Abstract
Echinoderms are closely related to chordates and comprise a major group of invertebrate deuterostomes. They are broadcast spawners and as such, each female accumulates millions of eggs and oocytes. These cells are readily isolated, and are often large, clear, and surrounded by accessory cells and extracellular coverings that do not prevent access to the oocyte. Sea stars are one type of echinoderm. Their oocytes are stored in prophase of meiosis, and since the natural meiotic stimulus has been identified as 1-methyladenine, these cells can be induced to complete meiotic maturation as individuals, or synchronously en masse. Microinjection and culture of these cells is feasible using quantitative or repetitive methods so that hundreds of oocytes and eggs can be modified each hour. Experimentation on this organism is extensive over a rich history of reproductive and developmental biology so that new investigators can easily incorporate this organism into their repertoire of research. This chapter will highlight the fundamental protocols to enable a new investigator to perform an array of approaches on this organism, including oocyte isolation, microinjection, and even single cell quantitative PCR.
Keywords: sea star, fertilization, meiosis, microinjection, polar body, single cell PCR
Introduction
The tree of life is rich in diversity. While the phylogenetic relationships are still being resolved, the broad variations in how organisms accomplish different tasks are hugely instructive as to how the process evolved and ultimately, to how we function. By studying yeast, flies, roundworms, frogs, and mice much has been learned that often not only directly applies to studies of humans but also provides significant insights to facilitate those human studies. Depending on the fundamental question being asked, the plethora of organisms in the world offer a wealth of research opportunities that may be easier, and provide faster inroads to a process than by studying the process in humans. No one organism is ideal for studying all questions of interest, and some are better suited for studying certain functions than others. Some of the major discoveries in the past 25 years have capitalized on the best organism for the question. For example, flies with rapid generation time are easily cultured and bred, which enabled investigators to first identify key regulatory genes involved in developmental processes of cell interactions, translational regulation, signal transduction cascades, and gene regulatory networks. Many of the genes identified in this work turned out to be proto-oncogenes, and greatly impacted the focus of cancer research. Research with this organism led to the Nobel Prize in Physiology or Medicine for three investigators in 1995. Studying the simple round worm, Caenorhabditis elegans enabled lineage tracing of each cell in the organism leading to a detailed understanding of organ development and apoptosis and three investigators shared the Nobel Prize in Physiology or Medicine in 2002 for this work.
A. General biology of sea stars
Sea stars are useful for several types of studies in reproduction. These include oocyte maturation, fertilization, embryologic development, and the evolutionary transitions in reproductive design. The research benefits of using sea stars include: the eggs and embryos are optically clear enabling excellent observation of individual cell movements, oocyte maturation and fertilization in vitro is under the investigators control, large quantities of gametes are easily obtained (one female sea star can provide millions of eggs), microinjection approaches of the oocyte uncomplicated, and embryos rapidly develop. In addition, sea stars are invertebrate deuterostomes i.e. they are members of Ambulacraria, which is closely related to chordates (Figure 1), and thus have an important evolutionary position for understanding reproductive features in humans. Male and female sea stars release their gametes through gonopores, located on the top (dorsal aspect) of the animal (Figure 2), and fertilization occurs externally in the seawater. Investigators can therefore spawn the animals individually in the lab, collect eggs and sperm, and fertilize en masse. Alternatively, the investigator may cut a small hole in the dorsal aspect of the arm, then with a fine forceps, lobes of the ovary (or testis) can be removed and the oocytes isolated in prophase of meiosis I. They can then be injected for expression or manipulation studies, or for better analysis of meiosis, treated with 1microMolar 1-methyladenine and matured within an hour. Such ease of in vitro manipulations has resulted in a rich classic and contemporary research literature. Investigators and interested readers are encouraged to use the many wonderful reviews of the ecology, phylogeny, developmental and reproductive biology developed in this class of organisms (e.g. Strathmann, 1987; Brusca and Brusca, 1990; Chia and Walker, 1991; Kanatani et al., 1969; Kishimoto, 1998; Stricker, 1999; Foltz et al., 2004).
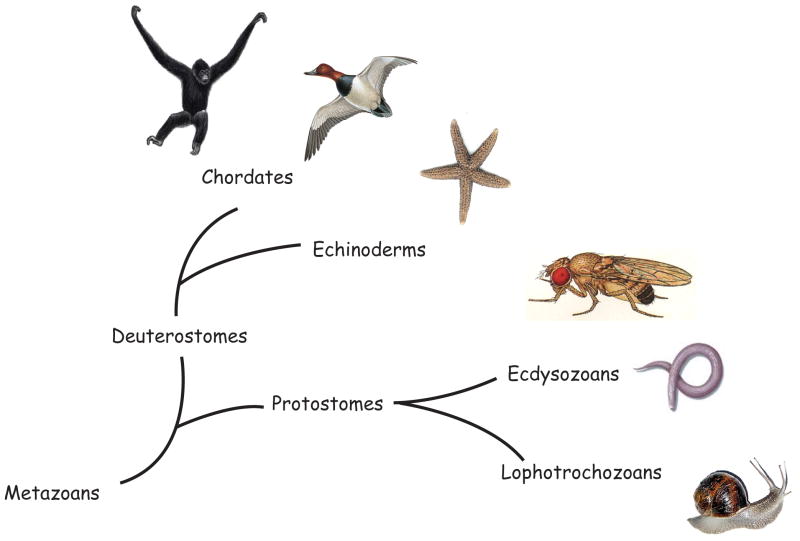
Figure 1.
Phylogenetic relationship amongst major organisms (Dunn et al., 2008). Fly from [insects.eugenes.org]; round worm [phschool.com]; mollusks [oum.ox.ac.uk]; bird [animals.howstuffworks.com]; sea star [okeefes.org]; primate [wildlifeextra.com]
Figure 2.
Adult sea star (Asterina miniata) shedding several million eggs. These cells are spawned having begun the process of meiosis and are fertilizable for the next few hours. The gonads line the sides of each arm and the gonopores (site of gamete release, asterisk) from each gonad is at the base of the body. Thus, the gonopores from adjacent arms are close to each other but shed oocytes from distinct gonads (double arrow pointing to the sites of the two adjacent gonopores).
B. Detailed methods for spawning eggs, and for isolating oocytes
1. Materials
Gravid sea stars. Vendors are available throughout the United States who can ship animals at different times of the year. These animals, like most, are gravid only during certain times of the year. Vendors include: Marine Resource Center of the Marine Biological Laboratories; http://www.mbl.edu/mrc/services/supply.html; Marinus Scientific; info@marinusscientific.com
-
Filtered natural seawater (FSW) or artificial seawater (ASW), and calcium-free artificial seawater. Sea water for these animals is 30–32 parts per thousand and should be well aerated. A standard refractometer, or a hydrometer (from a pet supply store) are adequate for this determination. We usually make the seawater using deionized water.
ASW (grams per liter) CaFSW: (grams per liter) NaCl: 28.32 NaCl: 26.5 KCl: 0.77 KCl: 0.7 MgCl2-6H2O: 5.41 MgSO4-7H2O: 11.9 MgSO4-7H2O: 7.13 NaHCO3: 0.5 CaCl2: 1.18 pH 8.0 NaHCO3: 0.2 pH 8.0 CaFSW should be made in plastic bottles as calcium can leach out of glassware. 3 mm sample corer (e.g. Fine Science Tools, Foster City, CA, model #18035-03)
Dissecting scissors (e.g. Noyes scissors are nice; Fine Science Tools, model #15012-12)
Blunt straight or curved forceps (e.g. Graefe forceps from Fine Science Tools, model #11051-10)
Single edged razor blades
1-methyladenine (Acros Organics USA, NJ), catalog # 201310250)
Glass/plasticware: 30 ml beakers to collect oocytes and for fertilization, pyrex baking dishes or similar container to hold animal during dissections
1.5 ml microfuge tubes
Pasteur pipets and pipet bulbs, or plastic transfer pipettes
Glass slides and coverslips
Nylon mesh or cheese cloth
Light microscope (compound or stereoscope with 100X magnification)
“Embryo safe” glassware: glass and plasticware that has never contacted chemicals like heavy metals/fixatives or detergents of any kind. Glassware can be made “embryo safe” by acid washing. Such “embryo safe” glassware used for oocytes and embryos should, henceforth, never be washed with detergent; only tap water, rinsing with distilled water, and air dry.
2. Collecting mature oocytes and sperm
The following comments deal with such commonly encountered sea stars as Asterina miniata. For more comparative treatises covering a greater diversity of oocyte types among asteroids, see Strathmann (1987) or Chia and Walker (1991). The arms of gravid sea stars are usually plump or resilient to gentle squeezing. Males and females cannot be distinguished externally, so the sex is determined empirically, or by gonad biopsy. A pair of gonads runs along the length of each arm of the animal and so one can test one arm of the adult, while not disturbing the others. Once sex has been determined, the animals can be separated into different aquaria or partitions within the aquaria and later used as needed.
To induce spawning in the animals, inject approximately 0.2 mLs of a 1mM solution (in sea water) of 1-methyladenine into each side of the base of an arm, and replace the animal into a container of seawater (not back into the aquarium as it may induce others to spawn). Within about 30–45 minutes the animal will begin spawning either eggs or sperm (Figure 2). Oocytes are collected into a beaker and are capable of fertilization for the next several hours (see below).
3. Collecting immature oocytes
To obtain a small amount of ovary or testis, push a 3 mm sample corer through the dorsal (top) aspect arm of the sea star near its base (Figure 3). Alternatively, you can cut an X with a razor blade in the same site and pry open the edges to expose the gonad below.
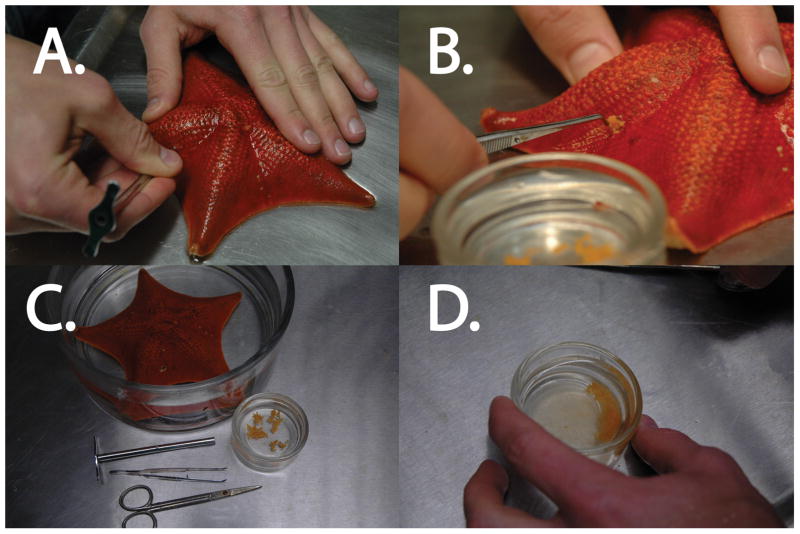
Figure 3.
Removal of sea star ovary (Asterina miniata). A. A hole is made in the arm of the sea star with a coring tool or straight razor. B. The ovary (or testis in a male) is removed with forceps and put in FSW. The ovary should have a plump appearance and readily spill out of the hole. C. Extraction of sea star ovary is done with very simple tools and upon extraction yields distinct lobes of ovary. D. Mincing the ovary with scissors will allow more oocytes to be isolated and subsequently exposed to 1-MA, if eggs are desired.
Using blunt forceps pull out a bit of gonad through the hole made in the arm using the sample corer. If the sea star is quite gravid, gonad material will spill out of the small hole. Ovary will be bright orange, yellowish, or tan depending on the species, but testis will be beige to white. The only other tissue loose in the arm is the gut, a grayish-brown, loose tissue. This gut runs alongside the gonads for much of the length of the arm. If it comes out of the incision, it should be returned into the arm. Squeezing the arm from below will sometimes help force the gonad out of the opening. Place pieces of ovary in filtered seawater (FSW) in a tall, narrow 30–50 mL glass beaker. Place testis fragments in a 1.5 ml microfuge tube on ice. This testis or “dry sperm” will be good all day on ice.
Return the sea star to its aquaria and push down on the surface near the opening to force out any air that may have entered. The sea star will heal within a few days and can be used as a source of gametes again.
Mince the ovary into small bits in FSW using small dissecting scissors. This will release the oocytes from the ovary. Full grown oocytes are ~ 180 microns in diameter and are arrested in prophase I of meiosis. The germinal vesicle (i.e. oocyte nucleus) of ~80 μm in diameter is clearly visible with a clear round object inside, which is the nucleolus. Healthy oocytes should have a smooth, uniform surface and a homogenous, slightly grainy cytoplasm. Outside of the oocyte jelly (the jelly is immediately outside of the cell, is transparent and can really only be seen by it excluding other objects) are flat follicle cells that release 1-methyladenine (1-MA) onto the surface of the oocyte. 1-MA is the “maturation hormone” that induces the oocyte to reenter the cell cycle and proceed through meiosis (Kanatani et al., 1969). If the goal is to work with oocytes, these follicle cells must be removed or they will activate maturation in the oocyte. If the goal is to work with matured oocytes, or eggs, they may be left on, even for fertilization, and additional 1-MA (1 microMolar) can be added for greater efficiency of maturation.
4. Removing follicle cells from the oocyte
In order to prevent the oocytes from maturing (i.e., re-entering meiosis) due to the release of 1-MA by the follicle cells, the follicle cells must be removed. This is accomplished using ice cold calcium-free artificial seawater (CaFASW). After mincing the ovary fragments and checking the quality of the oocytes, swirl the beaker to mix effectively and then let the chunks of ovary settle. Decant the suspension containing oocytes and debris into a fresh 30 ml beaker. Let the follicle - enclosed oocytes settle to the bottom of the new beaker, decant the FSW and then add ice cold CaFASW.
Place the beaker of CaFASW and oocytes on ice. The CaFASW will cause the follicle cells to loosen their adhesion to the jelly and the oocytes. After the oocytes have settled, repeat by decanting (and discarding) the supernatant, and replace the settled oocytes with new ice cold CaFASW, all the while inspecting the state of the oocytes using the microscope. When most of the oocytes appear free of follicle cells, pour off the CaFASW and replace with FSW. Depending on the individual animal, the time in CaFASW may vary from just a few minutes to as long as 30 minutes (do not leave oocytes in CaFSW for more than 30 minutes because prolonged exposure compromises the health of the oocyte). Often running the oocytes through cheese cloth also is effective at removing the follicle cells, especially after initial CaFSW treatment.
After the follicle cells have been removed, wash the oocytes 3 times with FSW and store them at 15–18°C. They will generally remain healthy at least for the rest of the day, and often for over 24 hours. Oocytes may be treated or microinjected during this time, and then following maturation (see below) can be fertilized.
5. Maturation of oocytes
In order for sea star oocytes to become fertilization-competent eggs, oocytes are typically exposed to the maturation-inducing hormone 1-methyladenine (1-MA) (for alternative methods of triggering maturation in sea stars, see Meijer et al. 1984). This hormone stimulates the oocytes to enter meiosis and undergo other changes that are collectively referred to as “oocyte maturation.” An obvious sign that an oocyte has begun maturation is breakdown of the germinal vesicle (GVBD).
To induce oocytes to mature, replace the FSW they are in with a solution of 1 microMolar 1-MA made in ASW. [1-methyladenine is made at 10mM in ASW. It is best to make small (100 microL) aliquots and store at −20°C. Stock aliquots usually retain activity for over 1 year if not thawed and refrozen.] GVBD will take place 20–40 min after exposure to 1-MA (the time to GVBD is temperature dependent and will vary from animal to animal). GVBD refers to the moment when the perimeter of the nucleus appears to disintegrate. Soon after GVBD, the remainder of the nucleus and the nucleolus will disappear. Within 10–15 min after GVBD, metaphase I of meiosis is reached. At metaphase I the oocyte can now be fertilized and is referred to as a mature oocyte or an egg. Note that the egg remains at metaphase I only for a few minutes as it then continues on in the meiotic cycle regardless of whether or not it is fertilized. If the egg is fertilized, it will finish meiosis and then begin the mitotic cleavages of embryogenesis. If an egg is not fertilized, it will finish meiosis and then undergo apoptosis at roughly 10 hours after 1-MA exposure (Sasaki and Chiba, 2001; Yuce and Sadler, 2001). In case of investigations involving pH changes or MAPK modulations, it is important to note that an arrest at metaphase-I can occur in intraovarian oocytes following 1-MA stimulation and that the completion of meiotic maturation without a metaphase arrest is a feature of oocytes removed from the ovary.
An alternative method to obtain mature oocytes is to put the pieces of ovary obtained from dissection into FSW containing 1-MA. Mincing the ovary into smaller pieces will enable greater recovery of oocytes. This will cause the ovary to ovulate defolliculated, maturing oocytes within about 30–60 minutes and it contains much less debris. Monitor the ovary closely for the release of the eggs and for GVBD. Transfer the released eggs (using a wide-bore pipette) into a beaker of FSW and prepare for fertilization.
6. Fertilization
For optimal fertilization, insemination of the eggs should occur at metaphase 1 (approximately 10–15 min after GVBD) although eggs can be fertilized any time during meiosis I. Eggs finish this stage about 1 hour after GVBD when the first polar body is extruded. Once eggs enter the second meiotic division, they often become polyspermic if inseminated and are referred to as “over mature”.
If eggs are in 1-MA, decant the 1-MA solution and add fresh FSW to approximately a 10% (volume/volume) suspension of oocytes. Clip the dissected testis (in a microfuge tube on ice) with small dissecting scissors. Spin the testis for 10 seconds at top speed in a microcentrifuge to separate released sperm from testis tissue. Immediately before insemination, dilute the sperm 1:1000 in a fresh tube containing FSW. Insemination can be performed at 15°C up to room temperature. Check that the diluted sperm have good motility by placing a drop on a slide and observing the sperm under a 10X or 20X objective then add 1:100 (sperm suspension to oocyte suspension) and gently mix by swirling with your pipette. Keep the eggs suspended by frequent, gentle stirring and monitor for elevation of the fertilization envelope every few minutes. Once formed (see Supplement Figure 1), allow the eggs to settle and gently wash them twice with large volumes of FSW to remove excess sperm. The fertilization envelope of A. miniata eggs elevates slowly and will not be fully elevated until several minutes after fertilization. The cells are now more stable to shear and insult. To get a synchronous population of developing embryos for biochemical studies there must be continuous aeration of the cultures and settling should be avoided. Rather the embryos should be lightly fertilized with constant stirring. For a description of methods to culture embryos and larvae, the reader is directed to Foltz et al., 2004.
7. Microinjection of sea star oocytes, eggs, and embryos
The large transparent cells, control over meiosis, and ease in microinjection makes these organisms prime for molecular manipulations. Many investigators have examined the gene regulatory networks, and have used molecular methods to test cellular mechanisms in this organism. Quantitative microinjection procedures are well defined. For details, see http://mterasaki.us/panda/injection/index.html (see also Foltz et al., 2004; Jaffe and Terasaki, 2004; Carroll and Hua, 2009).
We have recently incorporated a more high-throughput method to make injecting hundreds of oocytes, eggs or embryos easily performed. This approach enables a biochemical or molecular analysis of the phenotypes resulting from such molecular manipulations. We describe this here.
High-throughput sea star egg injections using Nitex® mesh
Constructing the injection plate
Sea star eggs do not develop well after chemical or charge attachment to plastic dishes; therefore a different method is needed to restrain the egg for injection. The protocol that we document here can be applied to oocytes, eggs, or fertilized eggs using the exact same conditions. A nylon matrix affixed to the bottom of a dish creates a grid of wells in which the cells are gently constrained. A. miniata eggs are 180micrometers in diameter and fit within a 200micrometers opening nylon mesh. If the mesh is smaller than 200micrometers the cells don’t enter the mesh and any larger can lead to multiple oocytes or loose cells in each well, hindering injection. The thickness of the mesh is also critical because it must be thick enough that the cell is prevented from being pushed around by the injection needle, yet thin enough that the needle is not blocked (Figure 4). A sample obtained from Seafer Filtration Inc. (#03-200/54, Depew NY) is thick enough (125micrometers) to contain the cell while injecting and thin enough to not hinder injection. The majority of the Nitex we have in the lab is PA 6.6 which has poor light stability and has a strong tendency to absorb moisture and can swell up to 4%, but to date, we have not encountered any difficulties. Dishes should be stored dry and in a drawer and should be replaced periodically if any poor performance is noted. Available screen sizes and technical notes (differences exist in strength, specific gravity, water absorption and resistances to: acid, alkali, light and solvents) can be found at: http://techlist.sefar.com/cms/newtechlistpdf.nsf/vwWebPDFs/openmesh_EN.pdf/$FILE/openmesh_EN.pdf. To prepare the injection dish use high vacuum grease (Dow Corning, Midland MI) and apply a very thin perimeter in a plastic petri dish (60mm #430589, Corning Inc., Corning NY). Place the Nitex square on top and gently press down. A rectangle of approximately 2×3cms can hold a large amount of FSW within the confines of the square with enough volume to resist evaporation (Figure 5).
Figure 4.
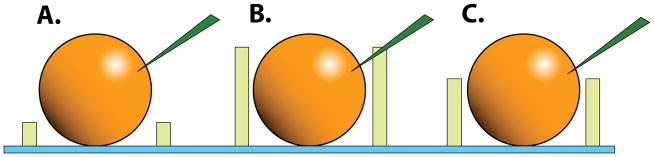
The importance of mesh thickness for efficient injections. A. In a thin mesh, the needle will simply push the egg over the surface of the mesh. B. Mesh that is too thick will prevent the needle from entering the egg; often this will result in pushing the egg through the mesh and trapping it underneath because the user is forced to use a steeper injection angle. C. The optimal thickness for injection is slightly more than half the diameter of the egg. At the optimal angle, the egg will be released from the needle by dragging the needle over the wall of the well.
Figure 5.
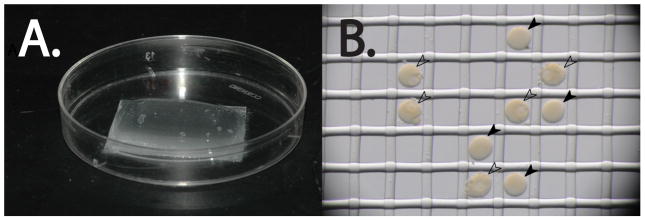
Nitex injection plates. A. The Nitex mesh is affixed to the bottom of the petri dish with vacuum grease. The FSW is confined to a mound of water within the boundaries of the mesh. B. Sea star eggs (black arrowheads) and oocytes (white arrowheads) fit within the mesh. Note the lack of GVs in the eggs when compared to the oocytes.
Injection setup
The needles that we use to inject are pulled glass capillaries with an internal filament, (1×90mm model GD-1, Narishige Scientific, Tokyo Japan). The needles are pulled on a vertical needle puller (model PC-10 Narishige Scientific, Tokyo Japan) in a two step pull, 64.3°C for 14sec, followed by a second pull at 79.8°C. Needles pulled in this manner have an open tip. Prior to loading the needle with the injection solution, the solution should be spun at maximum speed in a microcentrifuge for 10 minutes to pellet any debris that will clog the needle. The needle is loaded by applying a 0.5microL drop of the injection sample to the base of the needle whereupon the glass filament draws the solution down the entire length of the needle by capillary action into the tip of the needle. The needle is then loaded into a holder attached to a pressure injector (FemtoJet Eppendorf, Hamburg Germany) (Figure 6). The holder is attached to a coarse adjustment manipulator (model MMN-1 Narishige Scientific, Tokyo Japan) and a three-axis micromanipulator (model MMO-202ND Narishige Scientific, Tokyo Japan) to control the pitch and location of the needle in three dimensions. With the needle dimensions generated by the pull above, the injection pressure on the FemtoJet is set at 150–300hPa for 0.2–0.3sec with a constant pressure of 100hPa. During the injection, the needle will eventually clog and the time until this event depends on the injection solution that you are using. Often the injection will proceed more smoothly if you break the very tip of the needle by jamming the tip of the needle into the mesh. The non standard needle bore that this generates is why the injection pressure is widely distributed. Absolute volumes of injectate can be estimated based on the size of the bolus of fluid injected into the oocyte. No more than 5% of the oocyte volume should be injected. Quantitative injection methods are instead documented in Jaffe and Teraski (2004) and are important when absolute amounts of fluid needed to be injected into a small number of oocytes. The sample dish is loaded onto the microscope (Axiovert 25, Zeiss, Germany) and we lay a row of oocytes in the dish by use of a mouth pipette.
Figure 6.
Microinjection set up. The injection dish is clamped to the microscope stage with a minimum of water. The coarse adjustment is all that is needed to inject sea star eggs, while the fine adjustment is optional. The Femtojet is controlled by the mouse or by the foot pedal (not shown). To rapidly inject many eggs at a time, it is most efficient to align the needle in the field of view first. Once the pitch and position of the needle is setup, simply change the target by moving the stage control and the actual injection with the coarse z-axis control.
Injection protocol
The injection solution that we find to be ideal contains the following:
50% glycerol 2.0microL (the injection bolos is visible under bright field viewing conditions)
Experimental mix x microL (if injecting RNA, it should be as close to 1 microg/microL as possible)
10mM dextran 0.5microL (the fluorescent dextran allows for the identification of injected eggs)
H20 to 5microL (the water can be substituted for more RNA if necessary; water should be RNAse-free)
Fluorescently labeled dextran is available in a number of wavelengths. The most common ones we use are Texas Red (model D1863 Molecular Probes Invitrogen) and FITC (model D1820 Molecular Probes Invitrogen) for co-injected GFP and mCherry RNA constructs respectively. A shallow needle angle is ideal because a steeper angle can cause the egg to squirt out from underneath the needle. Depending on the stickiness of the eggs a certain percentage will stick to the needle upon injection and not fall off when dragged over the wall of the well. This requires raising the needle to the surface of the water which is why a small volume of water in the dish is ideal (Figure 5). Several hundred eggs can be injected in a single session and once accomplished, mouth pipette the eggs out of the dish by identifying the injected cells under a fluorescence stereo microscope. If there are many eggs you can also use a plastic Pasteur pipette to carefully suck up many at once. A quick rinse with DI water is enough to clean the dish of eggs and be ready for the next batch.
Once the injections are completed, multiple possibilities remain. If oocytes are injected, they can be cultured for up to three days in FSW before maturation and fertilization. In our hands, mRNA injections coding for fluorescent protein in oocytes will express the protein to detectable levels, but the level of expression and the rate of expression is much lower when compared to fertilized eggs (days compared to hours). If the goal of the experiment is to examine effects in embryogenesis, it is often easier to fertilize the eggs and then inject in order to ensure that effort is not wasted on injected eggs that subsequently do not fertilize. Embryos can be cultured in FSW for as long as needed and developmental changes are readily apparent (Supplementary Figure 2).
8. Sensitivity to Plastics and Environmental Exposures
Sea star oocytes are sensitive to contaminants in the holding tank as well as ex-vivo during micromanipulation. We found that any rust in the artificial seawater had a detrimental impact on sea star health, from aging tank components to a stray piece of metal from the lab. Interestingly, abnormalities in sea star oocyte competence were observed in the 2–3 weeks preceding a sudden rise in sea star mortality. Diminishing sea star health could be observed through increasing difficulty inducing spawning in sea stars, requiring higher doses and multiple injections of 1-MA, with diminishing success. Oocytes that were retrieved prior to a “die-off” in the tank were noted to have fragile cell membranes, poor fertilization rates, and abnormal or arrested embryonic development.
Proper handling of oocytes after spawning is also critical to both fertilization and development. We use 50mL polypropylene conical tubes to wash and transport oocytes; however we have observed that tubes manufactured by Corning, with Centristar caps induce activation of the fertilization envelope without exposure to sperm. Oocytes are then difficult to fertilize or micromanipulate. We found much better success with Falcon “Blue Max” conical tubes manufactured by Becton Dickinson. All tubes, glassware and plasticware should be rinsed with distilled water and ASW before coming into contact with oocytes.
9. Micromanipulation- Isolation of the Polar body
We initially hoped to isolate polar bodies from oocytes en masse, however the integrity of the vitelline layer (the oocyte’s extracellular matrix) prevented such approaches. Instead polar bodies were obtained individually using a micromanipulator and sharp dissection. Polar body biopsy can be performed for genetic analysis by suspending oocytes in artificial seawater and overlaying with mineral oil. The overlay prevents evaporation and solute concentration as the water evaporates when performing multiple biopsies. Cells are then stabilized with a holding pipette. Commercial pipettes designed for human oocyte micromanipulation can be purchased (Humagen, Charlottesville VA) and used, however the larger diameter of the sea star oocytes make them better suited for a holding pipette with a larger opening diameter (40–60micrometer). The narrower diameter of human holding pipettes can pinch the ooplasm and make it difficult to stabilize the sea star oocyte.
An angled biopsy pipette with an inner diameter of 20 micrometer (Cook medical, Bloomington, IN) can be used to sharply enter the vitelline layer and aspirate individual polar bodies (Figure 7). The polar body can then be transferred directly from the biopsy pipette into the lid of a 0.2mL PCR tube containing 11microL of reaction buffer. Sea star eggs (and polar bodies) are surrounded by an “egg jelly” outside of the vitelline layer that makes direct transfer of the polar body preferable to placing it on the dish for later removal. Polar bodies otherwise become stuck to the petri-dish, despite prior treatment of dishes with BSA.
Figure 7.
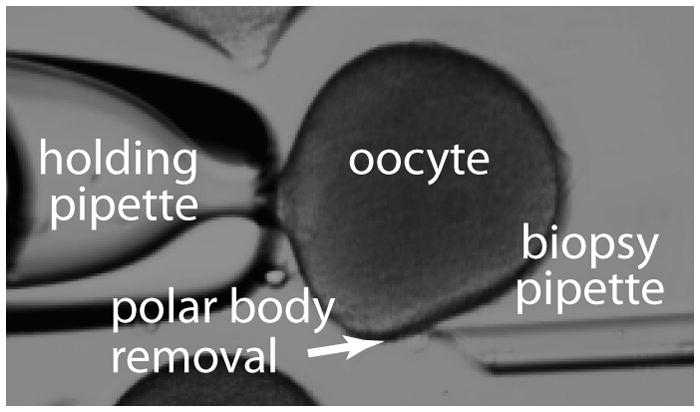
Polar body biopsy of a starfish oocyte.
10. Analysis of Genetic Expression
Isolating RNA from single cell samples is incredibly challenging as transcripts can be easily lost or degraded during the isolation process. When working with RNA, gloves should be worn and working surfaces should be treated with an RNase decontamination solution (RNase Zap, Ambion; Austin, TX) and rinsed with RNAse-free water. Several protocols and commercially available RNA isolation kits exist to isolate RNA from extremely limited quantities of cells, however, when dealing with single cells we have better success by proceeding with cDNA synthesis directly from the cell. This process maintains the specimen in a single tube and avoids specimen loss from incomplete transfer or degradation during RNA isolation. The oocyte or polar body is transferred directly into a reaction buffer and heat or chemically lysed; genomic DNA is degraded with DNase, which is then inactivated so that reverse transcription can follow directly to generate cDNA in the same tube where the cell was first isolated. We have found that cellular debris from such minute specimens does not significantly impact the reaction success.
Several commercial kits are available for single tube cDNA synthesis and we have tested the Superscript III, Cellsdirect cDNA synthesis kit (Invitrogen, Carlsbad, CA) as well as the Cells to Ct kit by Ambion (Austin, TX). Adding random hexamers (1microL at 10nanomolar) to the reaction optimizes the Cellsdirect protocol, which we have found to have the highest efficiency for first round cDNA synthesis. The addition of random hexamers is important when studying transcripts in oocytes as the oocyte has not fully activated the cytoplasmic polyadenylation system and mRNA polyA tails are generally short.
When translating our findings from the sea star to humans, we needed to alter our cDNA synthesis protocol slightly to allow for “pre-amplification” using a commercially available kit and validated primers. One benefit to working with an organism with a known genome is that more candidate genes exist and primers can be optimized for pre-amplification. The PreAMP protocol (Ambion) allows for greater sensitivity by linearly amplifying candidate cDNA transcripts for 10–15 cycles before proceeding with real time PCR. Using single cells, we found the amplification to be both efficient and linear up to 15 cycles. This noticeably increased our sensitivity to detect transcripts in human polar bodies. Therefore, despite the lower efficiency of the first cDNA synthesis step by the Cells to Ct kit (Ambion), the benefit of pre-amplification led us to favor this approach when working with human oocytes.
11. Conclusions
Diversity amongst organisms enables both comparative analysis of function, as well as a marked source of experimental testing. In our lab, sea star oocytes have proven effective for both uses. For an investigator new to the field, working on an organism such as a sea star enables a variety of techniques and protocols to be designed, tested, and mastered. A single starfish purchased for $5 can yield literally millions of oocytes that can be manipulated on the bench top. Following this mastery, such procedures can be modified for use with organisms such as mammals, in which few oocytes are available. Sea stars offer a distinct opportunity to study and master the process of meiosis, fertilization and early development. The genomes of multiple echinoderms have been sequenced, and the transcriptome and genome of the sea star will be approached within the coming year. Once available, the range of molecular, biochemical and function studies will be unlimited.
Supplementary Material
Time lapse video of the raising of the fertilization envelope of fertilized sea star eggs. Upon insemination of the egg, a series of reactions occur including the release of the contents of the cortical granules which results in the raising of the fertilization envelope, seen here. The fertilization envelope is the second of two mechanisms to block polyspermy. The video is 150x speed.
Time lapse video of sea star early development through hatched blastula. Note that it is a trivial matter to obtain large numbers of developmentally synchronized embryos. The video is approximately 3,000x speed depicting 2hpf to 22hpf.
Footnotes
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brusca RC, Brusca GJ. Invertebrates. Sinauer Associates, Inc. Publishers; Sunderland, MA: 1990. [Google Scholar]
- Carroll DJ, Hua W. Combining microinjection and immunoblotting to analyze MAP kinase phosphorylation in single starfish oocytes and eggs. In: Carroll DJ, editor. Microinjection: Methods and Applications. Vol. 518. Human Press; 2009. pp. 57–66. [DOI] [PubMed] [Google Scholar]
- Chia FS, Walker CW. Echinodermata: Asteroidea. In: Giese AC, Pearse JS, Pearse VB, editors. Reproduction of Marine Invertebrates. VI. Boxwood Press; Pacific Grove, CA: 1991. pp. 301–353. [Google Scholar]
- Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sørensen MV, Haddock SH, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- Foltz KR, Adams NL, Runft LL. Echinoderm eggs and embryos: procurement and culture. In: Ettensohn, Wessel, Wray, editors. Development of Sea Urchins, Ascidians and Other Non-Vertebrate Deuterostomes: An Experimental Analysis. Academic Press; 2004. pp. 40–74. [Google Scholar]
- Jaffe LA, Terasaki M. Quantitative microinjection of Oocytes, Eggs, and Embryos. In: Ettensohn, Wessel, Wray, editors. Development of Sea Urchins, Ascidians and Other Non-Vertebrate Deuterostomes: An Experimental Analysis. Academic Press; 2004. pp. 219–243. [Google Scholar]
- Harada K, Oita E, Chiba K. Metaphase I arrest of starfish oocytes induced via the MAP kinase pathway is released by an increase of intracellular pH. Development. 2003;130:4581–4586. doi: 10.1242/dev.00649. [DOI] [PubMed] [Google Scholar]
- Kanatani H, Shirai H, Nakanishi K, Kurokawa T. Isolation and identification of meiosis inducing substance in starfish Asterias amurensis. Nature. 1969;221:273–274. doi: 10.1038/221273a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Cell cycle arrest and release in starfish oocytes and eggs. Seminars in Cell and Developmental Biology. 1998;9:549–557. doi: 10.1006/scdb.1998.0249. [DOI] [PubMed] [Google Scholar]
- Meijer L, Pondaven P, Guerrier P, Moreau M. A starfish oocyte user’s guide. Cahiers de Biologie Marine. 1984;25:457–480. [Google Scholar]
- Sasaki K, Chiba K. Fertilization blocks apoptosis of starfish by inactivation of the MAP kinase pathway. Dev Biol. 2001;237:18–28. doi: 10.1006/dbio.2001.0337. [DOI] [PubMed] [Google Scholar]
- Strathmann MF. Phylum Echinodermata, Class Asteroidea. In: Strathmann MF, editor. Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast. The University of Washington Press; Seattle WA: 1987. pp. 535–555. [Google Scholar]
- Stricker S. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Yuce O, Sadler KC. Postmeiotic unfertilized starfish eggs die by apoptosis. Dev Biol. 2001;237:29–44. doi: 10.1006/dbio.2001.0361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time lapse video of the raising of the fertilization envelope of fertilized sea star eggs. Upon insemination of the egg, a series of reactions occur including the release of the contents of the cortical granules which results in the raising of the fertilization envelope, seen here. The fertilization envelope is the second of two mechanisms to block polyspermy. The video is 150x speed.
Time lapse video of sea star early development through hatched blastula. Note that it is a trivial matter to obtain large numbers of developmentally synchronized embryos. The video is approximately 3,000x speed depicting 2hpf to 22hpf.