Abstract
Antigen processing by intracellular proteases and peptidases and epitope presentation are critical for recognition of pathogen-infected cells by CD8+ T lymphocytes. First generation HIV protease inhibitors (PIs) alter proteasome activity, but the effect of first or second generation PIs on other cellular peptidases, the underlying mechanism and impact on antigen processing and epitope presentation to CTL are still unknown. Here we demonstrate that several HIV PIs altered not only proteasome but also aminopeptidase activities in PBMC. Using an in vitro degradation assay involving PBMC cytosolic extracts we showed that PIs altered the degradation patterns of oligopeptides and peptide production in a sequence-specific manner, enhancing the cleavage of certain residues and reducing others’. PIs affected the sensitivity of peptides to intracellular degradation, altered the kinetics and amount of HIV epitopes produced intracellularly. Accordingly the endogenous degradation of incoming virions in the presence of PIs led to variations in CTL-mediated killing of HIV-infected cells. By altering host protease activities and the degradation patterns of proteins in a sequence-specific manner, HIV PIs may diversify peptides available for MHC-I presentation to CTL, alter the patters of CTL responses, and may provide a complementary approach to current therapies for the CTL-mediated clearance of abnormal cells in infection, cancer or other immune disease.
Introduction
Highly active antiretroviral therapy (HAART) which is a combination of nucleoside reverse transcriptase inhibitors (NRTIs), non NRTIs, protease inhibitors (PIs) and integrase inhibitors given to HIV-infected patients efficiently suppresses HIV replication leading to partial immune restoration and turning the AIDS into a chronic disease (1).
HIV PIs by blocking the HIV aspartyl protease prevent the cleavage of HIV Gag and Pol polyproteins that include essential structural and enzymatic components of the virus. This blockage prevents the conversion of HIV particles into their mature infectious form (2). Currently 9 different HIV PIs are available on the market and used in HAART (3). Long-term treatment of responder patients with PI-containing HAART has been linked with several unpredicted side effects, such as hyperbilirubinaemia, hyper- or hypo-lipidemia (4), body fat redistribution (5), insulin resistance (6), osteopenia and osteoporosis (7, 8), more so with first generation PIs such as Saquinavir or Ritonavir than with newer PI such as Darunavir (9). The design of the first generation HIV PIs is based on the transition state mimetic of the Phe-Pro bond, the major substrate of HIV-I protease (10). Evidence showing the ability of the 20S proteasome to cleave similar bonds (11) raised questions about possible interactions between HIV PI and the proteasome catalytic sites.
Proteasomes play a key role in the degradation of full-length proteins and defective ribosomal products into peptides (12) that can be further shortened or degraded by cytosolic aminopeptidases and endopeptidases such as thimet oligopeptidase (TOP) (13, 14) or tri-peptidyl peptidase II (TPPII) (15). Some of these peptides are translocated by the transporter associated with antigen processing (TAP) complex into the ER, where they can be further trimmed by ER-resident aminopeptidases (ERAP1 or ERAP2) (16, 17) and, provided they contain appropriate anchor residues, loaded onto MHC-I and displayed at the cell surface..
Epitopes can be solely produced by the proteasome or a combination of proteasomes and aminopeptidases and/or endopeptidases although the sequence of degradation events leading to epitope production is poorly defined. Peptides produced during protein degradation can be subjected to hydrolysis by various peptidases, thus limiting the amount of peptides available for MHC-I presentation (18–20). The specificity of each peptidase is determined by length and motifs in the substrate. Proteasomes have the broadest cleavage capacity and often define the C-terminus of extended epitopes due to frequent cleavages after hydrophobic residues (21).
Aminopeptidases cleave N-terminal extensions of peptides shorter than 16aa and have well-defined hierarchy of cleavable residues and non-cleavable residues (22–24) that influence the kinetics of production of adjacent epitopes (24). We showed that specific motifs within and outside epitopes determine the sensitivity of peptide to degradation by cytosolic peptidases, the kinetics of epitope production and contribute to the amount of peptides available for presentation to CTL (18, 24, 25). In addition differences in peptidase activities among cell types also influence the kinetics and amount of epitope produced (26). The combination of specific sequences within proteins and intracellular peptidase hydrolytic activities shapes the kinetics of production and amount of peptides available for loading onto MHC-I. Therefore natural or artificial variations in cellular peptidase activities may change the balance between production and further cleavage of peptides, enhancing or impairing the presentation of various MHC-I epitopes. For instance the inhibition of the proteasome by N-acetyl-leucyl-leucyl-norleucinal (LLnL) or ERAP-1 knockout in mice altered the degradation of proteins and changed the repertoire of peptides presented on MHC class-I molecules and CTL responses (27–29). The 3 HIV PIs Ritonavir, Saquinavir and Nelfinavir inhibit the activity of purified mouse or human 20S proteasomes and the proteasome activity in immortalized cells (30–32) causing intracellular accumulation of polyubiquitinated proteins (33, 34). In mice infected with LCMV and treated with Ritonavir the cytotoxic immune response against two T cell epitopes of LCMV was reduced and prevented the expansion of LCMV reactive cytotoxic T cells (CTL) (30). One possible explanation for these changes in CTL responses might be PI-induced alteration of proteasome activity leading to modification of epitope production. No study has assessed in human primary cells the effect of PIs on post-proteasomal peptidases equally important in antigen processing, the link between PI-induced alterations of cellular peptidases, HIV protein degradation patterns and HIV epitope presentation to CTL (when HIV replication is not fully inhibited by HAART (35, 36)) or the effect of PIs on the processing of other pathogens that HAART-treated patients may encounter during co-infection.
Here we investigated the effect of 7 HIV PIs (Saquinavir, Ritonavir, Nelfinavir, Indinavir, Atazanavir, Darunavir and Kaletra) on proteasome and aminopeptidase activities of PBMC. Our results showed that HIV PIs variably altered not only proteasomal but also aminopeptidase activities. Furthermore using an in vitro epitope processing assay (25) we showed that HIV PIs changed HIV peptide degradation patterns, the cytosolic stability and amount of epitopes produced. In addition by measuring the lysis of PI-treated and HIV-infected cells by epitope-specific CTL we found that HIV PIs variably altered the presentation of HIV epitopes and the recognition by CTL. Finally we identified motifs whose cleavage is enhanced or reduced by HIV PIs, leading to increased or decreased production of neighboring epitopes.
Altogether these results show that by variably altering cellular protease activities HIV PIs modify HIV protein degradation patterns, epitope production and presentation leading to variations in CTL responses.
Materials and Methods
Preparation of antiretroviral drug stocks
The antiretroviral tablets used in this study were obtained from two sources: 1) NIH AIDS Research & Reference Reagent Program (NIAID) or 2) the Mass General Hospital (MGH) Inpatient pharmacy. The following reagents were donated by NIH AIDS Reagent Program: Indinavir, Lamivudine and Nelfinavir. From the MGH Inpatient pharmacy we obtained Ritonavir, Saquinavir, Atazanavir Sulfate, Delavirdine, Darunavir and Kaletra. The purified form of the antiretroviral drugs was obtained from Selleckchem (Ritonavir, Lopinavir, and Darunavir), Sigma (Saquinavir) and Santa Cruz (Nelfinavir). All drugs were dissolved in 100% DMSO. Kaletra was prepared by combining Lopinavir and Ritonavir with 5:1 ratio respectively, subsequently Kaletra concentrations mentioned in the manuscript correspond to Lopinavir amount. Stock solutions of 50 mM were kept at −20°C, and fresh aliquots were used for each experiment.
Peripheral blood mononuclear cell (PBMC) Isolation from Donors
The use of buffy coats from anonymous blood donors was approved under Protocol No. 2005P001218 by the Partners Human Research Committee (Boston, MA). Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats (Massachusetts General Hospital, Boston, MA) by Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation. The needed amount of PBMC for live cell proteolytic activity measurement was taken for immediate use and the remaining PBMC were used for cytosol extraction (25).
Measuring proteolytic activities in live cells or purified enzymes
Chymotryptic, tryptic, and caspase-like activities of proteasomes present in live PBMC or purified PBMC proteasome obtained using the protocol described in (37) were measured with specific fluorogenic substrates (50 µmol/L Suc-LLVY-Amc, 50 µmol/L BocLRR-Amc, and 75 µmol/L ZLLE-Amc, respectively; Bachem) [26,29]. Different amino acid cleavage activities required 5 µmol/L of X-Amc substrate where X represents any amino acid (26). PanCaspase activity was measured using 50 µmol/L of Ac-DEVD-AMC fluorogenic substrate. PBMC, purified PBMC proteasomes or purified aminopeptidase ERAP1 (obtained from R&D systems) were pretreated with increasing drug concentrations for 30 min at 37°C before adding the fluorogenic substrates. The specificity of the reaction was assessed by preincubating cells with inhibitors of proteasomes (MG-132; 10 µmol/L), aminopeptidases (bestatin; 120 µmol/L) or PanCaspase (Z-VAD-FMK; 20 µmol/L). Digitonin was added to the cells at final amount of 0.0025% to facilitate the entry of the drugs and the substrates into the cells. The fluorescence was measured at 37°C every 5 minutes for 5 hours using a VICTOR X Multilabel Plate Reader (Perkin Elmer, Boston, MA)
Drug toxicity measurement assay
PBMC at 500.000 cells/mL were incubated overnight with different PIs in 48-well plates. Cells were stained with Annexin and 7AAD (Annexin V APC Apoptosis Detection Kit I - BD Pharmingen) and the percentage of apoptotic and necrotic cells were determined by flow cytometry.
HIV epitope processing assay
2 nmol of purified peptide (Massachusetts General Hospital peptide core facility; >95% pure) were degraded in 20 ug of PBMC cytosol pretreated with different PIs for 30 min. The degradation reaction was stopped at various time points with 1% Formic Acid and degradation products were analyzed by LC-MS-MS. Peptides present in the digestion mix were purified by TCA precipitation at each time point. Equal amounts of peptide degradation samples at different time points were injected into a Nano-HPLC (NanoLC Ultra, Eksigent) and online nanosprayed into an Orbitrap mass spectrometry (LTQ Orbitrap Discovery, Thermo) at a flow rate of 400 nL/min. A Nano cHiPLC trap column (200 µm × 0.5 mm ChromXP c18-CL 5 µm 120 Å, Eksigent) was used to remove salts and contaminants in the sample buffers. Peptides were separated in a Nano cHiPLC column (75 µm × 15 cm ChromXP c18-CL 5 µm 300 Å, Eksigent) over a gradient of 2% to 40% buffer B (Buffer A: Water w/0.1% FA; Buffer B: Acetonitrile w/0.1% FA) in 20 min. Mass spectra were recorded in the range of 370 to 2000 Daltons. In the tandem mass spectrometry mode, the eight most intense peaks were selected with a window of 1 Dalton and fragmented. The collision gas is Helium and collision voltage is 35V. Peaks in the mass spectra were searched against the source peptides databases with Proteome Discoverer (Version 1.3, Thermo) and quantitatively analyzed. The integrated area of a peak generated by a given peptide is proportional to the relative abundance of the peptide present. Each degradation time point was run on the mass spectrometer at least twice.
Cytosolic peptide stability assay
2 nmol of purified peptide (Massachusetts General Hospital peptide core facility; >95% pure) were degraded at 37°C in 20 ug of PBMC cytosol pretreated with different PIs for 30 min. The reaction was stopped with 1% Formic acid at various time points. The degradation of the peptide was analyzed by RP-HPLC (Waters) as described previously (18). The peptide corresponds to one peak whose amount is proportional to the surface under peak. 100% corresponds to the surface under peak for each peptide at time 0. Peptides incubated at 37°C in buffer without extracts are similarly analyzed.
CTL induced killing assay
HLA-matched B Cells were incubated with PI for 30 minutes before being infected with VSVg-pseudotyped Gag-Pol-GFP expressing or NL4.3 HIV-GFP expressing in-house made virus. 36 hours post-infection half of the cells were stained with HLA-A/B/C APC (BD Biosciences) and analyzed by flow cytometry for the infection percentage and the HLA-A/B/C surface expression level. The other half of the cells were incubated with epitope-specific CTL clones at Effector to Target ratio to 4:1. All conditions were done in triplicates. To test the CTL specificity, non-infected cells were pulsed 30 minutes with decreasing concentration of CTL-matched or non-matched epitopes and subjected to CTL killing. To measure cell death Vybrant fluorescence-based cytotoxic assay kit (Invitrogen) was used. Percentage specific lysis was determined by using the following formula: [(experimental release−spontaneous release)/(maximum release−spontaneous release)]×100%. Maximum release was determined by lysis of all target cells with detergent (5% Triton) and spontaneous release was determined by incubating non-target cells (not infected) with CTL (38).
Statistical analysis
Data were analyzed using GraphPad Prism 5 software.
Results
HIV PIs alter cellular proteasome and aminopeptidase activities
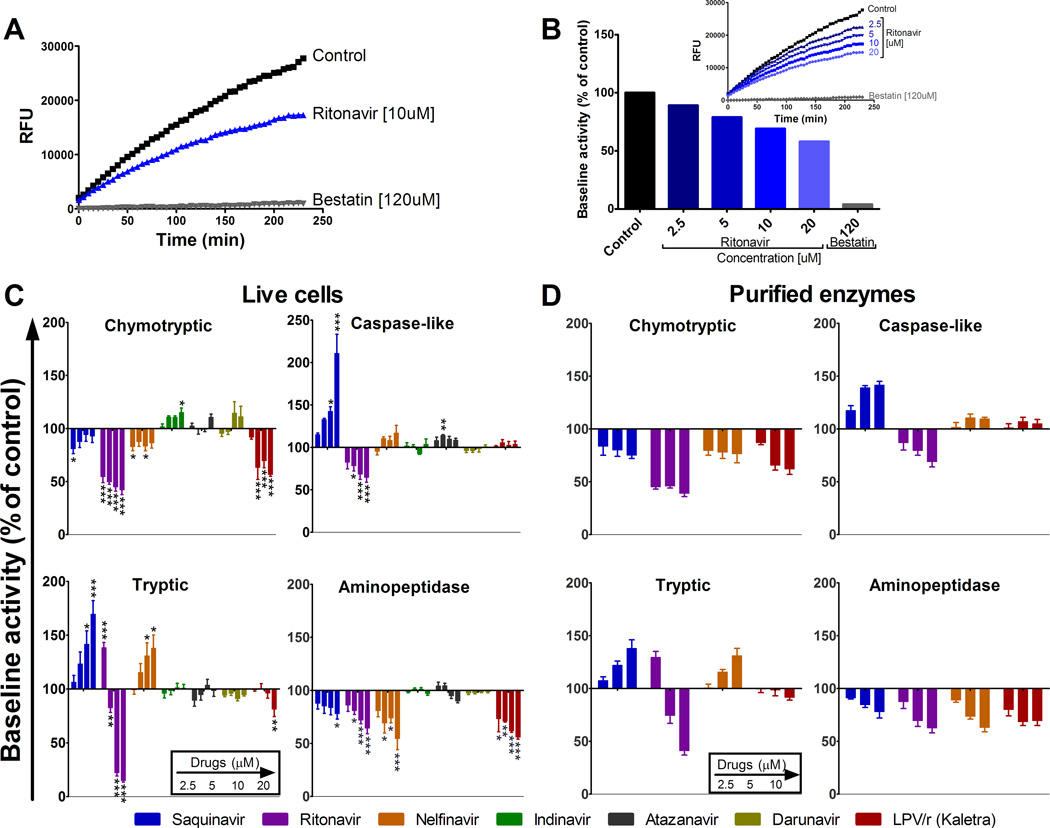
We first aimed to assess the effect of 7 HIV PIs (Saquinavir, Ritonavir, Nelfinavir, Indinavir, Atazanavir, Darunavir and Kaletra), 1 NRTI (Lamivudine) and 1 nNRTI (Delavirdine) on the main proteolytic activities —chymotryptic, tryptic, and caspase-like activities of proteasomes (i.e., cleaving after hydrophobic, basic, and acidic amino acids, respectively) — and aminopeptidase in freshly isolated PBMC from at least 6 different HIV-negative donors.
The concentrations of the PIs used in this study correspond to the level of PIs found in the plasma of ART-treated persons (39–43). Using annexin and 7AAD staining as markers for apoptosis and necrosis no toxicity was detected on PBMC treated at therapeutic concentrations of PIs ranging from 5–20 uM (supplemental figure 1A). Furthermore Pan-caspase activity measurement showed no caspase induction post PI treatment (supplemental figure 1B). Each protease activity was measured with a fluorogenic substrate composed of a peptide specific for each proteolytic activity and a fluorogenic coumarin-derivative moiety (24, 37) (Figure 1A). For each protease activity fluorescence was measured over time and the hydrolysis kinetics was calculated as the maximum slope of fluorescence emission after subtraction of fluorescence in the absence of cells. For each substrate one hundred percent represents the maximum slope of fluorescence emission by cells incubated with substrate and DMSO control.
FIGURE 1.
HIV PIs variably alter proteasome and aminopeptidase activities in human PBMCs. (A) Aminopeptidase substrate Leu-amc was added to PBMCs pretreated with DMSO (control, squares), 10 µM Ritonavir (triangles) or 120 uM Bestatin (inversed triangles) and incubated for 4 h at 37 °C, during which fluorescence emission was monitored every 5 min. (B) PBMCs were preincubated with increasing concentrations of Ritonavir or 120 µM of Bestatin before addition of Leu-amc. The maximum slope of fluorescence emission over 1 h was calculated for each condition. One hundred percent represents the maximum slope of fluorescence emission of the control (153.9). Maximum slope of fluorescence upon each treatment was compared to control. (C) PBMCs or (D) Purified proteasomes or ERAP1 were pretreated with DMSO (control) or increasing concentrations of each PI (Saquinavir, Ritonavir, Nelfinavir, Indinavir, Atazanavir, Darunavir and Kaletra –left to right bars on each graph-) before adding specific substrate for each activity (chymotryptic and caspase-like (top panels), tryptic and aminopeptidase (lower panels)). In each panel one hundred percent represents the maximum slope of DMSO treated PBMCs (1161.8 for chymotryptic, 194 for caspase-like, 475 for tryptic and 1063.6 for aminopeptidase) or purified proteasomes or ERAP1 (186.8 for chymotryptic, 27.1 for caspase-like, 91.8 for Tryptic and 507 for aminopeptidase). The maximum slope of treated PBMC was compared to that of control. Average of 6–8 healthy donors. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Dunnett’s post-test.
The specificity of substrate cleavage was checked by preincubation of cells with cognate inhibitors of proteasome (MG132) or aminopeptidase (Bestatin). In presence of the specific inhibitor (Bestatin) aminopeptidase activity was reduced by 20-fold compared to the control (max slope of 145.35 for control and 7.25 for Bestatin). Increasing concentrations of Ritonavir (2.5 µM to 20 µM) reduced aminopeptidase activities by 1.11 to 1.73 fold respectively (Figure 1B).
The effects of each PI at increasing concentrations on all three proteasome and aminopeptidase activities were assessed (Figure 1C). Chymotryptic activity of proteasome (Figure 1C, upper left panel) was decreased upon Saquinavir, Ritonavir, Nelfinavir or Kaletra treatment by 1.23-fold to 2-fold. In contrast Indinavir increased the chymotryptic activity by 1.15-fold and Atazanavir or Darunavir did not change it. Saquinavir and Atazanavir increased the proteasomal caspase-like activity (Figure 1C, upper right panel) by 1.15-fold to 1.9-fold whereas Ritonavir decreased it by 1.54-fold, unlike the latter the activity was not affected by Nelfinavir, Indinavir, Darunavir or Kaletra. Saquinavir and Nelfinavir increased the proteasomal tryptic activity (Figure 1C, lower left panel) by 1.38-fold to 1.7-fold. Ritonavir increased the activity at low concentration (2.5 µM) by 1.39-fold and decreased tryptic activity at higher concentrations by 5.5-fold. Kaletra also decreased the proteasomal tryptic activity by 1.24-fold. Proteasome tryptic activity was not affected by Indinavir, Atazanavir or Darunavir.
Saquinavir, Ritonavir, Nelfinavir and Kaletra decreased aminopeptidase activities (Figure 1C, lower right panel) by 1.21-fold to 1.83-fold, but no change was seen upon Indinavir, Atazanavir or Darunavir treatment. Delavirdine (nNRTI) and lamivudine (NRTI) did not have any significant effect on the proteasomal and aminopeptidase activities (unpublished data).
The effect of the PIs on chymotryptic, caspase-like, tryptic and aminopeptidase activities was was similar when using live PBMC, purified PBMC proteasome and aminopeptidase ERAP1 (Figure 1D) or PBMC cytosolic extracts (unpublished data). This shows that the alteration induced by PI are specific and validate the use of PI-treated cellular extracts as one approach to assess the impact of PIs on the processing of epitopes.
These results show that Saquinavir, Ritonavir, Nelfinavir and Kaletra altered proteasomal activities in human primary cells in agreement with previous studies testing first generation PIs on purified proteasomes or immortalized cell lines (31, 33). In addition we showed that these four PIs inhibited aminopeptidase activities known to play an important role in defining the composition of MHC class I peptide repertoire (17) Indinavir, Atazanavir and Darunavir, the newer PIs as well as reverse transcriptase inhibitors Lamivudine and Delavirdine did not significantly affect peptidase activities tested in PBMC.
Peptide degradation patterns are altered by HIV PIs
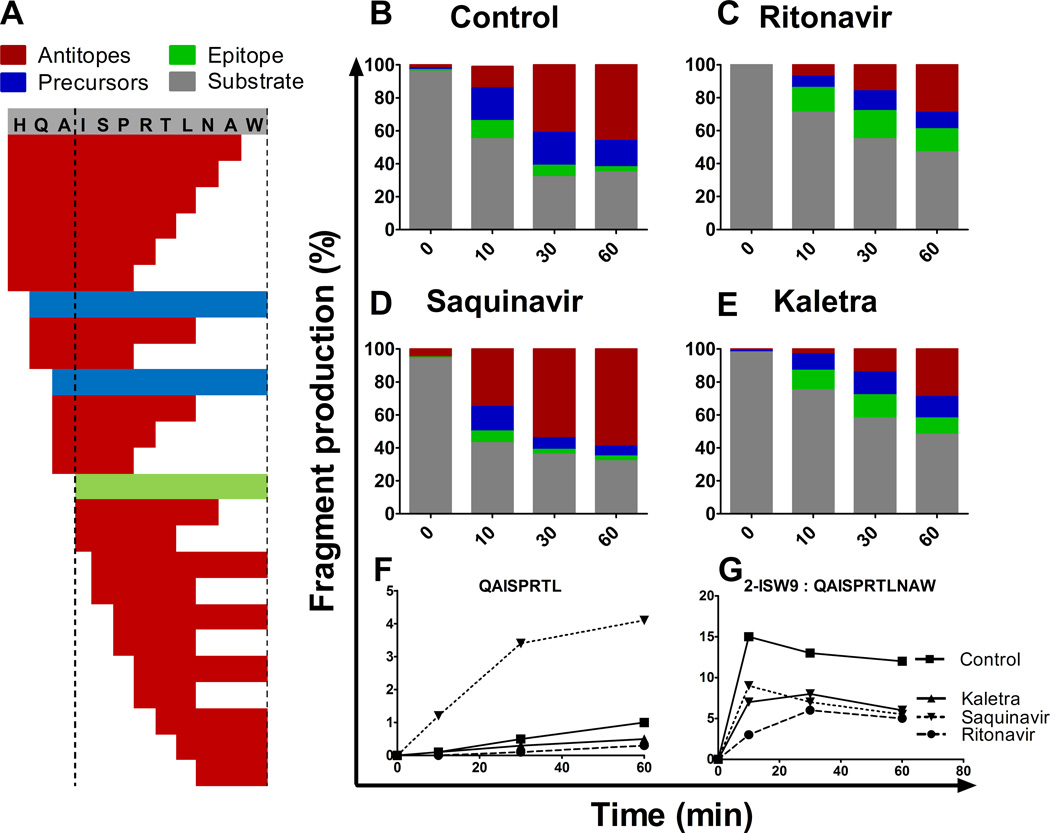
In order to assess the effect of HIV PI on peptide degradation patterns and epitope production we used PBMC cytosolic extracts to degrade 3ISW9 (HQAISPRTLNAW) fragment which is a precursor of a HLA-B57 restricted ISW9 epitope (ISPRTLNAW, aa 15–23 in Gag p24) that elicits frequent CTL responses in HLA-B57 HIV-infected individuals (18). Figure 2A shows degradation products of substrate 3ISW9 identified by LC-MS-MS after a 60-minute degradation in PBMC extracts in the absence of PIs. They included peptides encompassing epitope ISW9 -termed precursors, optimal epitope ISW9, and peptides containing only part of the optimal peptide that will not bind to HLA-B57 -termed antitopes.
FIGURE 2.
HIV PIs alter peptide degradation patterns. 3ISW9 (HQAISPRTLNAW) peptide containing HLA-B57-restricted ISW9 epitope (ISPRTLNAW) was degraded in PBMC cytosolic extracts preincubated with DMSO (A, B) or 10 µM of Ritonavir (C), Saquinavir (D) or Kaletra (E). Resulting degradation products at 0, 10, 30 and 60 min were analyzed by LC-MS-MS. Degradation peptides were categorized as substrate 3-ISW9 (grey), epitope ISW9 (green), precursors -peptides that include the epitope (blue) or antitopes (peptides including only part of the epitope (red) (A–E). Each peptide identified by a specific mass and charge corresponds to a peak of a specific intensity and the proportion of each category of peptides to the total peak intensity (ranging from 7.1E+8 to 8.4E+8 at a given time point) was calculated at each time point in the presence of DMSO (B), Ritonavir (C), Saquinavir (D) or Kaletra (E). Percentage of antitope QAISPRTL (E) and precursor 2-ISW9 (QAISPRTLNAW) (F) production over time upon PI treatment. This figure is representative of one of three independent experiments using PBMC extracts from 3 different donors and run in duplicates on the mass spectrometer.
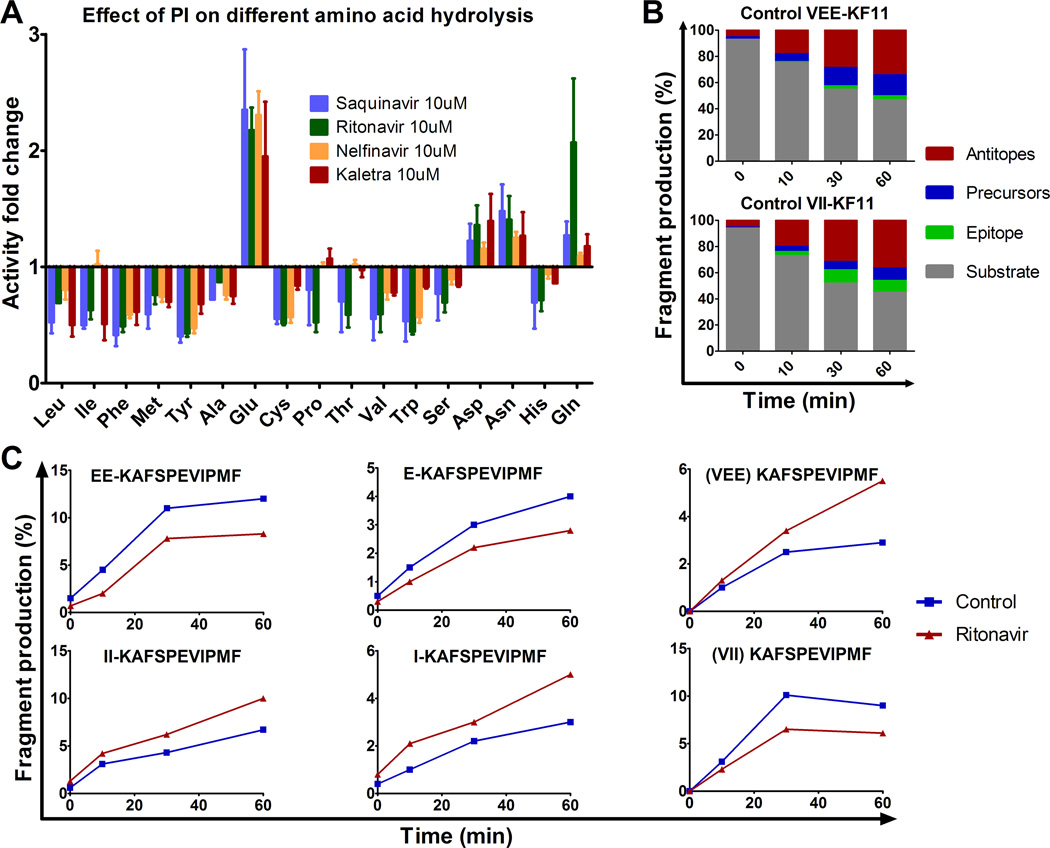
To assess and compare the production of all peptides over time in each condition we calculated the contribution of each peptide to the degradation products detected at a given time point. We first checked that the amount of peptide injected on the mass spectrometer directly correlated with the surface of the peptide’s corresponding peak (supplemental figure 2A–B). Three peptides were mixed at various ratios while keeping the total fentomol constant. The total intensity of all peaks were constant (<10% variation among mixes), and for each peptide the peak surface was proportional to the amount of the peptides (supplemental figure 2C), thus validating the measurement of the relative contribution of each peptide to the total intensity of degradation peptides in the presence of various drugs. In PBMC extracts treated with 10 µM Ritonavir or Kaletra 3ISW9 degradation started slower compared to DMSO control or Saquinavir-treated extracts (71–75% vs 43–55% 3ISW9 remaining at 10 minutes). However the production of ISW9 was increased by 4.6-fold or 3.36-fold at 60 min upon Ritonavir or Kaletra treatment respectively (14% and 10% of total peptides upon Ritonavir and Kaletra respectively compared to 3% in control) (Figure 2B–D), suggesting that peptide trimming was shifted towards epitope production. Saquinavir treatment of 10 µM produced 3.3-fold less ISW9 and 1.8-fold more antitopes compared to the control, suggesting that in the presence of Saquinavir precursors are being cut into peptides destroying epitopes (Figure 2C). These changes in the ratios of categories of peptides were confirmed by comparing individual fragment intensities in the presence of various PIs. For instance upon Saquinavir treatment the antitope QAISPRTL was produced up to 4–7-fold more than in control or Kaletra treatment whereas 2-ISW9 (QAISPRTLNAW) precursor was produced at least twice less (Figure 2E–F), suggesting that in the presence of Saquinavir precursors are being cut to antitopes from the C-terminal side (or over trimmed from the N-terminal side) whereas in presence of Ritonavir or Kaletra they are preferentially trimmed to epitopes. Two other epitopes are present in substrate 3ISW9, HLA-A25-restricted QW11 (QAISPRTLNAW) and HLA-B15-restricted HL9 (HQAISPRTL). In contrast to their positive effect on B57-ISW9 production Ritonavir and Kaletra reduced the production of A25-QW11 and B15-HL9 epitopes, suggesting that the effect of HIV PIs on epitope production is variable and peptide-dependent (supplemental figure 3). We had previously shown that variations in epitope production (due to mutations or variations in peptidase activities) measured by mass spectrometry correlated with changes in CTL-mediated killing of infected cells endogenously processing and presenting HIV epitopes (18, 24, 25). Altogether these results indicate that HIV PIs differently altered peptide degradation patterns, resulting in increased or decreased epitope production and changing the ratio of cytosolic peptides available for loading onto MHC-I.
The intracellular stability of optimal HIV epitopes is altered upon HIV PI treatment
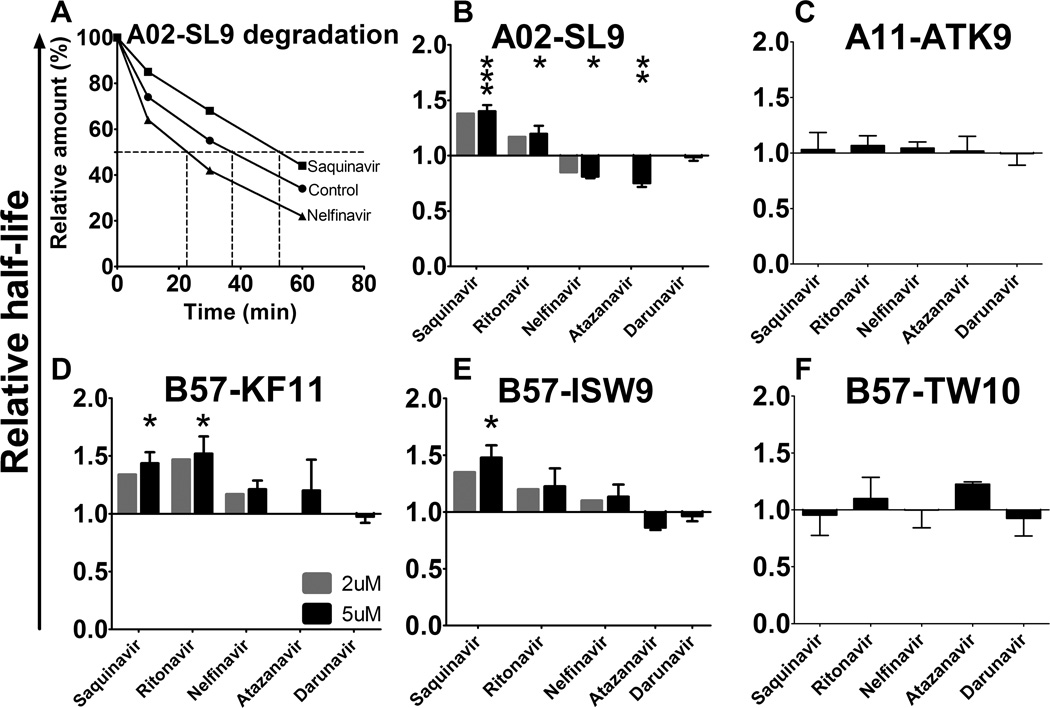
Peptides produced during protein degradation can be subjected to hydrolysis by multiple cytosolic proteases, thus altering the amount of peptides available for MHC-I presentation. The cytosolic stability of peptides is highly variable, defined by specific motifs, and contributes to defining the amount of peptides displayed to CTL (18, 19, 44). We hypothesized that PIs -by variably altering intracellular peptidase activities- might modify the cytosolic stability of peptides and consequently peptide availability for MHC-I loading.
In order to test the effect of HIV PI on the intracellular HIV epitope stability, highly purified peptides were incubated with cytosol from healthy human PBMC pretreated with DMSO (control) or HIV PIs. The amount of peptide remaining over time was measured by reverse-phase HPLC (RP-HPLC) profile analysis, where each peak defined by its elution time represents one peptide, and the surface area under the peak is proportional to the amount of peptide (25). The degradation of the peptide results in reduction of its peak and the appearance of additional peaks corresponding to truncated peptides. A value of 100% was assigned to the amount of input peptide present at time 0, and the amount of peptides remaining was calculated at each time point. The time at which 50% of the peptide was degraded defines its half-life. Figure 3A illustrate the degradation rates of HLA-A02 restricted SL9 (SLYNTVATL, aa 77–85 in Gag p17) in extracts preincubated with control DMSO, Saquinavir or Nelfinavir. Pretreating PBMC cytosol with Saquinavir decreased SL9 degradation rate, thus increasing its half-life to 52 min (compared to 37 min in control), whereas Nelfinavir treatment increased SL9 degradation and reduced its half-life to 24 min (Figure 3A). We measured the half-life of 5 optimally defined HIV epitopes that elicit frequent CTL responses in HIV-infected persons in the presence of absence of 2 or 5 uM HIV PIs: HLA-A02 restricted SL9, HLA-A11 restricted ATK9 (AIFQSSMTK, aa 158–166 in reverse transcriptase of HIV-1 polymerase), HLA-B57 restricted KF11 (KAFSPEVIPMF, aa 30–40 in Gag p24), HLA-B57 restricted ISW9 and HLA-B57 restricted TW10 (TSTLQEQIGW, aa 108–117 in Gag p24) (45). The half-lives of these epitopes in untreated cytosol which were highly variable (119.4 min, 37.2 min, 33.9 min, 25.7 min and 14.8 min for TW10, ATK9, SL9, KF11 and ISW9 respectively) as we previously showed (18) was compared to their half-lives upon different PI treatments. A02-SL9 epitope half-life was increased by 1.4-fold (p<0.001) and 1.2-fold (p<0.05) by Saquinavir and Ritonavir respectively, whereas Nelfinavir and Atazanavir reduced it by 1.25 fold (p<0.05) and 1.33-fold (p<0.01) respectively (Figure 3B). Darunavir did not change the half-life of SL9 and four other peptides. B57-KF11 half-life was increased by 1.44-fold (p<0.05) and 1.52-fold (p<0.05) by Saquinavir and Ritonavir respectively (Figure 3C). All other tested drugs did not show any effect on B57-KF11 half-life. B57-ISW9 half-life was increased by 1.48-fold (p<0.05) by Saquinavir (Figure 3D). Other PIs tested did not significantly change B57-ISW9 half-life. A11-ATK9 and B57-TW10 half-life was not changed by any of the PIs tested (Figure 3E–F). These results demonstrate that HIV PIs by changing activities of cellular proteases modified the cytosolic stability of several HIV epitopes, thus increasing or decreasing their availability for transfer into the ER, loading onto MHC-I and display to CTL.
FIGURE 3.
HIV PIs variably alter intracellular HIV epitope stability. (A) HLA-A02–restricted SL9 epitope (SLYNTVATL, aa 77–85 in HIV-1 Gag p17) was degraded in PBMC extracts pretreated with DMSO (control, circles), 5 µM of Nelfinavir (triangles) or 5 µM of Saquinavir (squares). Remaining peptide was quantified by RP-HPLC analysis after 0, 10, 30 and 60 minutes. 100% represents the amount of peptide at time 0 calculated as the surface under the peptide peak detected by RP-HPLC (815.986, 821.569, and 813.118 for DMSO, Saquinavir, and Nelfinavir, respectively). Times at which 50% of the SL9 peptide remained correspond to peptide half-lives (37 min, 52 min and 24 min for Control, Saquinavir and Nelfinavir respectively). (B–F) HLA-A02–SL9, HLA-B57-KF11, HLA-B57-ISW9, HLA-B57-TW10 and HLA-A11-ATK9 epitopes (from B to F respectively) were degraded in PBMC extracts pretreated with DMSO, 2 µM or 5 µM PI (Saquinavir, Ritonavir, Nelfinavir, Atazanavir or Darunavir). The cytosolic half-lives in control condition were 33.87, 25.66, 14.83, 119.4 and 37.21 minutes for SL9, KF11, ISW9, TW10 and ATK9 respectively. Fold differences of each epitope half-life upon treatment compared to control are presented in each panel. All data represent the average of 4 different experiments using 4 different PBMC extracts. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Dunnett’s post-test.
HIV PIs alter HIV epitope processing and presentation by HIV-infected cells to CTL
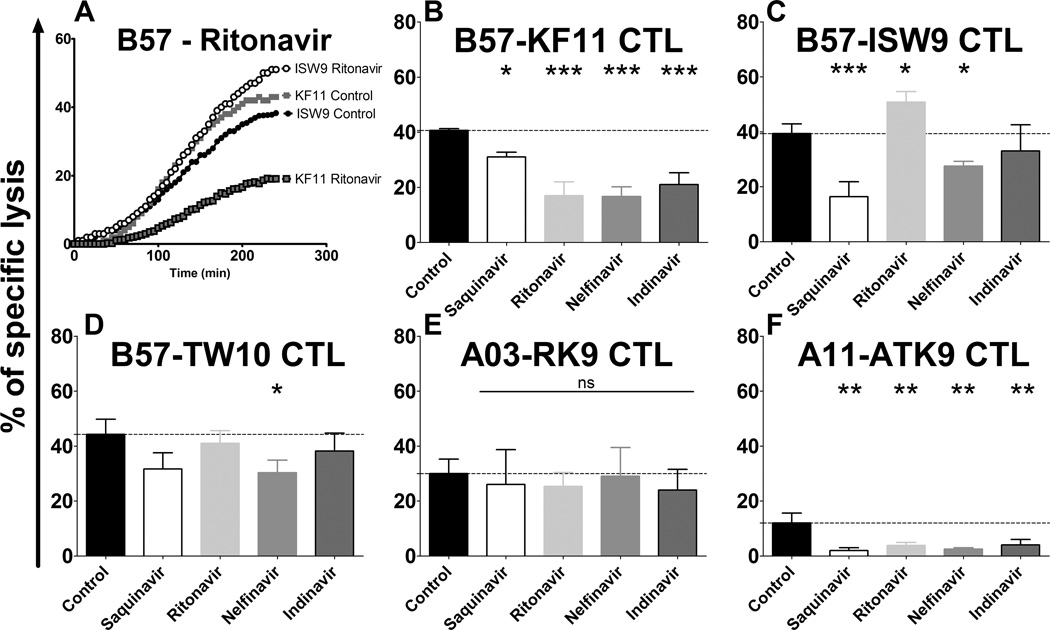
Having demonstrated that HIV PIs modified the degradation patterns of long peptides into epitopes and the intracellular stability of epitopes - 2 parameters that we previously identified to be critical to define the amount of MHC-bound peptides available for CTL recognition (18, 24, 25)- we next assessed whether HIV PIs could affect the endogenous processing and presentation of HIV epitopes and the subsequent CTL-mediated killing of HIV-infected cells. Since HIV PIs affect the late stages of replication we performed single round infections with non-replicative virus to monitor epitope presentation. Upon PI treatment single round infection with either vesicular stomatitis virus G glycoprotein (VSVg) pseudotyped lentivirus expressing HIV-1 Gag, Pol and GPF (VSVg-LV) or VSVg expressing HIV-1 NL4.3 without Env (VSVg-NL43Δenv) led to similar infection rates (67.3%–68.5%) and did not affect the surface expression of HLA-A/B/C (MFI≈60) (supplemental figure 4). The PIs blocked the replication and release of HIV-1 NL4.3 in Jurkat cells, confirming the stability and the activity of the PIs (data not shown).
HLA-matched B cell lines were pre-treated with DMSO or 5 µM PI before being infection with either VSVg-LV or VSVg-NL43Δenv and used as targets in a fluorescence-based killing assay with various epitope-specific CTL clone (38). Killing is monitored with an extracellular fluorogenic substrate that fluoresces after cleavage by an intracellular enzyme released by dying cells (46). Fluorescence is monitored every 5 minutes after addition of CTL to target cells, allowing for real-time measurement of CTL-mediated killing in conditions where the % lysis at 4 hours is similar those obtained in Cr-based killing assay (38). HIV PIs did not affect CTL-mediated lysis of uninfected cells pulsed with various amounts of cognate peptides, in accordance with the lack of effect of PIs on MHC-I expression (supplemental figure 4) and absence of toxicity on CTL (unpublished data). Fluorescence emission after synchronized addition of B57-KF11 or B57-ISW9 CTL to HIV-infected B57 expressing B cells was similar, indicative of similar kinetics of killing of infected targets by the two clones recognizing these two p24 epitopes (Figure 4A). However preincubation of target cells with Ritonavir before infection had opposite effects on the kinetics of killing by the two CTL clones. The kinetics of killing by B57-KF11 CTL was slower and the maximum lysis reduced upon Ritonavir treatment whereas they were enhanced for B57-ISW9 CTL (Figure 4A). We compared the specific lysis of HLA-B57 HIV-infected B cells 4 hours after parallel addition of three B57-restricted Gag-specific CTL clones in the presence of various PIs (Figure 4B–D), and similarly the killing of A03/11 HIV-infected cells B cells by A03 Gag-specific or A11-restricted RT-specific CTL (Figure 4E–F). Saquinavir, Ritonavir, Nelfinavir and Indinavir reduced the killing of HIV-infected cells by the B57-KF11 CTL by 1.31-fold (p<0.05), 2.4-fold (p<0.001), 2.44-fold (p<0.001) and 1.9-fold (p<0.001) respectively (Figure 4B). The killing of HIV-infected cells by B57-ISW9 CTL was inhibited 2.44-fold (p<0.001) and 1.2-fold (p<0.05) by Saquinavir and Nelfinavir respectively (Figure 4C). In contrast Ritonavir increased it by 1.2-fold (p<0.05) and Nelfinavir had no effect. The lysis of HIV-infected cells by B57-TW10 CTL was only decreased by Nelfinavir (1.46-fold, p<0.05) (Figure 4D). None of the drugs tested altered the recognition and killing of HIV-infected cells by A03-RK9 CTL, an epitope that is efficiently produced and highly stable in the cytosol (18, 25) (Figure 4E). The killing of HIV-infected cells by A11-ATK9 CTL -that was lower due to the lesser amount of RT present in incoming virions compared to Gag- was reduced by Saquinavir, Ritonavir, Nelfinavir and Indinavir by 8-fold (p<0.01), 4-fold (p<0.01), 4.8-fold (p<0.01) and 3-fold (p<0.01) respectively (Figure 4F). These results show that HIV PIs altered the endogenous processing and the presentation of HIV epitopes to CTL in various ways and underscore the link between drug-induced alterations of epitope production and subsequent changes in epitope-specific CTL responses. Ritonavir enhanced both in vitro production and intracellular stability of ISW9 (figures 2C and 3D) and led to enhanced killing of infected cells that endogenously processed ISW9 whereas the reduced in vitro production of ISW9 in the presence of Saquinavir correlated with the reduced killing of HIV-infected cells by B57-ISW9 CTL. Therefore PI-induced modulations of cellular peptidase activities leading to changes in peptide degradation patterns, epitope production or intracellular peptide stability affected epitope presentation and recognition of HIV-infected cells by CTL.
FIGURE 4.
HIV PIs variably alter the endogenous processing and presentation of HIV epitopes by infected cells to CTLs. (A) HLA-B57 B cells were treated with DMSO or 5 µM Ritonavir before being infected with VSVg-NL4.3-DEnv and used as targets in a fluorescence-based killing assay with KF11- and ISW9-specific CTLs. Fluorescence emission was recorded every 5 minutes from the moment ISW9-specific (circles) or KF11-specific (squares) CTL were added to HIV-infected cells pretreated with DMSO (no line) or with Ritonavir (black lines). Specific lysis was calculated as [(CTL-induced release−spontaneous release)/(maximum release−spontaneous release)]×100%. Maximum release was determined by lysis of all target cells with detergent (5% triton) and spontaneous release was determined by incubating non infected cells with CTLs. (B–F) HLA-matched B cells were treated with DMSO (control; black bars) or 5 µM PI (Saquinavir, Ritonavir, Nelfinavir, Atazanavir or Darunavir) before being infected with VSVg-NL4.3-DEnv and used as targets in fluorescence based killing assay with KF11-, ISW9-, TW10-, RK9- or ATK9-specific CTLs (B to F respectively). The lysis % of target cells at 4 hours after addition of an epitope-specific CTL were compared among cells pretreated with indicated PIs or DMSO control. All data represent the average of 4 experiments. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA with Dunnett’s post-test.
HIV PIs variably modify the cleavage of amino acids resulting in sequence-specific alterations of epitope production
Since HIV PIs variably affected the activities of cellular peptidases and the processing and presentation of epitopes in different ways, we hypothesized that alterations induced by PIs may be sequence-specific. To individually test the impact of each PI on amino acid cleavage we measured the hydrolysis of fluorogenic substrate X-amc (where X can be any amino acid) in PBMC pretreated with 10 µM of various PIs. We compared the hydrolysis of 17 aa in PBMC pretreated with Saquinavir, Ritonavir, Nelfinavir, Kaletra or control DMSO (Figure 5A). The hydrolysis of 13 aa was inhibited by 1.2- to 2.5-fold. Twelve out of 13 aa corresponded to substrates cleavable by aminopeptidases (24). The inhibition of their hydrolysis by HIV PIs is similar to that observed in figure 1-C using the standard leucine-amc substrate. The cleavage of Pro-amc performed by prolylpeptidases but not by aminopeptidases (47) was also reduced, suggesting that these PIs can also inhibit prolylpeptidase activities. In contrast the hydrolysis of Glutamic acid, Aspartic Acid, Glutamine and Asparagine –substrates not cleavable by aminopeptidases- was increased on average by 2.2-, 1.3-, 1.35-fold and 1.4-fold respectively (Figure 5A), becoming as cleavable as Valine and other residues slowly cleavable in the cytosol. Thus by modifying the cleavage of various amino acids in opposite ways certain PIs may change the patterns of peptide degradation in the cytosol. To test the relevance of these residue-specific changes on epitope processing we measured the production of a HIV epitope flanked by residues whose cleavage was either reduced (Isoleucine, I) or enhanced (Glutamic acid, E) by PIs. The processing of this epitope is proteasome and aminopeptidase-dependent and we previously showed that the trimming efficiency of extended KF11 (VXX-KF11) into KF11 correlated with that of fluorogenic X-amc (24). We compared the degradation of peptide 3KF11 (VEEKAFSPEVIPMF) which is a precursor of HLA-B57-restricted KF11 to that of an artificial mutant 2I-3KF11 (VIIKAFSPEVIPMF) in the presence or absence of 10 µM Ritonavir. In the absence of PI the degradation rates of 3KF11 and 2I-3KF11 proceeded with similar kinetics over 60 minutes, although on average 3.4-fold more epitope (KF11) and less 1KF11 precursors were produced from 2I-3KF11 due to faster N-terminal trimming of Isoleucine compared to Glutamic acid as we previously reported (24) (Figure 5B). However upon Ritonavir treatment 3KF11 degradation produced less precursors (on average 1.8-fold less for EEKAFSPEVIPMF and 1.4-fold less for EKAFSPEVIPMF) and 1.5-fold more epitopes compared to the control (Figure 5C upper panels), suggesting that Ritonavir by increasing the hydrolysis of glutamic acid increased the trimming of precursors towards epitope KF11. In contrast during 2I-3KF11 degradation, due to the decreased hydrolysis of Isoleucine upon Ritonavir treatment, the trimming of 2I-3KF11 towards epitopes was inhibited resulting in increased amounts of precursors (on average 1.6-fold more for IIKAFSPEVIPMF and 1.8-fold more for IKAFSPEVIPMF) and 1.46-fold less epitopes (Figure 5C lower panels). These opposite variations in in vitro production of KF11 (from 1.8-fold more to 1.5-less) fell within the range that affected endogenous processing and presentation of KF11 to CTL in our previous study (24). Together these results indicate that the effect of PI on peptide degradation is sequence-specific, thus increasing the production of some epitopes and decreasing the production of others and modifying the ratios of peptides available for loading onto MHC-I and presentation to CTL.
FIGURE 5.
HIV PIs modify HIV peptide degradation in a sequence-specific manner. (A) The hydrolysis of various amino acids was measured with fluorogenic substrate in PBMC pretreated with 10 µM Saquinavir (blue), Ritonavir (green), Nelfinavir (yellow) or Kaletra (red). Fluorescence emission was measured over 1 hour and the maximum slope of fluorescence was measured in each condition. The fold difference of the maximum slope of PI-treated PBMC over DMSO control was calculated for each condition. Data represent average results of 4 different experiments using 4 different PBMC donors. (B) The 3KF11 (VEEKAFSPEVIPMF) (upper panel) and 2I-3KF11 (VIIKAFSPEVIPMF) (lower panel) peptides were degraded using PBMC extracts. The resulting degradation products were analyzed by LC-MS-MS at 0, 10, 30 and 60 min. The distribution of substrate (3KF11 or 2I-3KF11, in grey), epitope (KF11, in green), precursors (blue) or antitopes (red) is shown at each time point. (C) The relative amount of epitope precursors (EEKAFSPEVIPMF and EKAFSPEVIPMF) and epitope KF11 (upper panels), and 2I-3KF11 epitope precursors (IIKAFSPEVIPMF and IKAFSPEVIPMF) and epitope (lower panels) were calculated at each time point of degradation with extracts pretreated with DMSO (control, blue) or with Ritonavir (red). Panels B and C are one experiment representative of two independent experiments using PBMC extracts from 2 different donors and run in duplicate on the mass spectrometer.
Discussion
The ability of CTL to clear virus-infected cells is dependent on the processing of viral antigens by cellular proteases and peptidases and peptide display by MHC class I. Any perturbation of cellular peptidase activity could modify protein degradation patterns and consequently epitope presentation. In this study we showed that several HIV PIs -by changing not only cellular proteasome but also aminopeptidase activities- altered HIV antigen degradation patterns and cytosolic stability of peptides in a sequence-specific manner, leading to variations in lysis of HIV-infected cells by CD8+ T cells.
Four out seven PIs affected at least one cellular peptidase activity. Saquinavir could either enhance or reduce proteasome and aminopeptidase hydrolytic activities while other PIs such as Kaletra mostly reduced proteasome and/or aminopeptidase activities, suggesting different interactions between each drug and each cellular enzyme. It was previously shown using molecular docking that Ritonavir can bind to the active center of the yeast proteasome PRE2 subunit that is homologous to human proteasome β5 subunit (48), elucidating the inhibition of the chymotryptic activity by Ritonavir (30, 48, 49). Likewise HIV PIs might interact with non-catalytic effector sites in the proteasome and aminopeptidases that were shown in enzymatic studies to regulate the different catalytic activities (50–52). This would provide a potential mechanism of either inhibition or enhancement of cellular peptidases by HIV PIs although molecular modeling and structural studies are required to test this hypothesis.
First generation PIs like Ritonavir, Saquinavir and Nelfinavir showed stronger effect on proteasome and aminopeptidase activities than newer PIs like Atazanavir and Darunavir. First generation PIs induced more rapid and profound side effects on lipid and glucose metabolism than newer PIs (4, 7, 8, 53–55). Rats treated with Ritonavir developed hyperlipidemia and displayed higher RNA expression of proteasome subunits (56, 57). Although there is no clear mechanistic link between the two observations ritonavir-induced proteasome inhibition may trigger a feedback loop leading to increased proteasome expression as observed with proteasome inhibitors (58). PI-induced proteasome inhibition may modify the half-life of proteins involved in glucose or lipid metabolism - such as the documented accumulation of sterol regulatory binding proteins (SREBP-1/2) inducing constitutive lipid biosynthesis in mice (59). Whether the modification of intracellular aminopeptidase activities would affect glucose or lipid metabolism remains unknown. Surface aminopeptidases such as membrane-bound ectoenzyme aminopeptidase N (APN/CD13) or intracellular aminopeptidases trafficking to the surface such as insulin-responsive aminopeptidase (IRAP) are involved in peptide cleavage, cholesterol uptake for APN (60, 61) or glucose transport uptake for IRAP (62). Considering the conservation between aminopeptidase catalytic sites it will be important to examine whether first and second generation PIs modify surface aminopeptidase activities as well as other peptidases in each subcellular compartment and affect the biological functions of these enzymes. Finally as various cell subsets present different levels of peptidase activities (26) it will be necessary to assess the effect of PIs not only on PBMC but also on specific cell subsets.
We showed that alteration of proteasome and aminopeptidase activities by HIV PIs modified both the degradation patterns of long HIV peptides as well as the sensitivity of epitopes to intracellular degradation prior to loading onto MHC-I, and therefore the amount of peptides available for display to CTL. The effect was both drug- and sequence-dependent. Variations in degradation patterns were explained by the intriguing observation that the cleavage of specific residues was enhanced while others were reduced. Twelve residues whose cleavage was reduced by four drugs corresponded to residues cleavable by aminopeptidases, thus suggesting that HIV PIs may reduce the efficiency of aminopeptidase-dependent trimming of many N-extended peptides into epitopes. Surprisingly these four drugs enhanced the cleavage of acidic residues, mostly E and to some extent D, H, Q which are poorly cleavable by aminopeptidases. Sequential incubation of cells with Ritonavir or Kaletra followed by aminopeptidase inhibitor reduced PI-enhanced cleavage of E by 53–59%, suggesting that PI modified aminopeptidase activities to facilitate the cleavage of acidic residues but also enhanced another unidentified peptidase activity. Ritonavir or Kaletra did not enhance caspase-like activity of the proteasome or the activity of caspases (which can cleave motifs containing acidic residues) at least when measured with a pan-caspase substrate (63) (Supplemental Figure 1), thus ruling out a major involvement of proteasomes and caspases in the changes in residue-specific cleavage patterns. Whether HIV PIs enhance additional cytosolic peptidases cleaving acidic residues or whether it may modify aminopeptidase hydrolytic capacity to enhance cleavage of acidic residues and reduce cleavage of other residues remain to be determined.
These findings have implications for the degradation of HIV proteins and beyond. First in the context of HIV protein degradation –specifically relevant for HIV-infected ART-treated persons with ongoing replication of drug-resistant mutated strains- we have shown that HLA-restricted mutations flanking residues tend to evolve from aminopeptidase-cleavable to poorly cleavable residues (24). In the presence of HIV PIs such as Kaletra used as booster in ART treatments we may expect that the production of the WT peptide would be decreased whereas flanking mutations leading to an acidic residue would enhance epitope production as shown in this study with an isoleucine to glutamic acid mutation. Alternatively a mutation towards an acidic residue within an epitope could enhance the intracellular degradation of the mutated epitope and the production of the wild type version. Overall these changes could affect the ratio of HIV peptides presented by infected cells. Mutated epitopes can elicit CTL responses (64–66), thus the change of ratio of WT and mutated peptides could contribute to shifts in immunodominance as seen after immune escape in acute HIV infection (67–70). Although the lack of appropriate longitudinal clinical samples precludes us to test this hypothesis PI-induced modification of epitope landscape may contribute to broadening of immune responses against HIV in ART-treated patients with ongoing viral replication. Additionally studying the impact of PI on HIV epitope presentation is relevant to approaches to purge HIV reservoirs by combining provirus reactivation in the presence of ART to prevent replication, and therapeutic vaccination to boost immune responses against HIV (71, 72). If ART needed to prevent replication after provirus reactivation calls for inclusion of HIV PIs it will be important to assess the repertoire of HIV epitopes presented in the context of these therapeutic strategies to define the complementary vaccination strategy better suited to clear reservoirs.
Secondly since certain PIs modify antigen processing in a sequence-specific manner the effect will likely be observed for the degradation of host proteins or proteins derived other pathogens. We compared the cytosolic stability of optimal epitopes derived from CMV, HCV, Influenza or EBV was variably affected by PI treatment, with Ritonavir/Kaletra increasing the cytosolic stability of several peptides (unpublished data). As intracellular peptide stability contributes to the amount of peptides displayed to CTL (18) HIV PIs may alter the presentation of epitopes derived from other pathogens infecting ART-treated persons. More than half of HIV+ individuals worldwide become co-infected with other pathogens like TB or HCV and need for effective drug combinations to curb both infections are needed (73–75). Assessing if and how ART–beyond reducing HIV viral load and cellular activation- may possibly contribute to diversifying immune responses against co-infecting pathogens by modifying the degradation patterns of these pathogens provides a new outlook of the use of HIV PIs. Similarly Saquinavir, Ritonavir and Nelfinavir -because of their inhibitory effect on the proteasome and other cellular targets- have been shown in previous studies and clinical trials to have beneficial effects on several cancers (31, 34, 76–81). In the repositioning of PIs as cancer therapy another potential benefit could be a PI-induced altered processing of cancer antigens (the intracellular stability of a MAGE3 epitope was modified by Ritonavir/Kaletra, unpublished data), potentially leading to presentation of a different cancer antigen derived peptides and new immune responses.
Our results indicate that HIV PIs by altering several cellular peptidase activities modify antigen processing and epitope presentation. Additional structural studies are needed to understand how HIV PIs modify peptide hydrolytic activity and specificity. However if HIV PIs allow to diversify epitope presentation they may provide complementary approaches to treat various immune disease –considering that temporary PI treatment would not induce toxicity and side effects observed in long-term HAART.
Supplementary Material
Acknowledgments
The authors thank Dr M. Hirsch, Dr S. Fortune, Dr D. Kavanagh, Dr F. Pereyra and Dr B. Zanoni for stimulating discussions and input on the manuscript.
The project was funded by grants AI084753 and AI084106 from NIAID to SLG.
Abbreviations
- PI
Protease inhibitor
Footnotes
Competing interests
All authors have no conflicting financial interests.
References
- 1.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Montero JV, Barreiro P, Soriano V. HIV protease inhibitors: recent clinical trials and recommendations on use. Expert Opin Pharmacother. 2009;10:1615–1629. doi: 10.1517/14656560902980202. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ena J, Benito C, Llacer P, Pasquau F, Amador C. Abnormal body fat distribution and type of antiretroviral therapy as predictors of cardiovascular disease risk in HIV-infected patients. Med Clin (Barc) 2004;122:721–726. doi: 10.1016/s0025-7753(04)74368-7. [DOI] [PubMed] [Google Scholar]
- 6.Yarasheski KE, Tebas P, Sigmund C, Dagogo-Jack S, Bohrer A, Turk J, Halban PA, Cryer PE, Powderly WG. Insulin resistance in HIV protease inhibitor-associated diabetes. J Acquir Immune Defic Syndr. 1999;21:209–216. doi: 10.1097/00126334-199907010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 8.Boesecke C, Cooper DA. Toxicity of HIV protease inhibitors: clinical considerations. Curr Opin HIV AIDS. 2008;3:653–659. doi: 10.1097/COH.0b013e328312c392. [DOI] [PubMed] [Google Scholar]
- 9.Orkin C, DeJesus E, Khanlou H, Stoehr A, Supparatpinyo K, Lathouwers E, Lefebvre E, Opsomer M, Van de Casteele T, Tomaka F. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14:49–59. doi: 10.1111/j.1468-1293.2012.01060.x. [DOI] [PubMed] [Google Scholar]
- 10.Erickson J, Neidhart DJ, VanDrie J, Kempf DJ, Wang XC, Norbeck DW, Plattner JJ, Rittenhouse JW, Turon M, Wideburg N, et al. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990;249:527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- 11.Diez-Rivero CM, Lafuente EM, Reche PA. Computational analysis and modeling of cleavage by the immunoproteasome and the constitutive proteasome. BMC Bioinformatics. 2010;11:479. doi: 10.1186/1471-2105-11-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 13.York IA, Mo AX, Lemerise K, Zeng W, Shen Y, Abraham CR, Saric T, Goldberg AL, Rock KL. The cytosolic endopeptidase, thimet oligopeptidase, destroys antigenic peptides and limits the extent of MHC class I antigen presentation. Immunity. 2003;18:429–440. doi: 10.1016/s1074-7613(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 14.Saric T, Graef CI, Goldberg AL. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem. 2004;279:46723–46732. doi: 10.1074/jbc.M406537200. [DOI] [PubMed] [Google Scholar]
- 15.Kawahara M, York IA, Hearn A, Farfan D, Rock KL. Analysis of the role of tripeptidyl peptidase II in MHC class I antigen presentation in vivo. J Immunol. 2009;183:6069–6077. doi: 10.4049/jimmunol.0803564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–483. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 17.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat Immunol. 2002;3:1177–1184. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 18.Lazaro E, Kadie C, Stamegna P, Zhang SC, Gourdain P, Lai NY, Zhang M, Martinez SA, Heckerman D, Le Gall S. Variable HIV peptide stability in human cytosol is critical to epitope presentation and immune escape. J Clin Invest. 2011;121:2480–2492. doi: 10.1172/JCI44932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herberts CA, Neijssen JJ, de Haan J, Janssen L, Drijfhout JW, Reits EA, Neefjes JJ. Cutting edge: HLA-B27 acquires many N-terminal dibasic peptides: coupling cytosolic peptide stability to antigen presentation. J Immunol. 2006;176:2697–2701. doi: 10.4049/jimmunol.176.5.2697. [DOI] [PubMed] [Google Scholar]
- 20.Reits E, Griekspoor A, Neijssen J, Groothuis T, Jalink K, van Veelen P, Janssen H, Calafat J, Drijfhout JW, Neefjes J. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity. 2003;18:97–108. doi: 10.1016/s1074-7613(02)00511-3. [DOI] [PubMed] [Google Scholar]
- 21.Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J Biol Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- 22.Hearn A, York IA, Rock KL. The specificity of trimming of MHC class I-presented peptides in the endoplasmic reticulum. J Immunol. 2009;183:5526–5536. doi: 10.4049/jimmunol.0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatz MM, Peters B, Akkad N, Ullrich N, Martinez AN, Carroll O, Bulik S, Rammensee HG, van Endert P, Holzhutter HG, Tenzer S, Schild H. Characterizing the N-terminal processing motif of MHC class I ligands. J Immunol. 2008;180:3210–3217. doi: 10.4049/jimmunol.180.5.3210. [DOI] [PubMed] [Google Scholar]
- 24.Zhang SC, Martin E, Shimada M, Godfrey SB, Fricke J, Locastro S, Lai NY, Liebesny P, Carlson JM, Brumme CJ, Ogbechie OA, Chen H, Walker BD, Brumme ZL, Kavanagh DG, Le Gall S. Aminopeptidase substrate preference affects HIV epitope presentation and predicts immune escape patterns in HIV-infected individuals. J Immunol. 2012;188:5924–5934. doi: 10.4049/jimmunol.1200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gall S, Stamegna P, Walker BD. Portable flanking sequences modulate CTL epitope processing. J Clin Invest. 2007;117:3563–3575. doi: 10.1172/JCI32047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazaro E, Godfrey SB, Stamegna P, Ogbechie T, Kerrigan C, Zhang M, Walker BD, Le Gall S. Differential HIV epitope processing in monocytes and CD4 T cells affects cytotoxic T lymphocyte recognition. J Infect Dis. 2009;200:236–243. doi: 10.1086/599837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 28.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006;103:9202–9207. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanchard N, Kanaseki T, Escobar H, Delebecque F, Nagarajan NA, Reyes-Vargas E, Crockett DK, Raulet DH, Delgado JC, Shastri N. Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. J Immunol. 2010;184:3033–3042. doi: 10.4049/jimmunol.0903712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andre P, Groettrup M, Klenerman P, de Giuli R, Booth BL, Jr, Cerundolo V, Bonneville M, Jotereau F, Zinkernagel RM, Lotteau V. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci U S A. 1998;95:13120–13124. doi: 10.1073/pnas.95.22.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaedicke S, Firat-Geier E, Constantiniu O, Lucchiari-Hartz M, Freudenberg M, Galanos C, Niedermann G. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62:6901–6908. [PubMed] [Google Scholar]
- 32.Kelleher AD, Booth BL, Jr, Sewell AK, Oxenius A, Cerundolo V, McMichael AJ, Phillips RE, Price DA. Effects of retroviral protease inhibitors on proteasome function and processing of HIV-derived MHC class I-restricted cytotoxic T lymphocyte epitopes. AIDS Res Hum Retroviruses. 2001;17:1063–1066. doi: 10.1089/088922201300343744. [DOI] [PubMed] [Google Scholar]
- 33.Piccinini M, Rinaudo MT, Anselmino A, Buccinna B, Ramondetti C, Dematteis A, Ricotti E, Palmisano L, Mostert M, Tovo PA. The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antivir Ther. 2005;10:215–223. [PubMed] [Google Scholar]
- 34.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62:5230–5235. [PubMed] [Google Scholar]
- 35.Hill A, McBride A, Sawyer AW, Clumeck N, Gupta RK. Resistance at virological failure using boosted protease inhibitors versus nonnucleoside reverse transcriptase inhibitors as first-line antiretroviral therapy--implications for sustained efficacy of ART in resource-limited settings. J Infect Dis. 2013;207(Suppl 2):S78–S84. doi: 10.1093/infdis/jit112. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis. 2013;207(Suppl 2):S49–S56. doi: 10.1093/infdis/jit107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisselev AF, Goldberg AL. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol. 2005;398:364–378. doi: 10.1016/S0076-6879(05)98030-0. [DOI] [PubMed] [Google Scholar]
- 38.Gourdain P, Boucau J, Kourjian G, Lai NY, Duong E, Le Gall S. A real-time killing assay to follow viral epitope presentation to CD8 T cells. Journal of Immunological Methods. 2013 doi: 10.1016/j.jim.2013.09.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acosta EP, Kakuda TN, Brundage RC, Anderson PL, Fletcher CV. Pharmacodynamics of human immunodeficiency virus type 1 protease inhibitors. Clin Infect Dis. 2000;30(Suppl 2):S151–S159. doi: 10.1086/313852. [DOI] [PubMed] [Google Scholar]
- 40.Cardiello PG, Monhaphol T, Mahanontharit A, van Heeswijk RP, Burger D, Hill A, Ruxrungtham K, Lange JM, Cooper DA, Phanuphak P. Pharmacokinetics of once-daily saquinavir hard-gelatin capsules and saquinavir soft-gelatin capsules boosted with ritonavir in HIV-1-infected subjects. J Acquir Immune Defic Syndr. 2003;32:375–379. doi: 10.1097/00126334-200304010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Hennessy M, Clarke S, Spiers JP, Kelleher D, Mulcahy F, Hoggard P, Back D, Barry M. Intracellular accumulation of nelfinavir and its relationship to P-glycoprotein expression and function in HIV-infected patients. Antivir Ther. 2004;9:115–122. [PubMed] [Google Scholar]
- 42.Jackson A, Watson V, Back D, Khoo S, Liptrott N, Egan D, Gedela K, Higgs C, Abbas R, Gazzard B, Boffito M. Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals. J Acquir Immune Defic Syndr. 2011;58:450–457. doi: 10.1097/QAI.0b013e3182364c67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Heeswijk RP, Veldkamp AI, Mulder JW, Meenhorst PL, Lange JM, Beijnen JH, Hoetelmans RM. Once-daily dosing of saquinavir and low-dose ritonavir in HIV-1-infected individuals: a pharmacokinetic pilot study. AIDS. 2000;14:F103–F110. doi: 10.1097/00002030-200006160-00003. [DOI] [PubMed] [Google Scholar]
- 44.Reits E, Neijssen J, Herberts C, Benckhuijsen W, Janssen L, Drijfhout JW, Neefjes J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20:495–506. doi: 10.1016/s1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- 45.Frahm N, Baker B, Brander C. Identification and Optimal Definition of HIV-Derived Cytotoxic T Lymphocyte (CTL) Epitopes for the Study of CTL Escape, Functional Avidity and Viral Evolution. HIV Molecular Immunology. 2008 [Google Scholar]
- 46.Batchelor RH, Zhou M. Use of cellular glucose-6-phosphate dehydrogenase for cell quantitation: applications in cytotoxicity and apoptosis assays. Anal Biochem. 2004;329:35–42. doi: 10.1016/j.ab.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Rosenblum JS, Kozarich JW. Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol. 2003;7:496–504. doi: 10.1016/s1367-5931(03)00084-x. [DOI] [PubMed] [Google Scholar]
- 48.Schmidtke G, Holzhutter HG, Bogyo M, Kairies N, Groll M, de Giuli R, Emch S, Groettrup M. How an inhibitor of the HIV-I protease modulates proteasome activity. J Biol Chem. 1999;274:35734–35740. doi: 10.1074/jbc.274.50.35734. [DOI] [PubMed] [Google Scholar]
- 49.Sato A, Asano T, Ito K, Asano T. Ritonavir interacts with bortezomib to enhance protein ubiquitination and histone acetylation synergistically in renal cancer cells. Urology. 2012;79:966, e913–e921. doi: 10.1016/j.urology.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 50.Ruschak AM, Kay LE. Proteasome allostery as a population shift between interchanging conformers. Proc Natl Acad Sci U S A. 2012;109:E3454–E3462. doi: 10.1073/pnas.1213640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jankowska E, Gaczynska M, Osmulski P, Sikorska E, Rostankowski R, Madabhushi S, Tokmina-Lukaszewska M, Kasprzykowski F. Potential allosteric modulators of the proteasome activity. Biopolymers. 2010;93:481–495. doi: 10.1002/bip.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kisselev AF, Akopian TN, Castillo V, Goldberg AL. Proteasome active sites allosterically regulate each other, suggesting a cyclical bite-chew mechanism for protein breakdown. Mol Cell. 1999;4:395–402. doi: 10.1016/s1097-2765(00)80341-x. [DOI] [PubMed] [Google Scholar]
- 53.Spector AA. HIV protease inhibitors and hyperlipidemia: a fatty acid connection. Arterioscler Thromb Vasc Biol. 2006;26:7–9. doi: 10.1161/01.ATV.0000198749.28422.29. [DOI] [PubMed] [Google Scholar]
- 54.Anuurad E, Bremer A, Berglund L. HIV protease inhibitors and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:478–485. doi: 10.1097/MED.0b013e32833dde87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noor MA, Flint OP, Maa JF, Parker RA. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 56.Waring JF, Ciurlionis R, Marsh K, Klein LL, Degoey DA, Randolph JT, Spear B, Kempf DJ. Identification of proteasome gene regulation in a rat model for HIV protease inhibitor-induced hyperlipidemia. Arch Toxicol. 2010;84:263–270. doi: 10.1007/s00204-010-0527-7. [DOI] [PubMed] [Google Scholar]
- 57.Lum PY, He YD, Slatter JG, Waring JF, Zelinsky N, Cavet G, Dai X, Fong O, Gum R, Jin L, Adamson GE, Roberts CJ, Olsen DB, Hazuda DJ, Ulrich RG. Gene expression profiling of rat liver reveals a mechanistic basis for ritonavir-induced hyperlipidemia. Genomics. 2007;90:464–473. doi: 10.1016/j.ygeno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, Kruger E. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of Mammalian proteasomes. J Biol Chem. 2003;278:21517–21525. doi: 10.1074/jbc.M301032200. [DOI] [PubMed] [Google Scholar]
- 59.Riddle TM, Kuhel DG, Woollett LA, Fichtenbaum CJ, Hui DY. HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J Biol Chem. 2001;276:37514–37519. doi: 10.1074/jbc.M104557200. [DOI] [PubMed] [Google Scholar]
- 60.Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jahne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, Orso E, Schmitz G. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem. 2005;280:1306–1320. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- 61.Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA. Intestinal cholesterol transport proteins: an update and beyond. Curr Opin Lipidol. 2007;18:310–318. doi: 10.1097/MOL.0b013e32813fa2e2. [DOI] [PubMed] [Google Scholar]
- 62.Yeh TY, Sbodio JI, Tsun ZY, Luo B, Chi NW. Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase. Biochem J. 2007;402:279–290. doi: 10.1042/BJ20060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 64.Feeney ME, Tang Y, Pfafferott K, Roosevelt KA, Draenert R, Trocha A, Yu XG, Verrill C, Allen T, Moore C, Mallal S, Burchett S, McIntosh K, Pelton SI, St John MA, Hazra R, Klenerman P, Altfeld M, Walker BD, Goulder PJ. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J Immunol. 2005;174:7524–7530. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 65.Allen TM, Yu XG, Kalife ET, Reyor LL, Lichterfeld M, John M, Cheng M, Allgaier RL, Mui S, Frahm N, Alter G, Brown NV, Johnston MN, Rosenberg ES, Mallal SA, Brander C, Walker BD, Altfeld M. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J Virol. 2005;79:12952–12960. doi: 10.1128/JVI.79.20.12952-12960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Connell KA, Hegarty RW, Siliciano RF, Blankson JN. Viral suppression of multiple escape mutants by de novo CD8(+) T cell responses in a human immunodeficiency virus-1 infected elite suppressor. Retrovirology. 2011;8:63. doi: 10.1186/1742-4690-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turnbull EL, Wong M, Wang S, Wei X, Jones NA, Conrod KE, Aldam D, Turner J, Pellegrino P, Keele BF, Williams I, Shaw GM, Borrow P. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J Immunol. 2009;182:7131–7145. doi: 10.4049/jimmunol.0803658. [DOI] [PubMed] [Google Scholar]
- 68.Liu MK, Hawkins N, Ritchie AJ, Ganusov VV, Whale V, Brackenridge S, Li H, Pavlicek JW, Cai F, Rose-Abrahams M, Treurnicht F, Hraber P, Riou C, Gray C, Ferrari G, Tanner R, Ping LH, Anderson JA, Swanstrom R, B CC, Cohen M, Karim SS, Haynes B, Borrow P, Perelson AS, Shaw GM, Hahn BH, Williamson C, Korber BT, Gao F, Self S, McMichael A, Goonetilleke N. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. J Clin Invest. 2013;123:380–393. doi: 10.1172/JCI65330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 70.Nowak MA, May RM, Phillips RE, Rowland-Jones S, Lalloo DG, McAdam S, Klenerman P, Koppe B, Sigmund K, Bangham CR, et al. Antigenic oscillations and shifting immunodominance in HIV-1 infections. Nature. 1995;375:606–611. doi: 10.1038/375606a0. [DOI] [PubMed] [Google Scholar]
- 71.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siliciano JD, Siliciano RF. HIV-1 eradication strategies: design and assessment. Curr Opin HIV AIDS. 2013;8:318–325. doi: 10.1097/COH.0b013e328361eaca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jalali Z, Rockstroh JK. Antiviral drugs and the treatment of hepatitis C. Curr HIV/AIDS Rep. 2012;9:132–138. doi: 10.1007/s11904-012-0111-2. [DOI] [PubMed] [Google Scholar]
- 74.Naidoo K, Baxter C, Abdool Karim SS. When to start antiretroviral therapy during tuberculosis treatment? Curr Opin Infect Dis. 2013;26:35–42. doi: 10.1097/QCO.0b013e32835ba8f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker NF, Meintjes G, Wilkinson RJ. HIV-1 and the immune response to TB. Future Virol. 2013;8:57–80. doi: 10.2217/fvl.12.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bono C, Karlin L, Harel S, Mouly E, Labaume S, Galicier L, Apcher S, Sauvageon H, Fermand JP, Bories JC, Arnulf B. The human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivo. Haematologica. 2012;97:1101–1109. doi: 10.3324/haematol.2011.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawabata S, Gills JJ, Mercado-Matos JR, Lopiccolo J, Wilson W, 3rd, Hollander MC, Dennis PA. Synergistic effects of nelfinavir and bortezomib on proteotoxic death of NSCLC and multiple myeloma cells. Cell Death Dis. 2012;3:e353. doi: 10.1038/cddis.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kraus M, Malenke E, Gogel J, Muller H, Ruckrich T, Overkleeft H, Ovaa H, Koscielniak E, Hartmann JT, Driessen C. Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis. Mol Cancer Ther. 2008;7:1940–1948. doi: 10.1158/1535-7163.MCT-07-2375. [DOI] [PubMed] [Google Scholar]
- 79.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi's sarcoma and tumour growth. Lancet Oncol. 2003;4:537–547. doi: 10.1016/s1470-2045(03)01192-6. [DOI] [PubMed] [Google Scholar]
- 80.Zeng J, See AP, Aziz K, Thiyagarajan S, Salih T, Gajula RP, Armour M, Phallen J, Terezakis S, Kleinberg L, Redmond K, Hales RK, Salvatori R, Quinones-Hinojosa A, Tran PT, Lim M. Nelfinavir induces radiation sensitization in pituitary adenoma cells. Cancer Biol Ther. 2011;12:657–663. doi: 10.4161/cbt.12.7.17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kimple RJ, Vaseva AV, Cox AD, Baerman KM, Calvo BF, Tepper JE, Shields JM, Sartor CI. Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16:912–923. doi: 10.1158/1078-0432.CCR-09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.