Introduction
Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) is a term that encompasses a constellation of abnormalities seen in progressive kidney disease that include altered levels of calcium, phosphorus, parathyroid hormone (PTH), and vitamin D; disturbances in bone modeling and remodeling, with the associated development of fractures or impaired linear bone growth (in children); and extraskeletal calcification in soft tissues and arteries. The kidney is responsible for maintenance of serum calcium and phosphorus within the normal range in people without kidney disease. In CKD stages 2 and 3, compensatory mechanisms in the form of elevated PTH, elevated fibroblast growth factor 23 (FGF-23), and decreased calcitriol result in normal to near-normal blood calcium and phosphorus levels. These compensatory mechanisms become overwhelmed in later stages of CKD, eventually failing and resulting in the group of abnormalities encompassed by CKD-MBD (Box 1).
Box 1. Definition of CKD-MBD.
A systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following:
|
Abbreviations: CKD, chronic kidney disease; CKD-MBD, chronic kidney disease-mineral and bone disorder; PTH, parathyroid hormone.
Reproduced from the KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (Kidney Int 2009;76(suppl 113)), with permission of Nature Publishing Group.
Suggested Reading
- Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006 Jun;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009 Aug;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007 Jan;14(1):3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Moe SM, Sprague SM. Brenner and Rector’s The Kidney. 8. WB Saunders Company; Philadelphia, PA: 2007. Chapter 52: Mineral bone disorders in chronic kidney disease; pp. 1784–1807. [Google Scholar]
- Moe SM. In: Chapter 8: Chronic Kidney Disease, Dialysis, and Transplantation. 3. Himmelfarb J, Sayegh M, editors. Elsevier Saunders; Philadelphia: 2010. pp. 98–114. [Google Scholar]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 Oct;42(4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
Biochemical Abnormalities of CKD-MBD
Phosphorus
Physiological Levels and Dietary Sources
Normal serum phosphorus concentration is 2.5–4.5 mg/dL; total body stores of phosphorus equal 700g
-
Of total body stores, 85% is in bone as hydroxyapatite, 14% intracellular, and 1% extracellular
-
Of the extracellular phosphorus, 70% is within phospholipids (organic), 30% is inorganic
15% of inorganic fraction is 15% protein-bound
85% of inorganic fraction complexed with cations or circulating in free monohydrogen or dihydrogen forms
This 85% is the fraction measured in phosphorus assays, and therefore not a reliable estimate of total body phosphorus, especially in CKD
-
A typical American diet contains ~1000–1400 mg/day of phosphorus; 2/3 excreted in urine, 1/3 in stool
Processed foods and foods rich in animal-based protein are high in phosphorus, thus difficult for patients with CKD to control serum phosphorus by diet alone while also eating the recommended amounts of protein
-
60%–70% dietary phosphorus is absorbed in all intestinal segments
Dependent on luminal concentration
Absorbed via sodium/phosphate cotransporter 2b (Npt2b)
Stimulated by calcitriol
Renal Handling
Inorganic phosphorus is filtered by glomeruli, then 70%–80% gets reabsorbed in proximal tubule via the Npt2a cotransporter
Npt2a is moved to or removed from the brush border to facilitate phosphorus reabsorption or excretion, respectively
20%–30% of filtered phosphorus is reabsorbed in distal tubule
Renal phosphorus excretion is sensitive to serum phosphorus levels; PTH and FGF-23 increase phosphorus excretion
Phosphorus depletion decreases its own excretion
FGF-23
-
Belongs to a group of molecules called phosphatonins
Phosphatonins are hormones that regulate phosphorus excretion
Three phosphatonins have been identified: sFRP-4, MEPE, and FGF-23 (the most studied)
Produced almost exclusively in osteocytes and bone lining cells, but also found in heart, liver, thyroid/parathyroid, intestine, and skeletal muscle
-
FGF-23 receptor on the proximal tubule requires a coreceptor (klotho) for signal transduction
Klotho is found in the distal renal tubule and parathyroid gland
Klotho is downregulated in aging and CKD
-
FGF-23 has the following actions
Downregulates luminal sodium/phosphate cotransporters in the proximal tubule, decreasing phosphorus reabsorption and therefore increasing its excretion
Inhibits 1α-hydroxylase (CYP27B1), decreasing the conversion of 25-hydroxyvitamin D ((25[OH]D) to 1,25-dihydoxyvitamin D (25(OH)2D; calcitriol)
Stimulates 24-hydroxylase (CYP24), leading to vitamin D degradation
Inhibits PTH secretion
FGF-23 gene expression in bone is stimulated by elevated phosphorus, PTH, and calcitriol, even in uremic animals
Local bone proteins also regulate synthesis
-
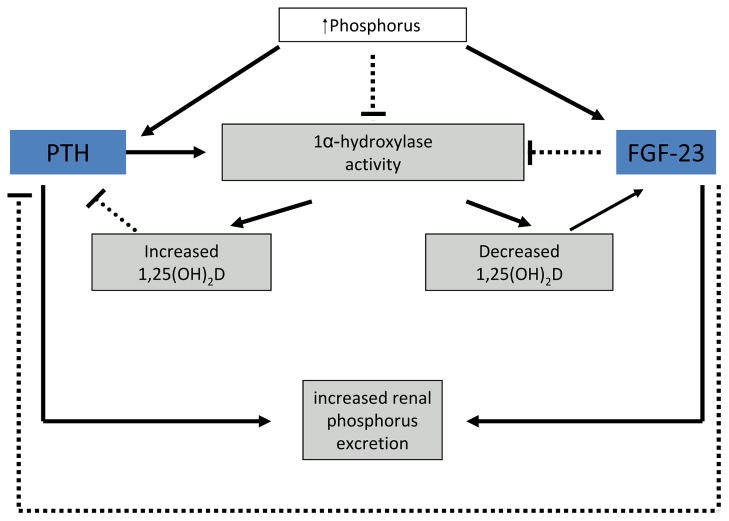
Figure 1 shows the regulation of serum phosphorus levels by PTH and FGF-23
Both FGF-23 and PTH lead to increased excretion of phosphorus
-
Regulatory feedback loops for both PTH and FGF-23 are dependent on calcitriol
PTH increases 1α-hydroxylase and therefore production of calcitriol, which in turn inhibits further PTH release
In contrast, FGF-23 inhibits 1α-hydroxylase and decreases calcitriol production, which will inhibit FGF-23 secretion
Hypocalcemia stimulates PTH, and therefore in low calcium, high phosphorus states the action of PTH predominates
In high calcium, high phosphorus states the action of FGF-23 predominates
Figure 1. Regulation of Serum Phosphorus.
A solid line indicates stimulation; a dashed line indicates inhibition. Adapted with permission of Elsevier from Moe SM, Sprague SM. Mineral bone disorders in chronic kidney disease. In: Brenner and Rector’s The Kidney, 8th ed. Philadelphia, PA: WB Saunders Company; 2007:1784–1807.
Phosphorus, FGF-23, and PTH in CKD
CKD and Phosphorus
Phosphorus homeostatic control is impaired at a glomerular filtration rate (GFR) as high as 60 mL/min (well before frank hyperphosphatemia develops)
As GFR falls below 60 mL/min, there is a gradual increase in serum phosphorus levels
During this period, “normal” phosphorus levels are maintained by continual increases in FGF-23 and PTH levels
Eventually this compensatory mechanism is overwhelmed when GFR decreases below 30 mL/min, and measured serum phosphorus levels may rise above the normal range
Hyperphosphatemia also leads to inhibition of calcitriol synthesis, which stimulates further PTH production; together these processes trigger secondary hyperparathyroidism in CKD to develop
Observational data suggest that hyperphosphatemia is connected to increased morbidity and mortality (all-cause and cardiovascular) in CKD
In different analyses of patients with CKD 5D, the level of phosphorus associated with increased mortality varies from > 5.5 mg/dL to > 7 mg/dL
Even in the non-CKD population, serum phosphorus in high-normal ranges is associated with increased risk of cardiovascular and all-cause mortality
No interventional study has shown that lowering phosphorus to a certain “target” are associated with better outcomes
CKD, FGF-23, and PTH
In early CKD, FGF-23 levels start rising
This coincides with its effects on increasing phosphorus excretion, decreasing calcitriol synthesis (thereby stimulating PTH), and facilitating the development of secondary hyperparathyroidism
In humans, FGF-23 and PTH appear to rise as GFR decreases
Dialysis patients have FGF-23 levels that may be up to 1000 fold greater than in non-CKD populations
In dialysis patients, serum FGF-23 levels are associated with mortality even when adjusted for the serum phosphorus levels and can predict development of secondary hyperparathyroidism and responsiveness to calcitriol therapy
Calcium
Physiological Levels and Dietary Sources
Serum calcium levels are controlled tightly in the 8.5–10.5 mg/dL range
Total body stores are ~1000 g (99% is in bone, 0.9% is intracellular, and 0.1% extracellular)
Extracellular calcium is measured as total calcium: 50% is free (the measured part), 10% is bound to anions, and 40% bound to albumin
Average dietary intake of calcium: 500–1000 mg/day
Calcium absorption occurs across intestinal epithelium via vitamin D-dependent TRPV5 and TRPV6 transporters, as well as paracellular pathways
Bioavailability of calcium from foods is altered by phytate and oxalate
Absorbed calcium enters 3 compartments: blood, soft tissue and bone
Renal Handling
-
Reabsorption
60%–70% is reabsorbed passively in proximal tubule with sodium and water reabsorption
10% is reabsorbed in the thick ascending limb via paracellular route
The remainder is reabsorbed through transcellular pathways in the distal convoluted tubule, the connecting tubule, and the cortical collecting duct via TRPV5 and TRPV6 calcium channels
TRPV6 predominates in the intestine whereas TRPV5 predominates in the kidney
-
Calcium sensing receptor (CaSR)
G-protein coupled protein that binds calcium to sense small changes in ionized calcium; decreased ionized calcium stimulates PTH secretion
CaSR is expressed in parathyroid cells, thyroid C cells, intestine, kidney, and likely bone
-
In the kidney, CaSR is in mesangial cells and throughout the tubules
Activation of CaSR on thick ascending limb decreases paracellular calcium reabsorption
Upregulation of CaSR in hypercalcemia inhibits ADH-induced free water reabsorption, leading to urinary dilution
Renal effects of CaSR are both dependant and independent of PTH
Calcium Abnormalities in CKD
In CKD stages 2–3, serum calcium levels are maintained in “normal” range at the cost of secondary elevations in PTH
Intestinal calcium absorption is impaired in CKD due to decreased calcitriol levels, but still proportional to calcium intake
Urinary calcium excretion decreases as CKD progresses due to PTH associated increased reabsorption and decreased filtered fraction of calcium
In CKD, intestinal absorption is not equal to urinary excretion
In CKD, the ability of bone to take up calcium depends on bone turnover
Patients with lower bone turnover (adynamic bone and mixed uremic osteodystrophy) are less able to take up calcium
When tubular excretion of calcium is decreased, these patients have a net positive calcium balance
Given net positive calcium balance in late CKD, the KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines recommend maximum total elemental intake of calcium of 2 grams a day (1.5 g from phosphate binder + 500 mg dietary calcium)
In patients with adynamic bone in whom calcium may be deposited in extracellular sites instead of being taken up by bone, it may be prudent to avoid calcium-based phosphate binders altogether, though there is no definitive evidence for this
KDIGO (Kidney Disease: Improving Global Outcomes) guidelines also recommend limiting calcium-based phosphate binders in this setting, but no absolute limit is given due to the lack of hard data
-
Observational studies in dialysis patients show an increase in the risk of all-cause mortality with high serum calcium
Levels at which this becomes significant vary in different analyses from >9.5mg/dL to >11.4 mg/dL
There are no studies that have treated patients to different calcium levels to determine mortality benefit
Vitamin D
Sources and Role
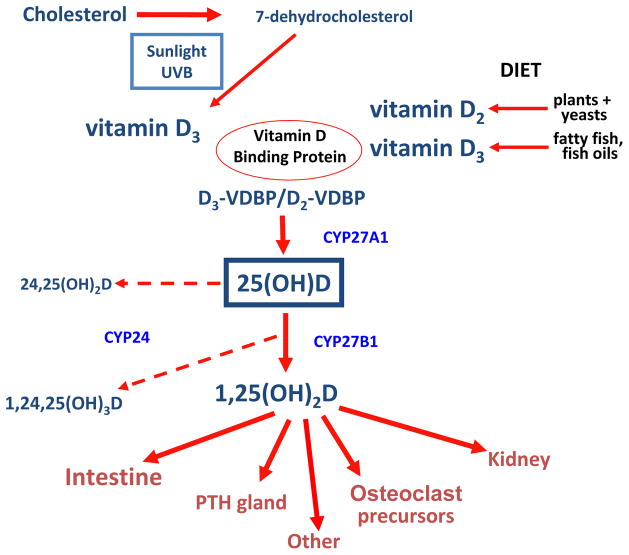
Cholesterol is converted to 7-dehydrocholesterol, which in the presence of sunlight is then converted to Vitamin D3 (cholecalciferol; nomenclature of vitamin D compounds provided in Box 2)
Vitamin D2 (ergocalciferol) is obtained from dietary sources
D2 and D3 are hydroxylated by CYP27A1 in the liver to 25(OH)D2 (ercalcidiol) and 25(OH)D3 (calcidiol), together termed 25(OH)D
Ercalcidiol and calcidiol have a half life of ~3 wk and are the best assessment of Vitamin D intake from sun and food
25(OH)D is converted by 1α-hydroxylase in the kidney to calcitriol (1,25-dihydroxycholecalciferol, or 1,25(OH)2D3) (See Fig 2)
-
1,25(OH)2D3 actions:
Increases TRPV5 and TRPV6, the calcium adenosine triphosphatase, and the sodium/calcium transporters in the intestine and kidney
This increases the absorption of oral calcium and the reabsorption of calcium in the renal tubules
Decreases PTH synthesis by binding to the Vitamin D receptor in the parathyroid gland, inhibiting PTH gene expression, and decreasing PTH cell proliferation
Box 2. Vitamin D Nomenclature Used by the KDIGO Work Group.
| Vitamin D: cholecalciferol and/or ergocalciferol |
| 25-Hydroxyvitamin D: the 25-hydroxylated metabolites of vitamin D; also known as ercalcidiol or calcidiol; abbreviated as 25(OH)D |
| Calcitriol: 1,25-dihydroxycholecalciferol; abbreviated as 1,25(OH)2D3. |
| Vitamin D analogs: derivatives of vitamin D2 and vitamin D3, of which the clinically investigated synthetic derivatives include doxercalciferol, paricalcitol, alfacalcidol, falecalcitriol, and 22-oxacalcitriol (maxacalcitol) |
Reproduced from the KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (Kidney Int 2009;76(suppl 113)), with permission of Nature Publishing Group.
Abbreviation: KDIGO, Kidney Disease: Improving Global Outcomes.
Figure 2. Overview of Vitamin D Metabolism.
Adapted with permission of Elsevier from Moe SM, Sprague SM. Mineral bone disorders in chronic kidney disease. In: Brenner and Rector’s The Kidney, 8th ed. Philadelphia, PA: WB Saunders Company; 2007:1784–1807.
Recommended Levels and Health Effects
In many studies, 25(OH)D deficiency is defined as <10 ng/mL and insufficiency as ≥ 10 ng/mL but < 20–32 ng/mL
-
Institute of Medicine (IOM) published report on vitamin D in 2010
Key conclusion: “While the average total intake of vitamin D is below the median requirement, national surveys show that average blood levels of vitamin D are above the 20 nanograms per milliliter that the IOM committee found to be the level that is needed for good bone health for practically all individuals”
Recommends daily dietary intake of 600 IU/day (800 IU/d for those over 70); maximum daily intake is 4000 IU
-
In vitro, 25(OH)D is thought to have a multitude of effects on the immune system, muscle activity, and endothelial function
Falls, cancers, immune diseases, and mortality in the general population have all been associated with low levels of Vitamin D
In the general population, Vitamin D supplementation may reduce risk of cancers
Nevertheless, the IOM 2010 report notes Vitamin D’s effects outside of bone health are not yet reliably studied and there are not definitive randomized controlled trials
Vitamin D and CKD
In observational studies in CKD, low 25(OH)D has been associated with progression to dialysis, cardiovascular events, and mortality
However, no study has shown a clinical benefit of treating patients with CKD to a specific Vitamin D level
Many patients with CKD have decreased levels of 1,25(OH)2D
Reduced phosphorus excretion leads to a rise in serum phosphorus and in FGF-23, which suppress 1α-hydroxylase activity and thereby decreases 1,25(OH)2D
Lower 1,25(OH)2D decreases intestinal calcium absorption, and the lower serum calcium stimulates PTH release, which restores 1,25(OH)2D levels (providing kidney function still adequate) and increases phosphorus excretion
As CKD progresses these compensatory mechanisms fail (see Fig 1)
-
KDIGO Guideline recommendations
25(OH)D levels should be measured at baseline in patients with CKD and then further testing as needed individualized based on replacement or treatment
Deficiency and insufficiency are to be corrected using treatment strategies recommended for the general population
1,25(OH)2D levels in CKD are variable depending on whether calcitriol or one of its analogs are administered, as paricalcitol can suppress levels
It is not recommended that 1,25(OH)D3 levels be measured routinely
PTH
Physiological Role
PTH is secreted by the parathyroid glands in response to hypocalcemia, hyperphosphatemia and/or calcitriol deficiency
Minute to minute concentrations of PTH are most sensitive to low ionized calcium concentrations
The sensitivity of this response may be blunted in the presence of hyperphosphatemia in CKD
Intact PTH (iPTH)
This 84-amino acid protein is cleaved from pre-pro PTH in the parathyroid gland
iPTH has a short half life (2–4 minutes)
Cleaved into amino-terminal, carboxy-terminal, and mid-length fragments, which are metabolized in the liver and kidney
amino-terminal fragments remain active; carboxy-terminal fragments accumulate in CKD
PTH Assays (see Fig 3)
Figure 3. Assays for parathyroid h ormone (PTH).
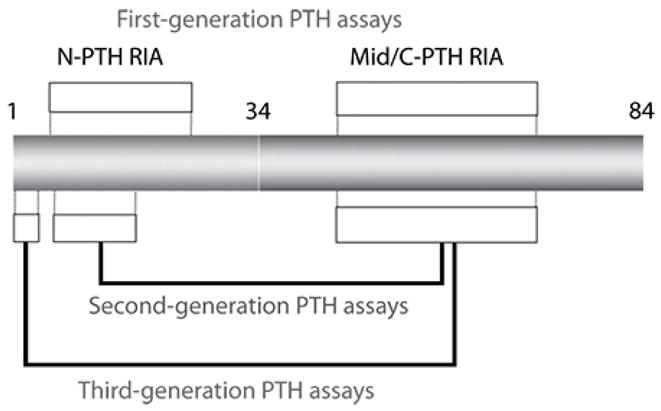
The intact PTH molecule is composed of 84 amino acids; different regions of the protein are targeted by first through third generation assays. Mid/C-PTH, mid/carboxyl terminus of PTH; N-PTH, amino terminus of PTH; RIA, radioimmunoassay. Reproduced with permission of Elsevier from Moe SM, Sprague SM. Mineral bone disorders in chronic kidney disease. In: Brenner and Rector’s The Kidney, 8th ed. Philadelphia, PA: WB Saunders Company; 2007:1784–1807.
-
First-generation assays
Radioimmunoassays using an antibody against the mid-region or carboxy-terminal end
Detects full-length PTH as well as the multiple carboxy- and amino-terminal fragments
Unreliable
-
Second-generation assay/intact PTH assays/two-step first generation immunoradiometric assays (IRMA)
Involve two antibodies, one that detects the amino terminus and the other the carboxy terminus
Most commonly used assay in clinical practice
However, in addition to detecting full-length PTH, it also detects fragments commonly referred to as 7-84 PTH
This 7-84 PTH may have antagonistic effects to full-length PTH on bone
Third generation assays/whole PTH assays/Biointact PTH assays only detect 1-84 PTH
-
Poor correlation between any of the PTH assays and bone histology in CKD
likely because a single time point PTH level may not correlate with bone remodeling, which occurs over several months
Also, significant assay to assay variability exists even in the same individual
Pathophysiology
PTH is significantly associated with mortality in observational studies at levels varying from >400 pg/mL to >600 pg/mL, depending on the population analyzed
There is inconsistent data on the underlying bone histology by biopsy in dialysis patients whose PTH levels were maintained in the 150–300 pg/mL range recommended by KDOQI guidelines
-
Given above issues, recent KDIGO guidelines recommend extremes of risk for PTH at less than 2 times the lower limit and greater than 9 times the upper limit the values of the specific assay used
However, trends of PTH within that range should be evaluated and medications adjusted as needed
Suggested Reading
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004 Aug;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr, Mehrotra R, Navaneethan SD. Vitamin D Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Controlled Trials. Clin J Am Soc Nephrol. 2010 Sep 28; doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida K, Hamano T, Mikami S, et al. Serum 25-hydroxyvitamin D as an independent determinant of 1-84 PTH and bone mineral density in non-diabetic predialysis CKD patients. Bone. 2009 Apr;44(4):678–683. doi: 10.1016/j.bone.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Qazi RA, Martin KJ. Vitamin D in kidney disease: pathophysiology and the utility of treatment. Endocrinol Metab Clin North Am. 2010 Jun;39(2):355–363. doi: 10.1016/j.ecl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008 Aug 7;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010 Sep;21(9):1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006 Jun;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- Moe SM. Confusion on the complexity of calcium balance. Semin Dial. 2010 Sep-Oct;23(5):492–497. doi: 10.1111/j.1525-139x.2010.00771.x. [DOI] [PubMed] [Google Scholar]
- Zidehsarai MP, Moe SM. Review article: Chronic kidney disease-mineral bone disorder: have we got the assays right? Nephrology (Carlton) 2009 Jun;14(4):374–382. doi: 10.1111/j.1440-1797.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- [Accessed on January 14th 2011];Institute of Medicine Report Dietary Reference Intakes for Calcium and Vitamin D. 2010 http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Report-Brief.aspx.
Bone Disease in CKD
Bone Biology
Cancellous bone is present in the epiphyses, and cortical bone in the shafts of long bones
Bone consists of crosslinked type 1 collagen fibers (90%) and proteoglycans, osteopontin, osteocalcin, osteonectin, and other noncollagenous proteins
Cells in bone are cartilage cells, osteoblasts, and osteoclasts
Mesenchymal cells in the bone marrow are differentiated to form osteoprogenitor cells and eventually mature osteoblasts
After bone formation, osteoblasts may undergo apoptosis or become a part of mineralized bone as osteocytes
Osteoclasts are formed from hematopoietic cells, fusing at bone to become multinucleated cells that reabsorb bone using enzymes
At any time, less than 20% of bone surface undergoes remodeling, a process that takes 3–6 months
Phases of bone remodeling are osteoclast resorption, reversal, maturation of osteoblasts, filling of lacunae with osteoid or unmineralized bone, mineralization, and finally a quiescent stage
Bones are chosen to undergo remodeling through the osteoprotegerin (OPG) and the RANK (receptor activator of nuclear factor-κB) system regulated by hormones (PTH, calcitriol, estrogen, glucocorticoids, as well as cytokines and interleukins)
Renal Osteodystrophy
The term is specific to bone pathology in CKD patients and is a component of CKD-MBD
-
Renal osteodystrophy is assessed by doing bone biopsies at the trabecular bone at iliac crest
Patients are given tetracycline 3 weeks and 3–5 days before bone biopsy
Tetracycline binds to hydroxyapatite and labels bone for visualization by fluorescence microscopy
The amount of bone formed between the 2 tetracycline labels is used to calculate bone turnover
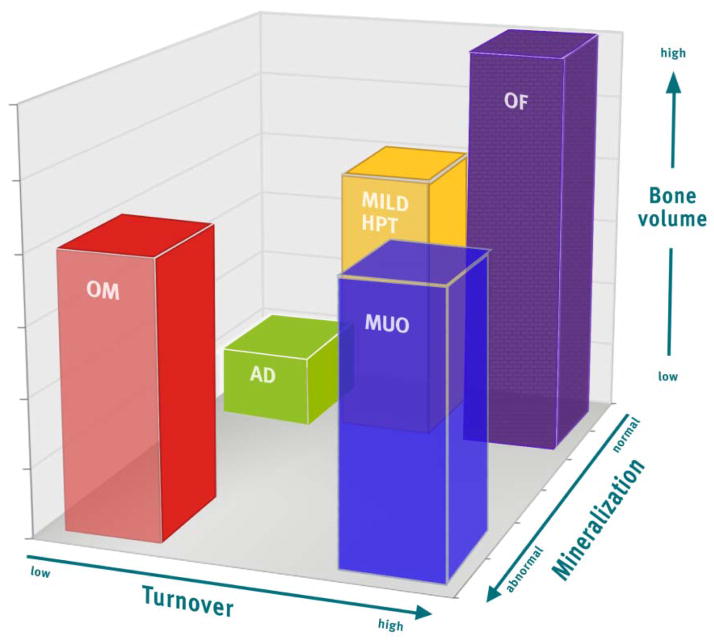
Three key parameters are used to assess bone (turnover, mineralization, volume; TMV system) and replace the terms adynamic bone, mild hyperparathyroidism, osteitis fibrosa, mixed uremic osteodystrophy, and osteomalacia (see Fig 4)
Biomarkers such as PTH and bone alkaline phosphatase are only modestly predictive of underlying bone histology but are the currently best available noninvasive tools for the assessment of renal osteodystrophy
-
Overall, impaired bone quality (altered architecture, remodeling, mass and volume) is seen in CKD
This can be superimposed on pre-existing age related changes in bone such as loss of bone mass due to osteoporosis
This translates to an increased prevalence of fractures in dialysis patients when compared with aged-matched general population
Figure 4. Turnover Mineraliz ation Volume (TMV) Classification System for Bone Histomorphometry.
The TMV system provides more information than the previously used classification scheme. Each axis represents one of the descriptors in the TMV classification: turnover (from low to high), mineralization (from normal to abnormal), and bone volume (from low to high). Individual patient parameters can be plotted on the graph, or means and ranges of grouped data can be shown. For example, many patients with renal osteodystrophy cluster in areas shown by the bars. The red bar (OM, osteomalacia) was previously described as low-turnover bone with abnormal mineralization. The bone volume may be low to medium, depending on the severity and duration of the process and other factors that affect bone. The green bar (AD, adynamic bone disease) was previously described as low-turnover bone with normal mineralization, and the bone volume in this example is at the lower end of the spectrum, but other patients with normal mineralization and low turnover will have normal bone volume. The yellow bar (mild HPT, mild hyperparathyroid-related bone disease) and purple bar (OF, osteitis fibrosa or advanced hyperparathyroid-related bone disease) were previously considered distinct categories, but in actuality represent a range of abnormalities along a continuum of medium to high turnover, and any bone volume depending on the duration of the disease process. Finally, the blue bar (MUO, mixed uremic osteodystrophy) is variably defined internationally. In the present graph, it is depicted as high-turnover, normal bone volume, with abnormal mineralization. In summary, the TMV classification system more precisely describes the range of pathologic abnormalities that can occur in patients with CKD. Reproduced with permission of Nature Publishing Group from Figure 1 in Moe et al. Kidney Int. 2006;69(11):1945–53.
Suggested Reading
- Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium-analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2010 Dec 2; doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SM. Review article: Bone density in patients with chronic kidney disease stages 4–5. Nephrology (Carlton) 2009 Jun;14(4):395–403. doi: 10.1111/j.1440-1797.2009.01159.x. [DOI] [PubMed] [Google Scholar]
- Ott SM. Bone histomorphometry in renal osteodystrophy. Semin Nephrol. 2009 Mar;29(2):122–132. doi: 10.1016/j.semnephrol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Moyses RM, et al. Osteoporosis in hemodialysis patients revisited by bone histomorphometry: a new insight into an old problem. Kidney Int. 2006 May;69(10):1852–1857. doi: 10.1038/sj.ki.5000311. [DOI] [PubMed] [Google Scholar]
- Bakkaloglu SA, Wesseling-Perry K, Pereira RC, et al. Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol. 2010 Oct;5(10):1860–6. doi: 10.2215/CJN.01330210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vascular Calcification in CKD
Background
Extraskeletal calcification is highly prevalent in CKD
Vascular calcification prevalence in dialysis patients ranges from 50%–90% in >20 studies that have addressed this using different modalities, and is even present in children on dialysis
Vascular calcification appears to start early in CKD and > 50% of patients initiated on hemodialysis already have evidence of coronary artery calcification (CAC)
Age and dialysis vintage are consistently associated with CAC
Use of calcium-based phosphate binders and elevated phosphorus are risk factors in some studies
-
Two types of vascular calcification
Intimal calcification leads to calcific plaques or circumferentially calcified atherosclerosis
Medial calcification is nonocclusive and leads to vascular stiffening; it can cause local ischemia and also affect the capacity of the vasculature to dampen increases in arterial pressure with each ventricular systole, leading to left ventricular hypertrophy
Traditionally, the CAC score obtained by electron beam CT is used to quantify calcification burden
Other available techniques can provide semi-quantitative evidence of calcification, including duplex ultrasonography, echocardiography, pulse wave velocity, and even plain X rays
A study of these techniques showed good correlation between lateral abdominal aortic X rays and electron beam CT in quantifying calcification
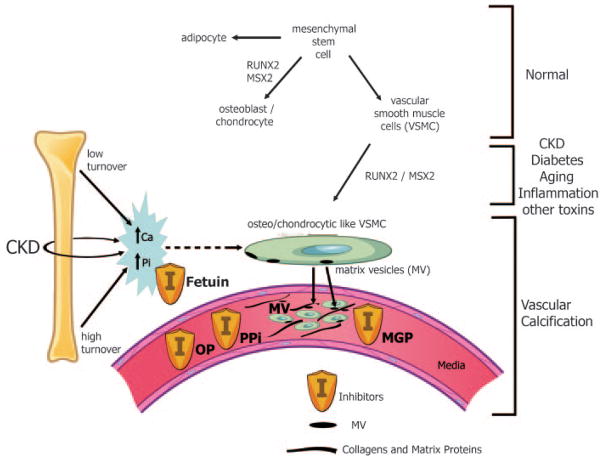
Pathogenesis of vascular calcification (see Fig 5)
Figure 5. Pathogenesis of Vascular Calcification.
Normally, mesenchymal stem cells differentiate to adipocytes, osteoblasts, chondrocytes, and vascular smooth muscle cells (VSMC). In the setting of CKD, diabetes, aging, inflammation, and multiple other toxins, these VSMC can dedifferentiate or transform into osteo/chondrocytic-like cells by upregulation of transcription factors such as RUNX-2 and MSX2. These transcription factors are critical for normal bone development and thus their upregulation in VSMC is indicative of a phenotypic switch. These osteo/chondrocytic-like VSMC then become calcified in a process similar to bone formation. These cells lay down collagen and noncollagenous proteins in the intima or media and incorporate calcium and phosphorus into matrix vesicles to initiate mineralization and further mineralize into hydroxyapatite. The overall positive calcium and phosphorus balance of most dialysis patients feeds both the cellular transformation and the generation of matrix vesicles. In addition, the extremes of bone turnover in chronic kidney disease (low and high or adynamic and hyperparathyroid bone, respectively) will increase the available calcium and phosphorus by altering the bone content of these minerals. Ultimately, whether an artery calcifies or not depends on the strength of the army of inhibitors (I) standing by in the circulation (fetuin A) and in the arteries (PPI = pyrophosphate, MGP = matrix Gla protein, and OP = osteopontin as examples). Reproduced with permission of the American Society of Nephrology from Figure 1 in Moe et al. J Am Soc Nephrol. 2008;19:213–216.
-
Features a phenotypic switch in which vascular smooth muscle cells (VSMC) dedifferentiate to osteo/chondrocytic-like cells
Switch associated with upregulation of transcription factors such as RUNX-2 and MSX-2
The most important stimulus appears to be hyperphosphatemia, but other uremic factors such as inflammation, cytokines, oxidative stress, and advanced glycation end products can also enhance this transformation
osteo/chondrocytic-like cells lay down collagen and noncollagenous proteins (extracellular matrix) in the intima or media
Calcium and phosphorus are incorporated into matrix vesicles to initiate mineralization in the form of hydroxyapatite
-
When the balance favors promineralizing factors (eg, elevations in calcium and phosphorus) over inhibitors of calcification (eg, fetuin A, matrix GLA protein, osteopontin, pyrophosphate), calcification occurs
Levels of calcium and phosphorus are influenced by the bone status in a particular individual; the extent of bone turnover alters the release of these minerals from bone
CKD patients who have low turnover bone disease appear to have the greatest risk of vascular calcification
It is likely that adynamic bone is not able to take up a high calcium loads and this excess calcium may become deposited in the vasculature
Observational studies have shown increased CAC and valvular calcification to be associated with increased mortality in patients with CKD
Calcification of large peripheral arteries is also associated with increased pulse wave velocity, increased pressure, and increased mortality
Calciphylaxis
Also called “calcific uremic arteriolopathy”; type of soft tissue/medial calcification in small skin arterioles, leading to tissue ischemia and ulceration
Debilitating, with mortality rates as high as 80%
Risk factors: hyperphosphatemia, obesity, female gender, dialysis vintage, warfarin use, and hypoalbuminemia
-
Potential treatments: parathyroidectomy, cessation of calcium-containing phosphate binders, frequent dialysis, hyperbaric oxygen therapy, use of bisphosphonates or calcimimetics, and use of sodium thiosulfate
No randomized trials have been performed for any of the potential treatments
A recent case series of 6 patients treated with sodium thiosulfate showed that the 2 responders who survived at 1 year of follow-up improved with respect to pain, wound size, and imaging
A systematic review of sodium thiosulfate (in press) in 14 dialysis patients showed decreased pain and improvement in skin lesions, though mortality rate remained unchanged at 70%
Suggested Reading
- Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: Emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost. 2010 Sep;104(3):464–470. doi: 10.1160/TH09-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008 Feb;19(2):213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010 Oct;21(10):1637–40. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: Emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost. 2010 Sep;104(3):464–70. doi: 10.1160/TH09-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009 May;75(9):890–897. doi: 10.1038/ki.2008.644. Epub 2009 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler M, Biggar PH. Review article: Getting the balance right: assessing causes and extent of vascular calcification in chronic kidney disease. Nephrology (Carlton) 2009 Jun;14(4):389–394. doi: 10.1111/j.1440-1797.2009.01149.x. [DOI] [PubMed] [Google Scholar]
- Jahnen-Dechent W, Schäfer C, Ketteler M, et al. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med. 2008 Apr;86(4):379–389. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010 Dec;6(12):723–735. doi: 10.1038/nrneph.2010.143. [DOI] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Liabeuf S, et al. Effects of uremic toxins on vascular and bone remodeling. Semin Dial. 2009 Jul-Aug;22(4):433–437. doi: 10.1111/j.1525-139X.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shanahan CM. Signalling pathways and vascular calcification. Front Biosci. 2011 Jan 1;16:1302–1314. doi: 10.2741/3790. [DOI] [PubMed] [Google Scholar]
- Shao JS, Cheng SL, Sadhu J, et al. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010 Mar;55(3):579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood AR, Wazny LD, Raymond CB, et al. Sodium thiosulfate-based treatment in calcific uremic arteriolopathy: a consecutive case series. Clin Nephrology. 2011 Jan;75(1):8–15. [PubMed] [Google Scholar]
- Noureddine L, Landis M, Patel N, et al. Efficacy of sodium thiosulfate for the treatment for calciphylaxis. Clin Nephrology. doi: 10.5414/cnp75485. (accepted for publication) [DOI] [PubMed] [Google Scholar]
CKD-MBD
Definition
Patients with CKD have biochemical abnormalities of calcium, phosphorus, Vitamin D, and PTH; bone changes associated with these abnormalities; and extraskeletal calcification
These 3 interrelated processes account for morbidity and mortality in CKD and are together called CKD-MBD
Management
KDOQI bone and mineral guidelines were published in 2003 and based largely on opinion of the work group members due to lack of strong evidence in the field
-
KDIGO guidelines were published in 2009 after a rigorous evidence review process based on the internationally used GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria
There was a lack of high quality evidence (RCTs) for patient-level outcomes for treatments
Hence, majority of guideline recommendations were weak in strength (see Table 1)
Table 1.
Recommendations for Ranges of Mineral Metabolism Parameters in CKD
| CKD Stage 3 | CKD Stage 4 | CKD Stage 5D | |
|---|---|---|---|
| Phosphorus | Maintain in “normal” range (2C) | Maintain in “normal” range (2C) | Lower towards the normal range (2C) |
| Calcium | Maintain in “normal” range (2C) | Maintain in “normal” range (2C) | Maintain in “normal” range (2C) |
| Intact PTH | Ideal level unknown | Ideal level unknown | Maintain within >2 and <9x the upper limit of normal (if there is a trend changing within that range, adjust prescription) (2C) |
Note: Grades are given in brackets (number refers to strength of recommendation, where level 1 is strong and level 2 is weak; letter refers to quality of evidence, where A is high, B is moderate, C is low and D is very low).
Based on the KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (Kidney Int 2009;76(suppl 113)).
Abbreviations: PTH, parathyroid hormone; CKD stage 5D, dialysis-dependent chronic kidney disease stage 5; CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes.
Suggested Reading
- Fadem SZ, Moe SM. Management of chronic kidney disease mineral-bone disorder. Adv Chronic Kidney Dis. 2007 Jan;14(1):44–53. doi: 10.1053/j.ackd.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Sprague SM. Renal bone disease. Curr Opin Endocrinol Diabetes Obes. 2010 Dec;17(6):535–539. doi: 10.1097/MED.0b013e3283400945. [DOI] [PubMed] [Google Scholar]
- Gal-Moscovici A, Sprague SM. Use of vitamin D in chronic kidney disease patients. Kidney Int. 2010 Jul;78(2):146–151. doi: 10.1038/ki.2010.113. [DOI] [PubMed] [Google Scholar]
- Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, Sprague SM, Goldfarb S. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD) Am J Kidney Dis. 2010 May;55(5):773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
Control of Hyperphosphatemia
Hyperphosphatemia is associated with poor cardiovascular outcomes, mortality, secondary hyperparathyroidism, and extra-skeletal calcification. Though the benefits of treating to certain target phosphorus levels have not been proven in RCTs, the KDIGO guidelines suggested that it is reasonable to treat hyperphosphatemia in patients with CKD (see Table 1). Currently available modalities for normalizing phosphorus include restriction of dietary phosphorus, phosphorus binders, and attempts to increase phosphorus removal in dialysis.
Diet
Dietary phosphorus restriction to 800–1000 mg/day recommended
Difficult to maintain this and consume adequate protein, since most foods high in protein tend to be high in phosphorus
-
Foods with a low phosphorus to protein ratio need to be encouraged and formal dietary counseling may be required to achieve this
Plant-based foods tend be low in their phosphorus to protein ratio
Additionally, phosphorus in plant-based foods is bound to phytate and may be less bioavailable since humans lack enzymes required to break the phosphorus-phytate bond
Preservatives present in many fast foods and processed foods tend to be high in phosphorus
Currently the US FDA does not mandate the reporting of phosphorus content on food labels, making it challenging to counsel patients
Phosphate Binders
Background
Dietary restriction is often insufficient to control elevated phosphorus in CKD; the next step includes the use of phosphate binders
An ideal binder should be minimally absorbed in the gut, have no side effects, and be effective in binding phosphorus at the lowest dose
Use of aluminum-based binders is now minimized in CKD due to evidence showing their toxicity in the form of osteomalacia, anemia, and dialysis encephalopathy
Magnesium carbonate and hydroxide have not been studied well, but there exists the risk of magnesium toxicity in patients with CKD; currently not widely used or recommended due to lack of long-term studies
Types of phosphorus binders in common use include calcium-based binders (calcium carbonate or acetate), anion-exchange resins (eg, sevalemer hydrochloride and sevelamer carbonate), and lanthanum carbonate; other binders are in development
Calcium-Based Binders
Commonly used forms are calcium acetate (25% elemental calcium: 169 mg of calcium per 667 mg capsule) and calcium carbonate (40% elemental calcium: 200 mg elemental calcium per 500 mg calcium carbonate)
No studies have examined calcium-based binders versus placebo or compared the two forms of calcium-based binders with extra skeletal calcification or with patient-centered outcomes such as mortality, fractures, hospitalizations etc
Both formulations have the potential to cause hypercalcemia as a side effect, but a metaanalysis showed calcium acetate may be less likely to do so
Gastrointestinal intolerance, notably constipation, may be a limiting side effect
Non-Calcium-Based Binders
-
Sevelamer
Previously formulated as sevelamer hydrochloride but now marketed as sevelamer carbonate
Side effects include GI intolerance
May also decrease LDL levels
Most trials were performed using the hydrochloride salt
-
Lanthanum Carbonate
Chewable; poorly, although not incompletely, absorbed and is cleared primarily by the liver
Initial concerns included toxicity similar to that of aluminum, however no liver toxicity, changes in cognition, or bone marrow suppression have been noted in human studies
No increased risk of osteomalacia have been noted in human studies
Calcium- versus non-calcium based binders
-
Two studies have examined the effect of calcium-based binders versus Sevalemer on mortality:
-
DCOR (Dialysis Clinical Outcomes Revisited) Study
2103 prevalent HD patients randomized to sevalamer or a calcium based binder (70% acetate and 30% carbonate forms)
Primary outcome of all-cause mortality or cause-specific mortality was not different between the two arms
However there was a significant drop out rate of about 50% in both arms, with only 1068 patients completing the study
When dialysis records were used to determine end points, a subgroup analysis of subjects > 65 years did show a survival advantage for sevelamer
However, another analysis that used Medicare claims to determine end points did not show mortality benefit in this group
The analysis of Medicare claims also showed all-cause hospitalizations were lower for the sevelamer subjects
-
RIND (Renagel in New Dialysis) study
Randomized 148 incident HD patients to sevelamer hydrochloride or calcium based binder
Showed an adjusted increased mortality in the calcium-based binder arm (HR, 3.1; P=0.016)
-
Therefore it is unclear at this time whether there is a mortality benefit of sevalamer compared with calcium based binders
There are no studies comparing the effect of calcium based binders versus Lanthanum or any of the other non-calcium, non-Sevelamer based binders with patient-centered outcomes
-
There is inconsistent data on the beneficial effect of sevelamer as compared with calcium based binders on vascular calcification, as shown in the following RCTs
A study of low phosphorus diet vs low phosphorus diet plus calcium carbonate vs low phosphorus diet plus sevalamer in 90 predialysis patients showed no progession of calcification in the diet plus sevalamer group though calcification progressed in the other 2 groups
TTG (Treat to Goal) study assessed the progression of calcification in 200 HD subjects randomized to sevalemer or calcium based binders; showed absolute increases in the CAC score in the calcium treated arm but not in the sevelamer arm
RIND study also showed a significant increase in calcification in the calcium based binder arm at 18 months compared with sevelamer
CARE-2 (Calcium Acetate Renagel Comparison) Study of long-term dialysis patients in the United States randomized to calcium acetate plus atorvastatin vs sevalamer plus a statin if needed to achieve LDL to 70 showed no difference in the progression of arterial calcification and similar lipid profiles in both arms
BRIC (Bone Remodeling and Coronary Calcification) study (calcium acetate versus sevalemer in 101 Brazilian dialysis patients) showed that the annual rate of CAC progression was not different between calcium based binders and sevalamer; however, this study allowed multiple medication and dialysate calcium changes based on baseline bone biopsy studies and thus was subject to considerable bias
-
Effect on bone of calcium based binders versus Sevelamer
BRIC study showed no significant changes in the two arms in turnover mineralization or bone volume
Another RCT of 119 HD patients randomized to sevalamer or calcium carbonate showed no changes in mineralization or volume at 1 year, but did show an increase in bone turnover in the sevelamer arm
-
Effect on bone of calcium based binders versus Lanthanum
RCT of 1 year of treatment favored Lanthanum carbonate over calcium based binders
RCT of 65 patients showed an improvement in turnover and volume, but worsened mineralization with Lanthanum arm
RCT of 20 subjects showed that no patients receiving lanthanum developed low turnover compared with 3 patients developing low turnover bone in the calcium arm
Therefore, bone changes in response to binder therapy are not consistent and are dependent on the individual patient and their initial bone status
-
In summary, there are limited data to suggest the use of one type of binder over another; however, in the presence of arterial calcification or adynamic bone disease, it is prudent to restrict the dose of calcium based binders till more conclusive data is available
In the KDOQI guidelines, maximal dose of elemental calcium was recommended at 1500 mg/day, with total calcium intake from diet plus binders recommended not to exceed 2000 mg/day
KDIGO guidelines recommend avoidance of calcium-based binders if there is arterial calcification, the PTH is persistently low, or in persistent or recurrent hypercalcemia; no daily ceiling was given due to the lack of balance studies
Clearance of Phosphorus in Dialysis
Patients receiving nocturnal hemodialysis (HD) remove twice the amount of phosphorus per week compared with those on thrice-weekly intermittent HD
Intermittent HD removes 1000 mg of phosphorus per session and because 1000 mg is also absorbed each day, net phosphorus balance is about 4000 mg per week
-
An RCT of 51 patients randomized to 6-times weekly nocturnal HD versus thrice weekly intermittent HD demonstrated significant and sustained decreases in serum phosphorus over a 6 month period
Also noted were a significant rate of discontinuation or lowering of phosphorus binder dose in the nocturnal hemodialysis group
No significant difference in PTH between groups
A frequent dialysis study that randomized 245 patients to daily versus thrice-weekly dialysis found a reduction in serum phosphorus levels in the frequent HD group (P=0.002)
With increasing popularity of nonconventional HD modalities, increased clearance of phosphorus by this route might complement diet and binder therapies; further studies are needed to provide more evidence for this
Suggested Reading
- Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002 Jul;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007 Nov;72(9):1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- St Peter WL, Liu J, Weinhandl E, Fan Q. A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: a secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis. 2008 Mar;51(3):445–454. doi: 10.1053/j.ajkd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005 Oct;68(4):1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007 Mar;71(5):438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- Russo D, Miranda I, Ruocco C, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007 Nov;72(10):1255–1261. doi: 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- Barreto DV, de Barreto FC, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification--results from the BRiC study. Nephron Clin Pract. 2008;110(4):c273–283. doi: 10.1159/000170783. [DOI] [PubMed] [Google Scholar]
- Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008 Jun;51(6):952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Frazao JM, Monier-Faugere MC, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008 Feb;19(2):405–412. doi: 10.1681/ASN.2006101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Haese PC, Spasovski GB, Sikole A, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl. 2003 Jun;(85):S73–S78. doi: 10.1046/j.1523-1755.63.s85.18.x. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Siami C, Swanepoel, et al. Improvements in renal osteodystrophy in patients treated with lanthanum carbonate for two years. Clin Nephrol. 2008 Oct;70(4):284–295. [PubMed] [Google Scholar]
- Freemont AJ, Hoyland JA, Denton J, et al. The effects of lanthanum carbonate and calcium carbonate on bone abnormalities in patients with end-stage renal disease. Clin Nephrol. 2005 Dec;64(6):428–437. [PubMed] [Google Scholar]
- Pierratos A. Daily (quotidian) nocturnal home hemodialysis: nine years later. Hemodial Int. 2004 Jan 1;8(1):45–50. doi: 10.1111/j.1492-7535.2004.00074.x. [DOI] [PubMed] [Google Scholar]
- Walsh M, Manns BJ, Klarenbach S, Tonelli M, Hemmelgarn B, Culleton B. The effects of nocturnal compared with conventional hemodialysis on mineral metabolism: A randomized-controlled trial. Hemodial Int. 2010 Apr;14(2):174–181. doi: 10.1111/j.1542-4758.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010 Dec 9;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Control of PTH
Rationale
Observational studies in CKD stages 3–5D demonstrate an association between PTH levels at extremes (less than 2 and greater than 9 times the normal assay limits) and mortality
Similar to phosphorus, there are no mortality studies that have randomized patients to different PTH cut-offs
An ideal PTH level would be correlated with a normal bone formation rate, but current assays of PTH are poorly correlated with bone formation rates
Therefore, KDIGO guidelines recommend “maintaining iPTH in the range of approximately 2–9 times limits for the assay”
Marked changes in PTH within that range should also be treated
The measures available for this include oral calcium, vitamin D, calcitriol, 1,25(OH)D2 analogs, calcimimetics, and parathyroidectomy
Treatment of Elevated PTH in CKD 3 and 4
PTH increases as an adaptive response to hyperphosphatemia; in individual patients this becomes maladaptive at a certain point and treatments for elevated PTH should be individualized and based on trends
KDIGO guidelines recommend correcting modifiable factors: treating hypocalcemia, elevated phosphorus, and vitamin D deficiency to attempt to reverse progressive hyperparathyroidism, however, there is a paucity of evidence to support this at the present time
Oral calcium has been used to suppress PTH in CKD 3–4, however its effects on arterial calcification are unclear
Treating hyperphosphatemia to lower PTH seems important physiologically but again has not been well studied
One 8-week trial of Lanthanum carbonate versus placebo in CKD 3–4 found a reduction in PTH
25(OH)D likely lowers but may not normalize PTH in CKD 3–5
Use of 25(OH)D to suppress PTH was studied retrospectively (meta-analysis) in 322 patients with CKD and found to lower PTH levels when given in conjunction with calcium
In CKD 3 and 4 patients with low 25(OH)D levels (< 30ng/ml) using ergocalciferol showed significant reductions in PTH in CKD3, however an RCT of 20 patients with CKD showed no significant effect of 25(OH)D therapy on PTH
KDIGO guidelines recommend that if PTH levels continue to rise in CKD 3–4, calcitriol or other vitamin D analogs may be used to suppress PTH
-
Role of Vitamin D analogs in treating elevated PTH in non-dialysis dependent CKD patients
Four placebo controlled RCTs of various vitamin D analogs (doxercalciferol, paricalcitol, alfacalcidol, calcitriol) all showed efficacy for PTH lowering as compared with placebo
No RCTs using Vitamin D analogs in pre-HD CKD address patient-level outcomes (mortality, hospitalizations, fractures, parathyroidectomy, quality of life) or vascular calcification
Two studies have shown improvement in bone turnover with Vitamin D analogs compared with the placebo
Observational studies have shown a lower risk of progression to ESRD and death in CKD stage 3–4 patients on a Vitamin D analog although no prospective studies have examined this
Theoretically it may be beneficial to correct both Vitamin D deficiency and calcitriol deficiency, but no studies have been performed to assess this
Calcimimetics also lower PTH as compared with placebo in CKD 3–4 but with a significant risk of hyperphosphatemia
Given this risk, further studies need to performed before this can be recommended
Suggested Reading
- Sprague SM, Coyne D. Control of secondary hyperparathyroidism by vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol. 2010 Mar;5(3):512–518. doi: 10.2215/CJN.03850609. [DOI] [PubMed] [Google Scholar]
- Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol. 2009 Jan;4(1):178–185. doi: 10.2215/CJN.02830608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis. 2009 Mar;53(3):408–416. doi: 10.1053/j.ajkd.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27(1):36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- Coburn JW, Maung HM, Elangovan L, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004 May;43(5):877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Coyne D, Acharya M, Qiu P, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006 Feb;47(2):263–276. doi: 10.1053/j.ajkd.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995 Feb 11;310(6976):358–363. doi: 10.1136/bmj.310.6976.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordal KP, Dahl E. Low dose calcitriol versus placebo in patients with predialysis chronic renal failure. J Clin Endocrinol Metab. 1988 Nov;67(5):929–936. doi: 10.1210/jcem-67-5-929. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008 Feb 25;168(4):397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- Shoben AB, Rudser KD, de Boer IH, et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008 Aug;19(8):1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calcitriol and Vitamin D analogs for Treating Elevated PTH in CKD-5D
Vitamin D analogs and calcitriol are traditionally used for their PTH-lowering effects and are effective in patients receiving dialysis
Retrospective data from multiple analyses show survival benefits in patients receiving any vitamin D analog
One study demonstrated survival benefit of paricalcitol compared with calcitriol, but another study showed no benefit of either paricalcitol or doxercalciferol over calcitriol
These studies are all retrospective and have not been confirmed in prospective analyses
Paricalcitol was observed to lead to less sustained hypercalemia than calcitriol in a secondary analysis of an RCT, though there was no difference in the number of subjects who had one episode of hypercalcemia
No head-to-head comparison of doxercalciferol, paricalcitol, or calcitriol have evaluated vascular calcification or patient-related endpoints
Therefore the KDIGO guidelines do not recommend one vitamin D analog over another or over calcitriol at this point
Suggested Reading
- Sprague SM, Coyne D. Control of secondary hyperparathyroidism by vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol. 2010 Mar;5(3):512–518. doi: 10.2215/CJN.03850609. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003 Jul 31;349(5):446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006 Nov;70(10):1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- Moe SM. Vitamin D, cardiovascular disease, and survival in dialysis patients. J Bone Miner Res. 2007 Dec;22( Suppl 2):V95–99. doi: 10.1359/jbmr.07s218. [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005 Apr;16(4):1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003 Apr;63(4):1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
Calcimimetics for Treating Elevated PTH in CKD-5D
-
Calcimimetics are allosteric activators of the extracellular CaSR, sensitizing the parathyroid gland to extracellular calcium
This decreases PTH release from the parathyroid
These actions are independent of vitamin D
Cinacalcet is the only FDA approved calcimimetic in the United States
RCTs have shown suppression of PTH, calcium, phosphorus, and calcium-phosphorus product
Retrospective analyses of pooled data of 1100 subjects from Phase 3 RCTs of Cinacalcet showed reductions in the risk of parathyroidectomy, fracture, cardiovascular hospitalization, and quality of life
An observational study found a significant survival benefit associated with cinacalcet use in dialysis patients receiving vitamin D analogs
ADVANCE (A Randomized Study to Evaluate the Effects of Cinacalcet plus Low-Dose Vitamin D on Vascular Calcification in Subjects with Chronic Kidney Disease Receiving Hemodialysis Study) showed no reduction in CAC in the cinacalcet/low-dose paricalcitol arm vs the flexible dose of Vitamin D analogs arm when analyzed by the Agatston method, but did show a reduction using the volumetric method
-
RCTs are needed on the effects of calcimimetics on patient-related outcomes and bone histology
EVOLVE (Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events), a global, phase 3, double-blind, randomized, placebo-controlled trial of 4000 subjects is examining impact of cinacalcet on mortality and cardiovascular events in hemodialysis patients with secondary hyperparathyroidism; EVOLVE is ongoing with results anticipated in late 2012 or 2013
KDIGO guidelines recommend that calcitriol, vitamin D analogs, or calcimimetics can be used in CKD 5D to lower PTH; the choice is dependent on serum calcium and phosphorus levels
Suggested Reading
- Brown EM. Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (CaSR) Biochem Pharmacol. 2010 Aug 1;80(3):297–307. doi: 10.1016/j.bcp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004 Apr 8;350(15):1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005 Oct;68(4):1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010 Sep;78(6):578–589. doi: 10.1038/ki.2010.167. [DOI] [PubMed] [Google Scholar]
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2010 Dec 8; doi: 10.1093/ndt/gfq725. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Parathyroidectomy for Treating Elevated PTH in CKD-5D
Parathyroidectomy is effective in optimizing PTH control and has been traditionally offered to patients with sustained PTH > 1000 pg/mL
Advantages are the lack of adverse effects from continuous Vitamin D analog or cinacalcet therapy
It would be difficult to perform a RCT of vitamin D/cinacalcet vs parathyroidectomy, and to date no such study has been performed
A retrospective analysis of USRDS data showed a lower mortality risk in patients who underwent parathyroidectomy
Current KDIGO guidelines recommend no specific level of PTH for which parathyroidectomy would be an absolute indication
Suggested Reading
- Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004 Nov;66(5):2010–2016. doi: 10.1111/j.1523-1755.2004.00972.x. [DOI] [PubMed] [Google Scholar]
Conclusion
CKD-MBD includes a constellation of biochemical and hormone abnormalities, impaired bone architecture, growth and fragility, and extraskeletal calcification. Management of CKD-MBD is important to decrease morbidity and mortality in CKD. This requires an integrated approach and an understanding of physiology since all three components are interrelated and affecting one typically affects the others. Studies focused on combination therapy to improve all aspects of CKD-MBD simultaneously will be the challenge of the future.
Acknowledgments
Support: None
Footnotes
Financial Disclosure: Dr Moe is a consultant and received honoraria and/or funding from Genzyme, Amgen, Shire, Litholink. Dr Moorthi declares that she has no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.