Abstract
Background
For unclear reasons, many Tourette syndrome (TS) children report near-complete tic remission by young adulthood. Immature maturation of brain networks, observed with resting-state functional MRI (rs-fc-MRI) in adolescents and adults with TS, might evolve to a mature pattern in adults who experience tic improvement or remission. We explored the feasibility of testing this hypothesis in our population of young adult TS males, each with prior clinical assessments completed during childhood as part of a separate TS Association Genetics Consortium study.
Methods
A total of 10 TS males (off tic suppressing drugs for at least 6 months) aged 19–32 years, mean follow-up interval 7.5 (2 to 13) years, and 11 neurologically normal controls were enrolled and underwent 3-Tesla structural and rs-fc-MRI sequences.
Results
The mean change in Yale Global Tic Severity Scale (YGTSS) was −31.5% (total) and −26.6% (YGTSS motor+vocal). Two subjects reported resolution of tic-related disability, with drops from mean 45 to 16.5 (YGTSS-total) and 25 to 11.5 (YGTSS motor+vocal.).
Rs-fc-MRI revealed significantly increased connectivity between the ipsilateral anterior and mid cingulate cortex and striatum, increased connectivity between local connections, and decreased connectivity between more distant connections; representing an immature connectivity pattern.
Discussion
Similar to previous reports, we found immature patterns of functional connectivity in adult TS subjects. Despite a lack of complete tic remission, two subjects exhibited dramatic drops in tic severity that correlated with tic-related disability improvement. More work is needed to elucidate the mechanism of such dramatic improvement in TS.
Keywords: Tourette syndrome, tic, OCD, ADHD, MRI
Introduction
Tics are stereotyped, repetitive involuntary movements (motor tics) or sounds (phonic tics) that are often performed in response to an internal feeling or urge (premonitory sensation) and only briefly suppressible.1 Tourette syndrome (TS) is defined by childhood onset of multiple motor and phonic tics lasting more than 1 year.2 Attention-deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) are often comorbid in TS,3 and, together with tic symptoms, comprise what is commonly known as the clinical “TS triad.”
Transient tics may be a common feature of normal brain development, as they can be seen in up to 20% of school-aged children.4 Childhood TS has a worldwide prevalence of about 1%,5 which may be explained in part by immature patterns of functional connectivity,6–9 a hyperactive dopamine-releasing system,10,11 and reduced densities of striatal inhibitory neurons12 and receptors.13 Despite these neurobiological abnormalities, most children experience improvement or complete remission of tic symptoms by the young adult years. A significant reduction in tic severity reportedly begins at a mean age of 16 years, with between 26 and 50% of patients being essentially free of tics (in remission) by early adulthood.14–17
It remains unclear what neurobiological factors mediate tic remission or persistence in young adults with TS; however, developmental failure of subcortical structures may play a role. This was suggested by the only longitudinal magnetic resonance imaging (MRI) study to date, which revealed a correlation between reduced caudate volumes in childhood and greater tic severity in adulthood.18 Cross-sectional volumetric studies in TS adults can be difficult to interpret, as it is unclear whether changes are a primary cause of tics or secondary to tic suppression.19 Resting-state functional connectivity MRI (rs-fc-MRI) has emerged as a potential method to measure brain network architecture in subjects with TS. During normal brain maturation, cortical network hubs develop fewer local connections and a higher number of more distant connections.20 rs-fc-MRI studies have demonstrated that TS adolescents and adults have higher numbers of local connections and fewer distant connections—a less mature pattern—of resting-state functional connectivity compared to controls.6,7 We performed a pilot study to confirm these findings in our local patient population and to justify the potential of rs-fc-MRI to study differential brain maturation between TS subjects with and without tic remission. Of particular interest was whether fronto-striatal connectivity would be abnormal given a prior report of increased activation of fronto-striatal regions in TS.21
Methods
We aimed to recruit individuals aged 18–35 with diagnosis of TS and tic-related impairment documented in childhood from a cohort of 80 eligible male patients previously assessed for TS Association International Consortium for Genetics (TSAICG) research in childhood to determine diagnosis and severity (averaged over the week prior to enrollment) of TS, as well as comorbid OCD or ADHD.22 TS subjects were off tic-suppressing drugs for at least 6 months. A total of 11 neurologically normal male controls that were age-matched within 2 years of participants were also recruited. The University of Utah Institutional Review Board approved the study, and informed consent was obtained from each subject.
Each TS subject completed a list of concomitant medications, the self-administered Conners Adult ADHD rating scale (CAARS), and the clinician-administered Yale Global Tourette Syndrome Scale (YGTSS) and Yale-Brown Obsessive Compulsive Scale (Y-BOCS).
Imaging was carried out using a Siemens 3 T Tim Trio scanner equipped with a 12-channel head coil. Structural/volumetric MRI scans were acquired for each subject using a 3D magnetization-prepared rapid acquisition with gradient echo (MPRAGE) T1-weighted sequence with the following parameters: repetition time/echo time (TR/TE) = 2,000/3.53 ms, 8-degree flip angle, field of view (FOV) 256×256 mm to cover the whole brain, yielding 224 slices, no gap, voxel size 1×1×1 mm.
To assess brain functional connectivity, rs-fc-MRI sequences were implemented using blood-oxygen-level dependent echo planar imaging (BOLD EPI) sequences with matrix dimensions of 64×64×40, TR = 2.0 s, and TE 30 ms. Data preprocessing was performed using MATLAB with AFNI and SPM8 toolboxes, including RETROICOR using physiologic heart rate and respiratory waveforms obtained during scanning,23 slice timing correction, motion correction (realigned and unwarped to separately obtain fieldmaps), coregistration to MPRAGE, and normalization of MPRAGE and functional images to the Montreal Neurological Institute (MNI) template. A general linear model was used to perform regression of the white matter, facial soft tissue, lateral ventricle, and six motion parameters from each voxel's data as previously described (PSTCor).24 Cross-correlation coefficients were computed following band-pass filtering of the data to evaluate frequency-dependent contributions to correlation using standard functional connectivity techniques. The rs-fc-MRI correlations were calculated between each of the averaged time series obtained from 116 regions from the automated anatomical labeling (AAL) brain atlas.25
Results
The study process is outlined in Figure 1. A total of 10 TS subjects (mean age 23.5±4.2 years) were successfully contacted and recruited. MRI data from one TS subject could not be used due to an incomplete examination. A total of 11 controls were enrolled and imaged, but one had to be excluded because a low-grade glioma was found during the structural MRI sequence. Subjects' clinical data are outlined in Table 1. The mean interval between baseline clinical assessment for TSAICG enrollment and the present study was 7.5 (range 2–13) years. Average tic severity over the past week was used to calculate changes from baseline. YGTSS data are outlined in Table 2. All TS subjects showed tic severity improvement. As a group, the mean reduction in YGTSS was 31.5% (total) and 26.6% for YGTSS total tic (motor+vocal) subscore. None were “tic free” in the past week or year, but two described resolution of tic-related impairment, with drops from mean 45 to 16.5 (YGTSS-total) and 25 to 11.5 (YGTSS motor+vocal).
Figure 1. Flowchart showing data recruitment and analysis.

From 80 subjects with prior YGTSS scores, 10 were imaged, with 9 TS subjects and 10 matched control subjects showing images with acceptable image quality.
Table 1. Subject Age and Comorbidity Data.
| Subject | Age | Medications | childhood OCD | Y-BOCS | ADHD diagnosis | Conners total | Conners inattentive | Conners Hyperactive |
|---|---|---|---|---|---|---|---|---|
| 1 | 21 | no | 17 | yes | 82 | 82 | 69 | |
| 2 | 21 | fluoxetine, dextroamphetamine, zolpidem | yes | 27 | yes | 82 | 82 | 69 |
| 3 | 20 | yes | 18 | no | 71 | 66 | 66 | |
| 4 | 24 | atomoxetine | yes | 0 | yes | 63 | 59 | 61 |
| 5 | 19 | yes | 20 | yes | 78 | 82 | 64 | |
| 6 | 28 | no | 0 | no | 71 | 59 | 74 | |
| 7 | 32 | yes | 0 | yes | 89 | 82 | 81 | |
| 8 | 27 | Nortryptiline | no | 0 | yes | 78 | 77 | 69 |
| 9 | 20 | no | 0 | yes | 46 | 46 | 46 | |
| 10 | 23 | no | 0 | yes | 44 | 46 | 44 |
Abbreviations: ADHD, Attention-deficit Hyperactivity Disorder; OCD, Obsessive-compulsive Disorder; Y-BOCS, Yale Brown Obsessive Compulsive Scale taken at the time of MRI.
Table 2. Subject Baseline and Follow-up Tic Severity.
| Subject | YGTSS1 motor | YGTSS1 vocal | YGTSS1 total tic | YGTSS1 tic impairment | YGTSS1 total | YGTSS2 motor | YGTSS2 vocal | YGTSS2 total tic | YGTSS2 tic impairment | YGTSS2 total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | 21 | 42 | 30 | 72 | 23 | 13 | 36 | 20 | 56 |
| 2 | 21 | 14 | 35 | 30 | 65 | 11 | 14 | 25 | 30 | 55 |
| 3 | 18 | 9 | 27 | 20 | 47 | 12 | 9 | 21 | 20 | 41 |
| 4 | 12 | 11 | 23 | 20 | 43 | 4 | 0 | 4 | 10 | 14 |
| 5 | 18 | 10 | 28 | 30 | 58 | 17 | 6 | 23 | 10 | 33 |
| 6 | 13 | 11 | 24 | 10 | 34 | 20 | 13 | 33 | 25 | 58 |
| 7 | 12 | 10 | 22 | 20 | 42 | 15 | 12 | 27 | 5 | 32 |
| 8 | 20 | 22 | 42 | 40 | 82 | 13 | 10 | 23 | 20 | 43 |
| 9 | 13 | 17 | 30 | 30 | 60 | 7 | 6 | 13 | 0 | 13 |
| 10 | 12 | 8 | 20 | 10 | 30 | 7 | 3 | 10 | 10 | 20 |
Abbreviations: YGTSS1, Baseline Yale Brown Global Tourette Syndrome Scale; YGTSS2, Follow-up YGTSS scores at the time of MRI.
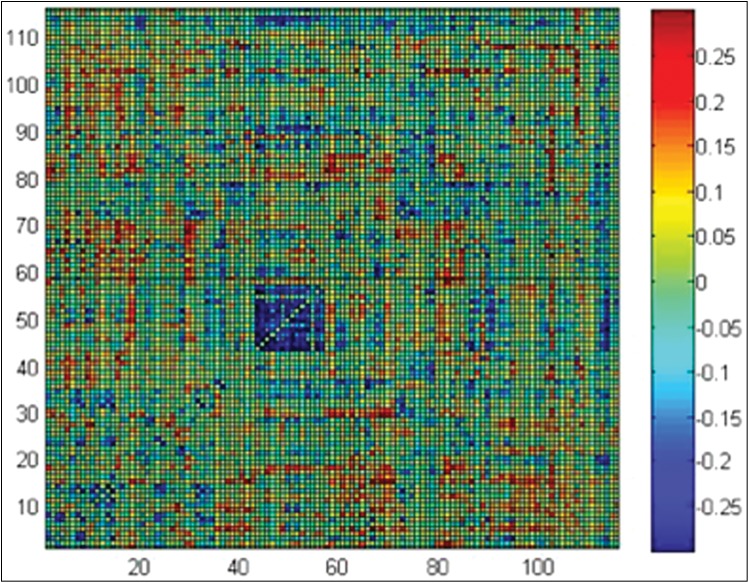
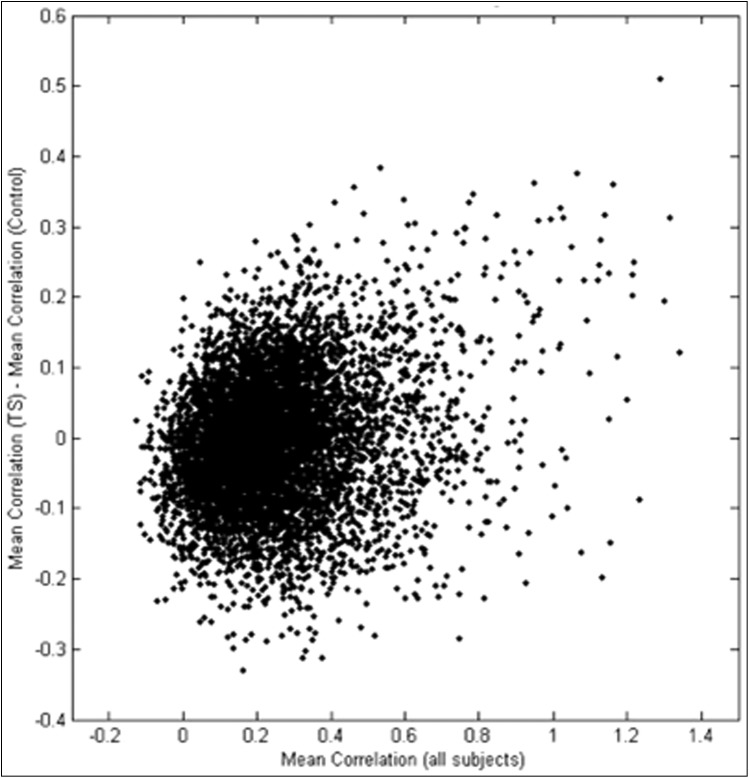
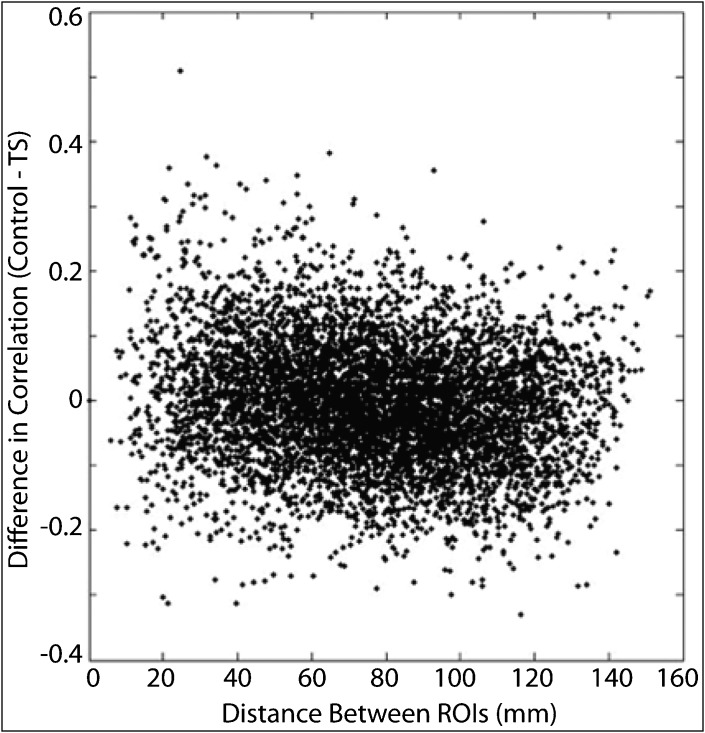
When examining functional connectivity between all 6,670 pairs of 116 regions from the AAL brain atlas, we found three specific differences: increased connectivity between nearby regions, decreased connectivity between more distant regions, and increased connectivity between ipsilateral corticostriatal projections. Regions that were closer together (r = 0.15) and more strongly correlated (r = 0.30) showed the greatest increases in correlation for TS subjects relative to control subjects (Figures 2–4). Regions with dense local interconnections, such as the occipital lobes, showed the greatest increases in connectivity in subjects with TS. For all connections averaged between occipital lobe ROIs, TS subjects showed significantly higher connectivity (p = 0.016). Although the sample size was not powered to identify individual connection differences, given an a priori hypothesis of abnormal function in corticostriatal regions associated with tics,6 we examined measurements between the anterior cingulate cortex and caudate nuclei. Functional connectivity was significantly increased in subjects with TS between the left anterior cingulate cortex and left caudate nucleus (p = 0.022, two-tailed t-test) and right mid cingulate cortex and right caudate nucleus (p = 0.0074, two-tailed t-test).
Figure 2. Difference in Fisher-transformed Correlation between Samples (Control – TS) for each Pair of 116 regions in the AAL brain atlas.
The blue square in the middle shows significantly higher correlation for TS subjects (p = 0.016) averaging correlation for all connections between occipital lobe regions (ROIs 43–56).
Figure 3. Differences in Correlation as a Function of Strength of Functional “Connection.”.
Scatter plot shows difference in correlation (Control – TS) as a function of mean correlation for the connection over all 20 participants.
Figure 4. Differences in Correlation as a Function of Distance of Functional “Connection.”.
Scatter plot shows difference in correlation (Control – TS) as a function of distance between the centroids of the ROIs for each functional “connection.”
Discussion
This was a pilot study designed to explore feasibility of using rs-fc-MRI to study tic remission (with potential recruitment of persistent and remitted cases of TS from participants previously assessed as children in the TSAICG study). Despite a small sample size, our results showed immature functional connectivity patterns in young adult males with persistent TS. These findings are in agreement with recent larger studies in adolescents (ages 10–15 years) and young adults (ages 19–40)6,7 and add to a growing body of literature showing that TS is a neurodevelopmental disorder.
Prior studies of brain connectivity in TS include findings of decreased structural connectivity (diffusion tensor imaging) in tracts involving the supplementary motor area with basal ganglia and in frontal cortico-cortical circuits.26 Functional connectivity exhibited “immature” distribution compared to controls, including connectivity values more similar to younger subjects than age-matched control subjects.7 In a study probing corticostriatal circuit connectivity, stronger functional integration was observed compared to controls.6 A study measuring fractional amplitude of low-frequency fluctuations (ALFF) found decreased ALFF in the posterior and anterior cingulate, superior and middle frontal cortex, and superior parietal lobule.27 ALFF is a composite marker of functional connectivity that examines individual regions rather than pairs of regions, and it is helpful in identifying individual brain areas where connectivity differences are most pronounced between groups, given that lower frequency fluctuation is a marker of functional network complexity.28 Although only a few studies have been performed, our results are broadly consistent with a pattern of increased frontostriatal connectivity with increased local connectivity (exemplified by short-range occipital connections and a negative correlation between distance and relative connectivity in TS) and decreased long-range connectivity.
Although our sample size was small, we did not observe any cases of complete tic remission. A previous longitudinal study found that 90% of adults still had objective evidence of tics (based on video review by a movement disorders expert), including half of those who felt that they were free of tics.29 Taken together, these findings raise concern that previous reports of complete remission14–17 may have overstated the degree of improvement seen in patients with TS. We did find two patients with prior tic-related disability in childhood who no longer reported tic-related disability as adults and had much more dramatic improvements in YGTSS scores than their peers. More work is needed to better understand the cause(s) of such dramatic improvements in TS. To date, 20 centers have enrolled children for the TSAICG who are now young adults. Our findings and high loss to follow-up (only 10 of 80 potentially eligible subjects were recruited) raise concerns about the feasibility of recruiting cases exhibiting dramatic improvement from these cohorts. More work is needed to better characterize and study the impact of early medical or behavioral interventions on the long-term clinical course of TS. Academic centers can utilize electronic medical record systems to collect and track standardized clinical measures (YGTSS, Y-BOCS) across lifespan of the patient; however, innovative funding mechanisms will be needed to support longitudinal studies of imaging and other biomarkers not routinely indicated in the clinical care of most TS patients.
Footnotes
Funding: Margolis Foundation; National Institute of Mental Health K08MH092697-02.
Financial disclosures: D.R.S. received consulting and/or speaker honoraria from Teva Neuroscience, Lundbeck, U.S. World Meds, Gerson Lehrman Group, and Williams Law Firm and research support from Teva Neuroscience, Auspex, UCB, Acadia, Phytopharm, Allon, Cure Huntington Disease Foundation, and National Institutes of Health. J.S.A. received research support from the National Institute of Mental Health (K08MH092697).
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Shprecher D, Kurlan R. The management of tics. Mov Disord. 2009;24:15–24. doi: 10.1002/mds.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision) [Google Scholar]
- 3.Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003;45:315–319. doi: 10.1111/j.1469-8749.2003.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 4.Kurlan R, McDermott MP, Deeley C, et al. Prevalence of tics in schoolchildren and association with placement in special education. Neurology. 2001;57:1383–1388. doi: 10.1212/WNL.57.8.1383. [DOI] [PubMed] [Google Scholar]
- 5.Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008;65:461–472. doi: 10.1016/j.jpsychores.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Worbe Y, Malherbe C, Hartmann A, et al. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–1946. doi: 10.1093/brain/aws056. [DOI] [PubMed] [Google Scholar]
- 7.Church JA, Fair DA, Dosenbach NU, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. Am J Psychiatry. 2011;168:1326–1337. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh R, Zhu H, Wang Z, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control in Tourette's syndrome. Am J Psychiatry. 2007;164:955–966. doi: 10.1176/appi.ajp.164.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer HS, Szymanski S, Giuliano J, et al. Elevated intrasynaptic dopamine release in Tourette's syndrome measured by PET. Am J Psychiatry. 2002;159:1329–1336. doi: 10.1176/appi.ajp.159.8.1329. [DOI] [PubMed] [Google Scholar]
- 11.Wong DF, Brasic JR, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–1251. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka Y, Kalanithi PS, Grantz H, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerner A, Bagic A, Simmons JM, et al. Widespread abnormality of the gamma-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135:1926–1936. doi: 10.1093/brain/aws104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erenberg G, Cruse RP, Rothner AD. The natural history of Tourette syndrome: a follow-up study. Ann Neurol. 1987;22:383–385. doi: 10.1002/ana.410220317. [DOI] [PubMed] [Google Scholar]
- 15.Leckman JF, Zhang H, Vitale A, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 16.Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160:65–69. doi: 10.1001/archpedi.160.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burd L, Kerbeshian PJ, Barth A, Klug MG, Avery PK, Benz B. Long-term follow-up of an epidemiologically defined cohort of patients with Tourette syndrome. J Child Neurol. 2001;16:431–437. doi: 10.1177/088307380101600609. [DOI] [PubMed] [Google Scholar]
- 18.Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plessen KJ, Lundervold A, Gruner R, et al. Functional brain asymmetry, attentional modulation, and interhemispheric transfer in boys with Tourette syndrome. Neuropsychologia. 2007;45:767–774. doi: 10.1016/j.neuropsychologia.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fair DA, Cohen AL, Dosenbach NU, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzone L, Yu S, Blair C, et al. An FMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. Am J Psychiatry. 2010;167:341–349. doi: 10.1176/appi.ajp.2009.08121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharf JM, Yu D, Mathews CA, et al. Genome-wide association study of Tourette's syndrome. Mol Psychiatry. 2013;18:721–728. doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::AID-MRM23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 26.Cheng B, Braass H, Ganos C, et al. Altered intrahemispheric structural connectivity in Gilles de la Tourette syndrome. Neuroimage Clin. 2013;4:174–181. doi: 10.1016/j.nicl.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui Y, Jin Z, Chen X, He Y, Liang X, Zheng Y. Abnormal baseline brain activity in drug-naive patients with Tourette syndrome: a resting-state fMRI study. Front Hum Neurosci. 2014;7:913. doi: 10.3389/fnhum.2013.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JS, Zielinski BA, Nielsen JA, Ferguson MA. Hum Brain Mapp. 2013. Complexity of low-frequency blood oxygen level-dependent fluctuations covaries with local connectivity. [Epub ahead of print]. doi: 10.1002/hbm.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappert EJ, Goetz CG, Louis ED, Blasucci L, Leurgans S. Objective assessments of longitudinal outcome in Gilles de la Tourette's syndrome. Neurology. 2003;61:936–940. doi: 10.1212/01.WNL.0000086370.10186.7C. [DOI] [PubMed] [Google Scholar]