2008.05.130. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97 (24:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitambi SS, McCulloch KJ, Peterson RT, Malicki JJ. Small molecule screen for compounds that affect vascular development in the zebrafish retina. Mech Dev. 2009;126 (5-6:464–477. doi: 10.1016/j.mod.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]