Abstract
Objective
To estimate the cost-effectiveness of a trial of labor after one previous cesarean (TOLAC) when incorporating long-term events and outcomes.
Methods
A Markov model comparing TOLAC with elective repeat cesarean delivery (ERCD) was developed for a hypothetical cohort with no contraindication to a TOLAC. Women were selected from a prospective study to derive probability estimates for potential events through three subsequent pregnancies. Probabilities for cerebral palsy and stress urinary incontinence, cost data, and quality adjusted life years (QALYs) were obtained from the literature. The primary outcome was cost-effectiveness measured as the marginal cost per QALY gained, with a $50,000 threshold per QALY used to define cost-effectiveness.
Results
The TOLAC strategy dominated the ERCD strategy at baseline, with $164.2 million saved and 500 QALYs gained per 100,000 women. The model was sensitive to six variables: the probability of uterine rupture and successful TOLAC among women with no prior vaginal delivery, the frequency of stress urinary incontinence, and the costs of failed TOLAC, successful TOLAC, and ERCD. When the probability of TOLAC success was at the base value, 67.2%, TOLAC was preferred if the probability of uterine rupture was 3.1% or less. When the probability of uterine rupture was at the base value, 0.8%, the TOLAC strategy was preferred as long as the probability of success was 47.2% or more. Probabilistic sensitivity analysis confirmed the base-case analysis.
Conclusions
Under baseline circumstances, TOLAC is less expensive and more effective than an ERCD when considering long-term consequences when the likelihood of success is 47.2% or more.
Keywords: cost-effectiveness, trial of labor, elective repeat, accreta
Introduction
In the United States, approximately 1 out of 5, or almost 300,000 women per year planning the delivery of their second child have had a prior cesarean delivery and are therefore faced with the choice of whether or not to attempt a trial of labor [1,2] The ramifications of this decision on maternal and infant outcomes have been reviewed in several papers and summarized in the Agency for Healthcare Research and Quality (AHRQ) evidence report and technology assessment which concluded that “vaginal birth after a previous cesarean is a reasonable and safe choice for the majority of women with prior cesarean” [3]. In order to adequately counsel women both the short-term and long-term maternal and infant effects of this decision must be considered. The downstream effects include not only adverse perinatal outcomes from the index delivery, such as cerebral palsy, but adverse outcomes in future pregnancies, such as placenta previa and accreta.
Previous decision analyses have compared trial of labor after a previous cesarean (TOLAC) with elective repeat cesarean delivery (ERCD), but have been limited in their inclusion of inputs and the incorporation of long-term health consequences related to the initial delivery approach [4-8]. For example, three analyses provided the outcome of the initial decision without considering further pregnancies [4-6], and another did not take into consideration patient preferences [7]. A decision model comparing these two delivery strategies estimated the probabilities of maternal consequences throughout reproductive life, but did not include costs, preferences or infant outcomes [8].
The present cost-effectiveness analysis was undertaken in order to incorporate relevant long-term outcomes and to determine the future health and economic consequences of choosing a TOLAC as opposed to an ERCD among women with one previous cesarean.
Methods
We developed a decision analytic model comparing a TOLAC with an ERCD for a hypothetical cohort of 100,000 women who had no contraindication to a TOLAC and whose only previous delivery was through a low transverse cesarean incision. To model downstream effects from this initial decision, a Markov model was developed to account for potential events related to this initial choice throughout a woman's life. This analysis was based on the societal perspective, incorporating all health outcomes and economic costs regardless of who experienced the outcome or paid the costs [9]. The primary outcome was cost-effectiveness, measured as the marginal cost per quality-adjusted life-year (QALY) gained, with a marginal cost per QALY ratio of less than $50,000 (a frequently used threshold in the United States) used to define cost-effectiveness [10].
The decision tree was developed using TreeAge Pro 2012 (TreeAge Software, Inc., Williamstown, MA). Probabilities for the decision tree were obtained primarily from data collected in 1999 through 2002 in a registry (the Cesarean Registry) by institutions of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Nineteen clinical centers throughout the United States participated in this observational study, in which data were collected on all women with a prior cesarean delivery. The study was approved by the institutional review board of each participating center where study personnel abstracted data from patient charts under a waiver of informed consent. Further detail on the Cesarean Registry can be obtained from previously published articles [11,12].
The Study Populations
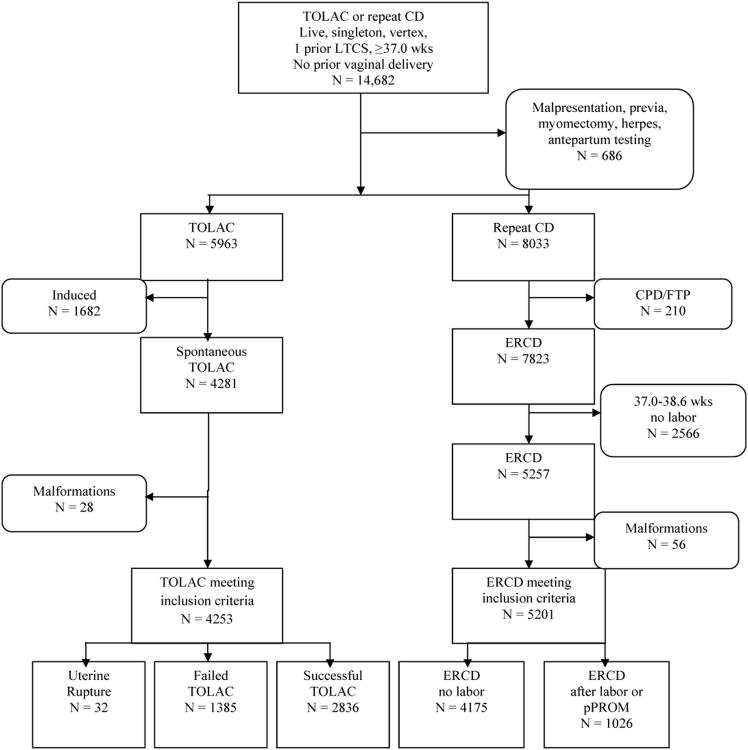
The initial decision represented a woman's approach to delivery: either a TOLAC or an ERCD (Fig. 1). Women who were eligible for this choice had a singleton, term vertex gestation, and one prior low transverse incision without a prior vaginal delivery (n=14,682). A gestation was considered term if delivery occurred at or beyond 37 weeks' gestation. An ERCD was defined as a cesarean delivery without any indication other than the prior cesarean. Thus, women who had a repeat cesarean for indications such as placenta previa or active herpes were excluded (n=686). In order to ensure that women who underwent ERCD truly had no indication for cesarean other than their choice, those who reported to have a cesarean that was elective but who had an additional reported indication implying this was not the case (i.e. cephalopelvic disproportion, failure to progress, cord prolapse, non-reassuring tracing or abruption) were excluded (n=210). Also, women were ineligible for the cohort if they had a scheduled cesarean prior to 39 weeks without spontaneous labor or premature rupture of membranes given that elective delivery prior to 39 weeks is associated with known adverse outcomes unrelated to mode of delivery (n=2566) [13]. Women with induced labor also were excluded since this intervention has been associated with a lower probability of success and a higher probability of uterine rupture, and is not a probabilistic possibility but a choice that a woman and her provider can make (n=1682) [14,15]. Women carrying fetuses with congenital malformations (i.e. trisomy, clubbed foot, neural tube defect) were removed since these conditions, unrelated to mode of delivery, could influence the newborn outcome (n=84). This process left 9454 women for analysis, of whom 4253 (45.0%) had a TOLAC and 5201 (55.0%) had an ERCD. The maternal and infant outcomes in the decision tree were contingent on the mode of delivery (i.e., vaginal delivery, cesarean delivery, or delivery in the context of uterine rupture).
Figure 1.
Flow chart illustrates the development of the index pregnancy study groups.
TOLAC, trial of labor after a previous cesarean; CD, cesarean delivery; LTCS, low transverse cesarean section; CPD, cephalopelvic disproportion; FTP, failure to progress; ERCD, elective repeat cesarean delivery; pPROM, premature rupture of the membranes.
Subsequent health states were determined for future pregnancies, with the assumption that for all modes of delivery after two births, 22% of women will have a third child, and after three births, 14% of women will have a fourth child [16]. In the model, those women who experienced a successful TOLAC were potentially eligible for another TOLAC. Women who initially chose an ERCD, or who experienced a cesarean or uterine rupture during their chosen TOLAC underwent an indicated repeat cesarean in subsequent pregnancies. The cycle length between each pregnancy was two years, representing the average interval between childbearing, whereas other health states had a one year cycle length. Maternal age at the beginning of the Markov model was assumed to be 28 years of age, the median maternal age of women in the Cesarean Registry entering the model. The model was terminated after 78 years, representing the life expectancy at birth in 2007 of 77.9 years [17] and when over 92% of the infants entered the death state. The maternal and infant branches of the tree were considered to be independent events. Figure 2 shows as an example the TOLAC 2 health state, representing women undergoing a trial of labor after one previous cesarean with a prior vaginal delivery.
Figure 2. Example of the TOLAC 2 arm of the decision tree.
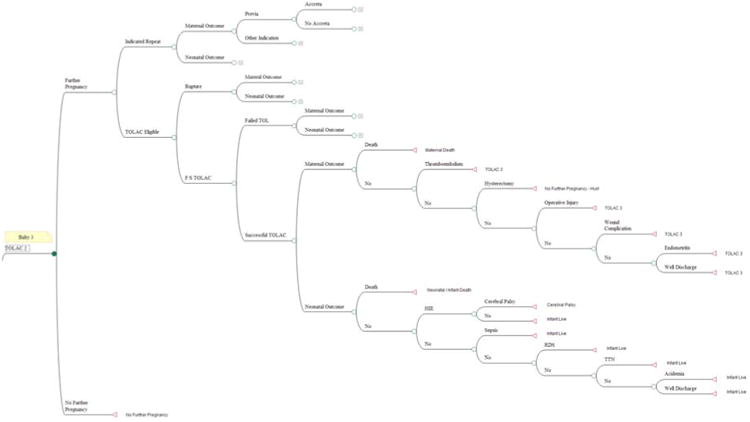
CP, cerebral palsy; IR-2CDPV, indicated repeat cesarean with 2 prior cesareans and one or more prior vaginal deliveries; RDS, respiratory distress syndrome; TOLAC, trial of labor after a previous cesarean; TTN, transient tachypnea of the newborn
Maternal and Infant Probabilities and Outcomes
Maternal and infant probabilities for these subsequent health states were obtained based on data from the Cesarean Registry that accounted for mode of delivery, the number of prior cesareans, and presence of prior vaginal deliveries. In addition, the model accounted for the chance in subsequent pregnancies that a woman developed indications for a cesarean (e.g., breech presentation or active herpes lesion), or that a placenta previa and/or accreta occurred (Tables 1-5). The ranges used in sensitivity analysis were obtained from the 95% Blyth-Still-Casella binomial confidence intervals (from Stat-Xact, Cytel Software) based on the proportion of events [18]. Since the probability of successful TOLAC and uterine rupture have previously been shown to be variables to which the results of decision analytic models of TOLAC are sensitive, these two variables were varied across a range (0-5.0%) wider than that which would have been derived from the dataset alone.
Table 1. Maternal outcome probability estimates.
| Mode of Delivery | Hysterectomy | Operative Injury | Wound Complication | Endometritis | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Baseline | Range | Baseline | Range | Baseline | Range | Baseline | Range | |
|
| |||||||||
| TOLAC 1 | |||||||||
| Rupture | 32 | 3.13 | 0.160-16.12 | 15.63 | 6.37-31.89 | 0 | 0-10.89 | 12.5 | 4.39-28.15 |
| Failed TOLAC | 1385 | 0.217 | 0.059-0.607 | 1.23 | 0.717-1.95 | 1.30 | 0.811-2.01 | 7.80 | 6.46-9.31 |
| Successful TOLAC | 2835 | 0 | 0-0.122 | 0 | 0-0.122 | 0.035 | 0.002-0.194 | 1.76 | 1.32-2.31 |
| TOLAC 2 and 3 | |||||||||
| Indicated repeat | 454 | 0.220 | 0.011-1.22 | 0.661 | 0.180-1.86 | 0.661 | 0.180-1.86 | 5.51 | 3.60-7.90 |
| Rupture | 47 | 12.77 | 5.71-24.51 | 14.89 | 6.76-28.26 | 2.13 | 0.109-10.70 | 10.64 | 4.29-22.67 |
| Failed TOLAC | 905 | 0.332 | 0.090-0.931 | 0.553 | 0.218-1.27 | 0.995 | 0.495-1.82 | 7.62 | 6.03-9.49 |
| Successful TOLAC | 7095 | 0.127 | 0.063-0.240 | 0.014 | 0.001-0.077 | 0.042 | 0.012-0.118 | 0.691 | 0.511-0.908 |
| ERCD | 5199 | 0.231 | 0.129-0.390 | 0.231 | 0.129-0.390 | 0.846 | 0.616-1.12 | 1.67 | 1.34-2.06 |
| IR-2CDNPV | 6148 | 0.342 | 0.212-0.521 | 0.407 | 0.273-0.590 | 1.30 | 1.03-1.61 | 2.47 | 2.10-2.89 |
| IR-3CDNPV | 1728 | 1.22 | 0.754-1.82 | 0.984 | 0.574-1.56 | 1.74 | 1.17-2.46 | 2.37 | 1.71-3.17 |
| IR-2CDPV | 1184 | 0.676 | 0.292-1.30 | 0.760 | 0.378-1.41 | 1.35 | 0.815-2.14 | 2.96 | 2.07-4.04 |
Data presented as percent; all data from the Cesarean Registry. Data were missing for one woman in the TOLAC 1, successful TOLAC group and two women in the ERCD group.
TOLAC, trial of labor after a previous cesarean; ERCD, elective repeat cesarean delivery; IR-2CDNPV, indicated repeat cesarean with 2 prior cesareans and no prior vaginal delivery; IR-3CDNPV, indicated repeat cesarean with 3 prior cesareans and no prior vaginal delivery; IR-2CDPV, indicated repeat cesarean with 2 prior cesareans and one or more prior vaginal deliveries
Table 5. Infant outcome probability estimates.
| Mode of Delivery | RDS | TTN | Acidemia | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | Baseline | Range | Baseline | Range | Baseline | Range | |
|
| |||||||
| TOLAC 1 | |||||||
| Rupture | 32 | 0 | 0-9.47 | 0 | 0-9.47 | 9.38 | 2.60-23.70 |
| Failed TOLAC | 1384 | 1.01 | 0.588-1.65 | 1.81 | 1.21-2.63 | 0.578 | 0.250-1.11 |
| Successful TOLAC | 2836 | 0.670 | 0.404-1.04 | 0.741 | 0.459-1.12 | 0.071 | 0.013-0.244 |
| TOLAC 2 and 3 | |||||||
| Indicated repeat | 544 | 6.07 | 4.21-8.36 | 2.94 | 1.78-4.68 | 0.368 | 0.065-1.28 |
| Rupture | 48 | 2.08 | 0.107-10.47 | 0 | 0-6.64 | 12.50 | 5.59-24.03 |
| Failed TOLAC | 907 | 2.54 | 1.67-3.74 | 1.87 | 1.10-2.98 | 0.662 | 0.289-1.38 |
| Successful TOLAC | 7096 | 1.02 | 0.795-1.28 | 0.832 | 0.634-1.06 | 0.085 | 0.037-0.183 |
| ERCD | 5201 | 0.461 | 0.296-0.681 | 1.31 | 1.02-1.65 | 0.250 | 0.133-0.421 |
| IR-2CDNPV | 6203 | 1.76 | 1.45-2.11 | 1.94 | 1.61-2.30 | 0.306 | 0.185-0.475 |
| IR-3CDNPV | 1767 | 2.72 | 2.01-3.56 | 2.21 | 1.57-3.00 | 0.396 | 0.186-0.782 |
| IR-2CDPV | 1196 | 2.34 | 1.61-3.34 | 1.25 | 0.704-2.05 | 0 | 0-0.289 |
Data presented as percent and represent conditional probabilities given prior mode of delivery; all data from the Cesarean Registry
Data were missing for one infant in the TOLAC 1, failed TOLAC group.
CD, cesarean delivery; TOLAC, trial of labor after a previous cesarean; ERCD, elective repeat cesarean delivery; IR-2CDNPV, indicated repeat cesarean with 2 prior cesareans and no prior vaginal delivery; IR-3CDNPV, indicated repeat cesarean with 3 prior cesareans and no prior vaginal delivery; IR-2CDPV, indicated repeat cesarean with 2 prior cesareans and one or more prior vaginal deliveries
The maternal outcomes that were considered included: endometritis (clinical diagnosis of puerperal uterine infection in the absence of findings suggesting another source), wound complication (seroma, hematoma or infection), operative injury (broad ligament hematoma, cystotomy, or bowel or ureteral injury), peripartum hysterectomy, uterine rupture (disruption or tear of the uterine muscle and visceral peritoneum or a uterine muscle separation with extension to adjacent structures), placenta previa, placenta accreta, thromboembolism and maternal death. All probabilities except for the final two outcomes were obtained from the Cesarean Registry. Due to the rarity of thromboembolism and maternal death, these probabilities were estimated from the literature [3,19]. Stress urinary incontinence (SUI) also was incorporated into the model and estimated, from the literature, as the marginal increase in long-term persistent SUI for TOLAC compared to ERCD to the end of life [20]. Since the data demonstrating this long-term increase have been considered inconclusive, however, these probabilities were not included in the base-case scenario, but in the sensitivity analysis [21].
Neonatal outcomes that were evaluated were: transient tachypnea of the newborn (TTN), respiratory distress syndrome (RDS), infection (i.e., suspected or confirmed sepsis), acidemia (arterial cord pH less than 7.0), hypoxic ischemic encephalopathy (HIE), and neonatal death. Because cerebral palsy (CP) could occur as a long-term consequence of an event (HIE) at the time of delivery, the probability of CP was incorporated in the model by estimating, from the literature, that 12% of the infants with HIE would ultimately be diagnosed with CP [22].
Costs
With the exception of CP and SUI, the following costs, based on mode of delivery, were incorporated into the model: hospital, obstetrician, pediatrician, anesthesiologist, maternal and caregiver opportunity costs. A summary of these costs by mode of delivery and outcome is provided in Table 6. Further detail regarding the basis for these costs is provided in the Supplementary Material (S1-S2). Hospital costs were obtained from the 2009 AHRQ's Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUPnet), a nationwide database of hospital inpatient stays containing approximately 95% of all hospital discharges in the United States [23]. These costs represent direct and indirect hospital costs. Obstetrician and pediatrician costs were obtained from the 2010 Current Procedural Terminology from the American Medical Association (AMA) [24]. Since the Cesarean Registry did not contain data that would allow estimation of anesthesia costs, these costs were derived from the literature [4]. Maternal and caregiver postpartum opportunity costs were derived from the Bureau of Labor Statistics using the 2009 median hourly wage and salary averages for women 25 to 34 years old and for all individuals 16 years and older, respectively [25]. Since the costs associated with maternal and infant death are hard to quantify, as these events occur in such a large variety of circumstances, a range of 0 to $1 million was used, with hospital baseline estimates of $20,000 and $50,000 respectively, approximating a high hospital cost outcome such as hysterectomy or HIE. For CP, hospital costs after delivery were estimated as twice the base cost of HIE, with the addition of approximately $9000 for pediatrician fees and $23,800 of direct and indirect costs per year for the next 49 years [26-28]. Costs associated with SUI were obtained from the literature and accorded a yearly cost of $400, with a range of 0 to $1600 [29]. All costs are presented in 2009 US dollars, with adjustments for inflation, when needed, according to the medical care component of the Consumer Price Index [30]. In sensitivity analysis, due to the lack of standard errors for most values, the majority of costs were ranged from 50% to 400% of the base-case estimate. For maternal and well-infant discharge, the upper limit of the range was set at 150%, since it was assumed costs above this range would imply an adverse outcome. Although the ranges included values that appeared beyond plausible in some cases, such a wide range ensured that threshold analyses could be judiciously performed [31].
Table 6. Cost estimates by mode of delivery.
| Outcomes | Uterine Rupture | Failed TOLAC | Successful TOLAC | ERCD/Indicated Repeat* | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Baseline | Range | Baseline | Range | Baseline | Range | Baseline | Range | |
|
| ||||||||
| Maternal | ||||||||
| Death | 27.9 | 0-1,000.0 | 27.9 | 0-1,000.0 | 24.1 | 0-1,000.0 | 27.41 | 0-1,000.0 |
| Thromboembolism | 19.1 | 9.6-76.5 | 18.4 | 9.2-73.5 | 13.3 | 6.7-53.3 | 16.61 | 8.3-66.4 |
| Hysterectomy | 20.4 | 10.2-81.5 | 19.6 | 9.8-78.6 | 14.6 | 7.3-58.4 | 17.9 | 8.9-71.5 |
| Operative injury | 16.4 | 8.2-65.6 | 15.7 | 7.8-62.6 | 10.6 | 5.3-42.5 | 13.9 | 6.9-55.6 |
| Wound complication | 18.1 | 9.0-72.3 | 17.3 | 8.7-69.4 | 12.3 | 6.2-49.2 | 15.6 | 7.8-62.3 |
| Endometritis | 18.2 | 9.1-73.0 | 17.5 | 8.8-70.0 | 12.5 | 6.2-49.9 | 15.7 | 7.9-63.0 |
| Well (no adverse outcome) | 13.9 | 6.9-20.8 | 13.1 | 6.6-19.7 | 8.1 | 4.0-12.1 | 11.4 | 5.7-17.0 |
| Infant | ||||||||
| Death | 52.2 | 0-1,000.0 | 52.2 | 0-1,000.0 | 52.2 | 0-1,000.0 | 52.2 | 0-1,000.0 |
| CP, acute care at birth | 82.4 | 41.2-329.7 | 82.4 | 41.2-329.7 | 82.4 | 41.2-329.7 | n/a | n/a |
| CP, ongoing care per yr | 23.8 | 11.9-95.1 | 23.8 | 11.9-95.1 | 23.8 | 11.9-95.1 | n/a | n/a |
| HIE | 40.9 | 20.4-163.5 | 40.9 | 20.4-163.5 | 40.9 | 20.4-163.5 | 40.9 | 20.4-163.5 |
| Sepsis | 8.6 | 4.3-34.2 | 8.6 | 4.3-34.2 | 8.6 | 4.3-34.2 | 8.6 | 4.3-34.2 |
| RDS | 25.9 | 12.9-103.5 | 25.5 | 12.7-101.9 | 25.5 | 12.7-101.9 | 25.5 | 12.7-101.9 |
| TTN | 9.1 | 4.5-36.3 | 8.7 | 4.3-34.6 | 8.7 | 4.3-34.6 | 8.7 | 4.3-34.6 |
| Acidemia | 7.7 | 3.9-30.9 | 7.3 | 3.7-29.3 | 7.3 | 3.7-29.3 | 7.3 | 3.7-29.3 |
| Well (no adverse outcome) | 0.9 | 0.5-1.4 | 0.9 | 0.4-1.3 | 0.9 | 0.4-1.3 | 0.9 | 0.5-1.3 |
Currency in dollars ($thousands); ERCD, elective repeat cesarean delivery; TOLAC, trial of labor after a previous cesarean;
CP, cerebral palsy; HIE, hypoxic ischemic encephalopathy; RDS, respiratory distress syndrome; TTN, transient tachypnea of the newborn
For maternal outcomes placenta previa and accreta in the indicated repeat groups, $5064 and $8561 in additional costs applied, respectively
Quality of Life
Disutilities or utility decrements were assigned based on the literature (Table 7) [4,32-34]. All women experiencing placenta accreta were assigned the hysterectomy disutility. Those infants who experienced infant death, CP and HIE were assigned disutilities of 0, 0.44, and 0.75, respectively. QALYs were determined based on the utilities and life expectancy. It was assumed mode of delivery per se did not alter maternal or neonatal life expectancy, which was estimated using 2007 life table estimates [17]. For infants with CP, however, a life expectancy of 50 years was assumed [27].
Table 7. Disutility estimates by mode of delivery or outcome.
| Disutility | Disutility Days | ||||
|---|---|---|---|---|---|
|
| |||||
| Mode of Delivery/Outcome | Baseline | Range | Baseline | Range | Reference |
| Maternal | 0 | 0-0.5 | All | All | Assumed |
| ERCD/indicated repeat | 0.45 | 0.25-0.65 | 21 | 14-180 | 4 |
| Uterine rupture | 0.49 | 0.29-0.69 | 21 | 14-180 | 4 |
| Failed TOLAC* | 0.47 | 0.27-0.67 | 21 | 14-180 | 4 |
| Successful TOLAC | 0.35 | 0.15-0.55 | 7 | 2-42 | 4 |
| Hysterectomy† | 0.49 | 0.29-0.69 | 21 | 14-180 | 4, 32 |
| Urinary stress incontinence | 0.19 | 0-0.29 | All | All | 33 |
| Cerebral palsy | 0.44 | 0.26-0.61 | All | All | 34 |
| HIE | 0.75 | 0.35-0.95 | 42 | 14-180 | Assumed |
| Infant | 0 | 0-0.75 | All | All | Assumed |
ERCD, elective repeat cesarean delivery; TOLAC, trial of labor after a previous cesarean; n/a; not applicable;
HIE, hypoxic ischemic encephalopathy
Extrapolated from Chung et al., midway between ERCD and rupture.4
Blend of Harris et al. and Chung et al. at 55% and 45% respectively to represent the proportion of women with a hysterectomy that would and would not have desired another pregnancy.4, 32 For Chung et al. assumed the disutility and disutility days in the Table and from Harris et al. disutilities of 0.31 (0.14-0.48) until age 50. Results in a disutility and range for first two years of 0.35 (0.15-0.55) and subsequently 0.17 (0.08-0.26) until age 50.
Sensitivity Analyses
To test the robustness of the results obtained from the base-case model, sensitivity analyses were performed. One-way sensitivity analysis was conducted on all probabilities, costs and QALYs by varying one variable at a time from the low to high value in its range, while holding other variables fixed. As recommended by the United States Panel on Cost Effectiveness in Health and Medicine, all costs and QALYs were discounted at 3% annually in the base case, with a range of 0-7% tested in sensitivity analysis [35]. Multivariable sensitivity analysis also was conducted by varying more than one probability at time. This included probabilistic sensitivity analysis (PSA) using Monte Carlo simulation with 10,000 iterations to determine how often the base-case strategy was preferred. Simulation was conducted using the beta or uniform distribution for the probabilities where appropriate and the gamma distribution for costs.
Results
Base-Case Scenario Analysis
The base-case analysis revealed that, for a hypothetical cohort of 100,000 women whose first birth was a low-transverse cesarean, the choice of a TOLAC resulted in 80,229 fewer cesareans as well as fewer cases of the long-term maternal outcomes hysterectomy (271), placenta previa (93), placenta accreta (80) and maternal death (10) (Table 8). Conversely, TOLAC was associated with 816 additional uterine ruptures, as well as the long-term adverse neonatal outcomes of infant death (111), HIE (76) and CP (9). Overall, the TOLAC strategy was dominant, since it was both less expensive and more effective than the ERCD strategy, with $164.2 million and 500 QALYs saved per 100,000 women (Table 9).
Table 8. Maternal and infant outcomes per 100,000 women by mode of delivery.
| Outcome | Second Child (Index Pregnancy) | Third Child | Fourth Child | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| TOLAC | ERCD | TOLAC | ERCD | TOLAC | ERCD | TOLAC | ERCD | |
|
| ||||||||
| Deliveries | 100000 | 100000 | 21979 | 21947 | 3065 | 3049 | 125044 | 124996 |
| Cesarean deliveries | 33315 | 100000 | 9796 | 21947 | 1656 | 3049 | 44767 | 124996 |
| Uterine rupture | 752 | 0 | 52 | 0 | 12 | 0 | 816 | 0 |
| Maternal death | 2 | 10 | 1 | 3 | 0 | 0 | 3 | 13 |
| Thromboembolism | 89 | 178 | 23 | 39 | 4 | 5 | 116 | 222 |
| Hysterectomy | 94 | 231 | 87 | 171 | 35 | 85 | 216 | 487 |
| Operative injury | 513 | 230 | 58 | 104 | 20 | 37 | 591 | 371 |
| Wound infection | 439 | 841 | 121 | 283 | 26 | 52 | 586 | 1176 |
| Endometritis | 3716 | 1645 | 427 | 530 | 63 | 70 | 4206 | 2245 |
| Previa alone | 0 | 0 | 47 | 124 | 13 | 29 | 60 | 153 |
| Accreta | 0 | 0 | 27 | 79 | 15 | 43 | 42 | 122 |
| Infant death | 47 | 0 | 186 | 127 | 29 | 24 | 262 | 151 |
| Cerebral palsy | 9 | 0 | 0 | 0 | 0 | 0 | 9 | 0 |
| HIE | 69 | 0 | 6 | 0 | 1 | 0 | 76 | 0 |
| Sepsis | 4974 | 2860 | 1648 | 1610 | 277 | 315 | 6899 | 4785 |
| RDS | 736 | 448 | 311 | 356 | 54 | 74 | 1101 | 878 |
| TTN | 1013 | 1267 | 265 | 385 | 39 | 58 | 1317 | 1710 |
| Acidemia | 269 | 239 | 43 | 60 | 6 | 10 | 318 | 309 |
TOLAC, trial of labor after a previous cesarean; ERCD, elective repeat cesarean delivery; HIE, hypoxic ischemic encephalopathy; RDS, respiratory distress syndrome; TTN, transient tachypnea of the newborn infant
Table 9. Overall cost-effectiveness analysis results at 3% discount rate.
| Strategy | Cost ($) | Incremental Cost ($) | Effectiveness | Incremental Effectiveness | C/E | Incremental C/E (ICER) |
|---|---|---|---|---|---|---|
| TOLAC | 14472 | 0 | 69.053 | 0 | 210 | |
| ERCD | 16113 | 1642 | 69.048 | -0.005 | 233 | -328200 per QALY |
C, cost; E, effectiveness; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year
Sensitivity Analysis
These results were robust to all changes except for 6 variables as determined by one-way sensitivity analysis. These variables and their threshold values were; the probability of uterine rupture during TOLAC for women without a prior vaginal delivery (3.1%), the probability of successful TOLAC for women without a prior vaginal delivery (46.4%), the cumulative increase in frequency of SUI among women undergoing a TOLAC (0.8%), and the cost of failed TOLAC ($19,246), the cost of successful TOLAC ($10,485), and the cost of ERCD ($9420).
Bivariable analysis on the probability of uterine rupture and successful TOLAC in women without a prior vaginal delivery indicated that when the probability of uterine rupture was at 0% the TOLAC strategy was preferred if the probability of success was 40.0% or more. When the probability of uterine rupture was at the base value, 0.8%, TOLAC was preferred if the probability of success was 47.2% or more. When the probability of uterine rupture was set at 1.5% and 3.0%, TOLAC was preferred if the probability of success was 53.6% and 67.2% or more, respectively. When the probability of success was set at the base value, 67.2%, TOLAC was preferred when the probability of uterine rupture was 3.1% or less. The sensitivity of the three SUI variables also was assessed. For example, when the probability, cost and disutility of SUI were set at the upper limit (22.0%, $1600, 0.29, respectively), ERCD was preferred since the TOLAC strategy cost $701.1 million and 156,400 QALYs more per 100,000 women. When the frequency of SUI was considered to be 11% greater for those undergoing TOLAC compared to ERCD, as suggested by Press et al. [20], and the cost and disutility set at the base values ($400, 0.19), ERCD was preferred. For 100,000 women this strategy cost $56.0 million more but in contrast saved 50,900 QALYs, and therefore resulted in an incremental cost per QALY of $1100. If the disutility was set at 0.05, with the frequency set at the threshold (0.8%) and the cost at the base value ($400), TOLAC was the preferred strategy since ERCD cost $1566 more with increasing effectiveness of 0.005, with an incremental cost-effectiveness ratio of approximately $326 thousand. Moreover, with a disutility of 0.05, TOLAC was preferred over the entire cost range (0-$1600) only if the frequency of SUI was 1.8% or less.
Probabilistic sensitivity analysis (Table 10) of the 5 sensitive variables (excluding the frequency of stress urinary incontinence) resulted in TOLAC preferred 69.4%, 68.5% and 68.0% of the time at cost-effectiveness thresholds of $100, $50 and $25 thousand, respectively. When including the frequency of stress urinary incontinence TOLAC was preferred 2.9%, 5.0% and 9.0% of the time. When all of the variables without stress urinary incontinence were subjected to PSA, TOLAC was preferred 63.0%, 64.6% and 65.7% (Fig. 3). When all variables were subjected to PSA, however, TOLAC was preferred 4.6%, 7.3% and 11.8% of the time (Fig. 4). Changing the range of cost variables to 50-150% did not appreciably change the results.
Table 10. Monte Carlo scenarios - percentage TOLAC found cost-effective.
| Cost-Effectiveness Threshold | |||
|---|---|---|---|
| 25,000 | 50,000 | 100,000 | |
| Simulation description - variables varied | |||
| Cerebral palsy* | 100.0 | 100.0 | 100.0 |
| Five sensitive† | 68.0 | 68.5 | 69.4 |
| All variables without stress urinary incontinence | 65.7 | 64.6 | 63.0 |
| Six sensitive‡ | 9.0 | 5.0 | 2.9 |
| Stress urinary incontinence§ | 7.8 | 5.0 | 3.2 |
| All variables, costs 50-400% | 11.8 | 7.3 | 4.6 |
| All variables, costs 50-150% | 11.2 | 6.8 | 4.8 |
The probability, cost and disutility of cerebral palsy
The probability of uterine rupture and successful TOLAC without a prior vaginal delivery, the cost of failed TOLAC, the cost of successful TOLAC, the cost of ERCD all without complications
The probability of uterine rupture and successful TOLAC without a prior vaginal delivery, the cost of failed TOLAC, the cost of successful TOLAC, the cost of ERCD all without complications, the total frequency of stress urinary incontinence
The probability, cost and disutility of stress urinary incontinence
Figure 3.
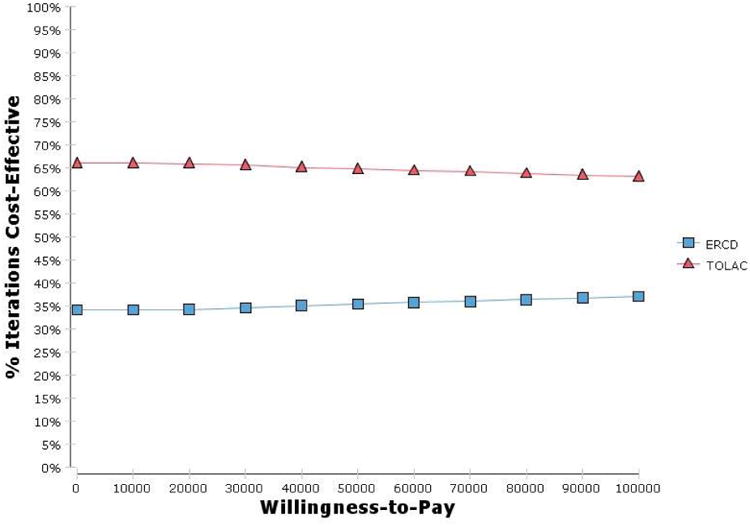
Cost-effectiveness acceptability curve for all variables without stress urinary incontinence, costs 50-400%. ERCD, elective repeat cesarean delivery; TOLAC, trial of labor after a previous cesarean.
Figure 4.
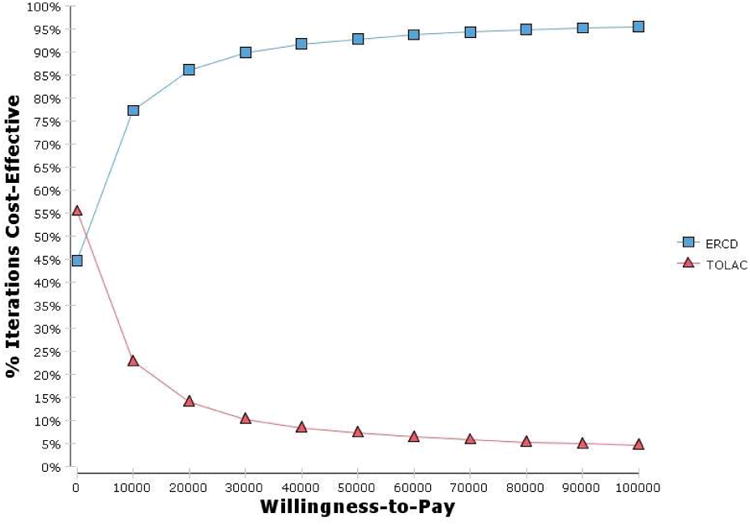
Cost-effectiveness acceptability curve for all variables, costs 50-400%. ERCD, elective repeat cesarean delivery; TOLAC, trial of labor after a previous cesarean.
Discussion
Under the base-case assumptions, for 100,000 women with one prior low transverse cesarean delivery, choosing to deliver a second child by TOLAC was the most cost-effective strategy when the future consequences of this decision were considered, saving $164.2 million and 500 QALYs. This analysis improves upon prior analyses in several ways. First, our study incorporates several maternal and infant long-term downstream outcomes, such as placenta accreta and CP that have not been assessed simultaneously. The maternal and infant probabilities used to inform the model were obtained mainly from an observational study conducted to specifically answer questions related to the mode of delivery after a previous cesarean. This allowed us to uniquely identify cohorts of women that fit specific criteria throughout the reproductive life cycle to populate the model. Moreover, whereas previous studies relied on cost data from a select set of institutions, this analysis utilized cost data from the AHRQ and the AMA. The present analysis demonstrated that although TOLAC was cost-effective under the base-case assumptions, it was sensitive to several variables which, if altered sufficiently, resulted in the alternate strategy of ERCD being preferred. As in previous analyses, the results were found to be sensitive to the probability of uterine rupture and successful TOLAC. Specifically, when the probability of TOLAC success was below 46%, TOLAC was no longer cost-effective. In addition, the results were found to be sensitive to the three cost variables for mode of delivery. The thresholds represented 18%, however, to almost 50% of the base values, implying that these values would be unlikely to occur. Of note, the results were affected by the addition of stress urinary incontinence. Since the evidence for a marginal increase in the setting of TOLAC compared with ERCD has been considered inconclusive by review panels this was investigated in sensitivity analysis [21]. This analysis suggests that if TOLAC influences SUI by more than 0.8%, with a cost of $400 and a disutility of 0.19 per year, the preferred strategy changes to an ERCD. In contrast, it has been suggested that ERCD may be associated with higher rates of childhood asthma and lower rates of breastfeeding, both of which could attenuate any marginal increase for incontinence in the TOLAC arm [36]. They were not modeled here, however, as well since the scientific evidence for these marginal effects are also considered insufficient and even less robust than for incontinence [36,37]. A direct comparison with previous analyses comparing TOLAC with ERCD is difficult due to the different parameters and model structures utilized. The Grobman et al. life-cycle model found that TOLAC would save $179 million per 100,000 women, however, QALYs were not included in the analysis.[7] The analyses by Chung et al. [4], Gilbert et al. [5] and Fawsitt et al. [6] which incorporated only the pregnancy immediately following one prior cesarean found the TOLAC strategy to be cost-effective. Chung et al. reported ERCD would cost $112,023 per QALY (no total QALYs were provided) whereas Gilbert et al. reported $138.6 million saved and 1703 QALYs gained. Fawsitt et al. found TOLAC to be the dominant base case strategy saving almost €221 million and 1615 QALYs per 100,000 women, however no neonatal outcomes were incorporated in the analysis [6]. Pare et al.; without incorporating costs or QALYs in their decision analysis, concluded that for a woman with a single prior cesarean and planning only one more pregnancy an ERCD was preferred since it results in fewer hysterectomies [8]. In contrast, if several future pregnancies were desired TOLAC was preferred due to the overall reduction in cases of hysterectomy and placenta accreta.
Limitations
The group of women modeled for the delivery of their second child did not include those undergoing labor inductions given that this is a choice and not a probabilistic occurrence. Instead it was predicated on women in spontaneous labor and included probabilities of success and rupture with this type of labor after a previous cesarean and without a prior vaginal delivery. The sensitivity analysis, however, allows insight into whether induction would be cost-effective. The 2010 AHRQ evidence report estimated that the frequency for rupture for those induced at any gestational age to be 1.5% [3]. Even at this frequency, the preferred strategy changed to an ERCD, only when the probability of success was approximately less than 53%. Women induced with a favorable cervix would be expected to have a chance of success greater than that threshold. Additionally, the model has been developed to compare delivery approaches and not choices in timing of delivery (awaiting spontaneous labor for TOLAC versus scheduled cesarean delivery for ERCD). Thus, we have compared the experience of TOLAC with repeat cesarean, as this is a choice that women may confront. Conversely, we have not incorporated expectant management beyond 40 weeks into the model, as this is not a requirement of a TOLAC strategy but a different choice altogether.
For feasibility and clarity, the maternal and infant probabilities from the Cesarean Registry were based on a hierarchy and therefore for several arms of the tree no more than one complication could be experienced by an individual. This effect, however, would be de minimus at best since 11 (0.12%) of the mothers and 220 (2.3%) of the infants in the primary cohort experienced more than one outcome and thereby were too small in number to appropriately include in the decision tree. In addition, women in the rupture arm as well as the indicated repeat could experience uterine rupture, placenta previa or accreta with one other maternal morbidity. This study collected data on hospital deliveries with a gestational age greater than or equal to 20 weeks or resulting in an infant weighing 500 grams or more. Therefore, this analysis was not able to incorporate any potential marginal impact on fertility by mode of delivery, such as early miscarriages and ectopic pregnancies. In addition, external validity may be limited since the majority of the hospitals that participated in the Cesarean Registry were teaching hospitals and may not be representative of all settings in the United States health care system.
Utilities relating to TOLAC and ERCD are very limited [37,38] and although we feel we used the best available sources from the literature they have their weaknesses. A majority of the utilities came from Chung et al. [4] and their description of how the utilities were collected includes the instrument used, the Quality of Well-Being classification system, but they do not indicate how many people or what type (physicians or patients) were included in data collection. In addition, the assigned disutility days could potentially be short We attempted to rectify this by assigning a wide range to be tested in sensitivity analysis. For example, Chung et al. assigned 21 disutility days to ERCD and hysterectomy whereas we incorporated a range of 14 to 180 days. In addition, for a proportion of the hysterectomy population we also incorporated disutilities to represent women who would have desired another pregnancy (Table 7). Moreover, the disutility assigned to infants for cerebral palsy was based on parental surveys of children between 2 and 24 months of age with deafness and various degrees of developmental delay rather than directly from children themselves throughout their lifetime [34]. Ideally, a population-based collection of utilities by mode of delivery would be paramount.
Conclusions
We found that when considering the future reproductive consequences of a TOLAC compared with an ERCD to deliver a second child, TOLAC is more cost-effective under a wide variety of circumstances. This conclusion is sensitive to several variables that must be considered in the cost-effective assessment; accordingly, further efforts should be directed at research that will determine the actual values for these variables.
Table 2. Other maternal probability estimates.
| Outcome | Baseline | Range | Reference |
|---|---|---|---|
|
| |||
| Likelihood of third pregnancy | 0.22 | 0.11-0.88 | 16 |
| Likelihood of fourth pregnancy | 0.14 | 0.07-0.56 | 16 |
| Rupture | |||
| 1 prior low transverse incision | 0.752 | 0-5.0 | CR |
| 1 prior CD, ≥ 1 prior vaginal delivery | 0.378 | 0-5.0 | CR |
| Successful TOLAC | |||
| 1 prior low transverse incision | 67.19 | 20.0-1.0 | CR |
| 1 prior CD, ≥ 1 prior vaginal delivery | 88.69 | 20.0-1.0 | CR |
| Indicated repeat | |||
| 1 prior CD, ≥ 1 prior vaginal delivery | 6.01 | 5.53-6.52 | CR |
| Urinary stress incontinence | 0 | 0-22.0 | 20 |
| Thromboembolism | |||
| Successful TOLAC* | 0.0445 | 0.0358-0.0530 | 19 |
| Rupture, Failed TOLAC, ERCD, Indicated repeat* | 0.178 | 0.143-0.212 | 19 |
| Maternal death | |||
| Failed TOLAC for TOLAC 1† | 0.0040 | 0.0008-0.0201 | 3 |
| Successful TOLAC for TOLAC 1† | 0.0008 | 0.0002-0.0040 | 3 |
| Rupture for TOLAC 1† | 0.0081 | 0.0017-0.0403 | 3 |
| ERCD† | 0.0096 | 0.0021-0.0432 | 3 |
| Failed TOLAC and Indicated repeat for TOLAC 2 and 3‡ | 0.0104 | 0.0025-0.0425 | 3 |
| Successful TOLAC for TOLAC 2 and 3‡ | 0.0030 | 0.0007-0.0122 | 3 |
| Rupture for TOLAC 2 and 3‡ | 0.0209 | 0.0049-0.0851 | 3 |
Data presented as percent and represent conditional probabilities given prior mode of delivery; CR, data from the Cesarean Registry
CD, cesarean delivery; TOLAC, trial of labor after a previous cesarean; ERCD, elective repeat cesarean delivery
Thromboembolism rate for cesarean delivery four times successful TOLAC19
From term maternal death studies.3 Assumption risk 5 times higher for failed TOLAC compared with successful TOLAC, failed TOLAC 33% of all TOLAC, rupture risk twice failed TOLAC
From all maternal death studies.3 Assumption risk 3.5 times higher for failed TOLAC compared with successful TOLAC, failed TOLAC 11% of all TOLAC, rupture risk twice failed TOLAC
Table 3. Previa and accreta probability estimates.
| Outcome | N/Denominator | Baseline | Range | Reference |
|---|---|---|---|---|
|
| ||||
| Previa (with or without accreta) | ||||
| One prior CD | 254/35420 | 0.717 | 0.632-0.810 | CR |
| Two prior CD | 77/8335 | 0.924 | 0.730-1.15 | CR |
| Three prior CD | 52/2217 | 2.35 | 1.76-3.03 | CR |
| Accreta | ||||
| Previa, one prior CD | 28/254 | 11.02 | 7.45-15.33 | CR |
| Previa, two prior CD | 30/77 | 38.96 | 28.05-50.75 | CR |
| Previa, three prior CD | 31/52 | 59.62 | 45.10-72.99 | CR |
| Maternal death | ||||
| Previa only* | n/a | 0.0104 | 0.0025-0.0425 | 3 |
| Accreta and previa | 1/89 | 1.12 | 0.058-5.52 | CR |
| Thromboembolism | ||||
| Previa only | n/a | 0.178 | 0.143-0.212 | 19 |
| Accreta and previa | n/a | 0.178 | 0.143-0.212 | 19 |
| Hysterectomy | ||||
| Previa only, 1 prior CD | 5/226 | 2.21 | 0.876-4.84 | CR |
| Previa only, 2 prior CD | 7/47 | 14.89 | 6.76-28.26 | CR |
| Previa only, 3 prior CD | 4/20 | 20.00 | 7.14-41.11 | CR |
| Operative injury | ||||
| Previa only | 2/294 | 0.680 | 0.121-2.38 | CR |
| Accreta and previa | 17/89 | 19.10 | 12.08-28.10 | CR |
| Wound complication | ||||
| Previa only | 5/294 | 1.70 | 0.672-3.78 | CR |
| Accreta and previa | 2/89 | 2.25 | 0.401-7.33 | CR |
| Endometritis | ||||
| Previa only, 1 prior CD | 7/226 | 3.10 | 1.46-6.20 | CR |
| Previa only, 2 prior CD | 2/47 | 4.26 | 0.761-13.94 | CR |
| Previa only, 3 prior CD | 1/20 | 5.00 | 0.256-23.06 | CR |
| Accreta and previa | 2/89 | 2.25 | 0.401-7.33 | CR |
Data presented as percent and represent conditional probabilities given prior mode of delivery or outcome; CR, from the Cesarean Registry
From all maternal death studies.3
Table 4. Infant outcome probability estimates.
| Mode of Delivery | Neonatal Death | HIE | Sepsis | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | Baseline | Range | Baseline | Range | Baseline | Range | |
|
| |||||||
| TOLAC 1 | |||||||
| Rupture | 32 | 3.13 | 0.160-16.12 | 3.13 | 0.160-16.12 | 18.75 | 8.50-34.74 |
| Failed TOLAC | 1384 | 0.072 | 0.004-0.397 | 0.072 | 0.004-0.397 | 7.08 | 5.79-8.51 |
| Successful TOLAC | 2836 | 0 | 0-0.122 | 0.035 | 0.002-0.194 | 3.81 | 3.13-4.56 |
| TOLAC 2 and 3 | |||||||
| Indicated repeat | 544 | 2.21 | 1.24-3.75 | -- | -- | 20.04 | 16.75-23.51 |
| Rupture | 48 | 2.08 | 0.107-10.47 | 6.25 | 1.73-16.95 | 29.17 | 16.95-43.21 |
| Failed TOLAC | 907 | 0.882 | 0.382-1.70 | 0.221 | 0.039-0.765 | 13.45 | 11.30-15.84 |
| Successful TOLAC | 7096 | 0.90 | 0.70-1.15 | 0 | 0-0.049 | 5.95 | 5.41-6.52 |
| ERCD | 5201 | 0 | 0-0.066 | -- | -- | 2.86 | 2.43-3.34 |
| IR-2CDNPV | 6203 | 0.580 | 0.408-0.796 | -- | -- | 7.38 | 6.75-8.06 |
| IR-3CDNPV | 1767 | 0.792 | 0.460-1.29 | -- | -- | 10.41 | 9.03-11.91 |
| IR-2CDPV | 1196 | 1.25 | 0.704-2.05 | -- | -- | 12.21 | 10.40-14.17 |
Data presented as percent and represent conditional probabilities given prior mode of delivery; all data from the Cesarean Registry. Data were missing for one infant in the TOLAC 1, failed TOLAC group. CD, cesarean delivery; TOLAC, trial of labor after a previous cesarean; ERCD, elective repeat cesarean delivery; IR-2CDNPV, indicated repeat cesarean with 2 prior cesareans and no prior vaginal delivery; IR-3CDNPV, indicated repeat cesarean with 3 prior cesareans and no prior vaginal delivery; IR-2CDPV, indicated repeat cesarean with 2 prior cesareans and one or more prior vaginal deliveries
Acknowledgments
The authors thank the following core committee members participated in protocol development and coordination between clinical research centers (Francee Johnson, B.S.N., Julia Gold B.S.N./A.P.N.), data management (Sandra Meadows), protocol/data management and statistical analysis (Elizabeth Thom, Ph.D., John C. Hauth, M.D., Dwight J. Rouse).
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
The George Washington University Biostatistics Center — E. Thom, H. Juliussen-Stevenson, M. Fischer, L. Leuchtenburg
Northwestern University — A. Peaceman, M. Socol, D. Gradishar, G. Mallett
The Ohio State University — J. Iams, F. Johnson, S. Meadows, H. Walker
University of Alabama at Birmingham — D. Rouse, J. Hauth, A. Northen, S. Tate
University of Texas Southwestern Medical Center — K. Leveno, S. Bloom, J. Gold, D. Bradford
University of Utah — M. Belfort (Utah Valley Regional Medical Center), F. Porter (Intermountain Healthcare), B. Oshiro (McKay-Dee Hospital Center), K. Anderson (University of Utah Health Sciences Center), A. Guzman (McKay-Dee Hospital Center)
University of Pittsburgh — S. Caritis, K. Lain, M. Cotroneo, D. Fischer, M. Luce
Wake Forest University Health Sciences — P. Meis, M. Harper, M. Swain, C. Moorefield, K. Lanier, L. Steele
Thomas Jefferson University —A. Sciscione, M. DiVito, M. Talucci, M. Pollock
Wayne State University — M. Dombrowski, G. Norman, A. Millinder, C. Sudz, B. Steffy
University of Cincinnati — M. Miodovnik, T. Siddiqi, H. How, N. Elder
Columbia University — M. Miodovnik, F. Malone, M. D'Alton, V. Pemberton, V. Carmona, H. Husami
Brown University — M. Carpenter, H. Silver, J. Tillinghast, D. Catlow, D. Allard
University of Miami — M.J. O'Sullivan, G. Burkett, J. Gilles, J. Potter, F. Doyle, S. Chandler
University of Tennessee — W. Mabie, R. Ramsey
University of Texas at San Antonio — D. Dudley, O. Langer, D. Conway, S. Barker, M. Rodriguez
University of North Carolina — K. Moise, K. Dorman, S. Brody, J. Mitchell
The University of Texas Health Science Center at Houston — L. Gilstrap, M. Day, M. Kerr, E. Gildersleeve
Case Western Reserve University-MetroHealth Medical Center — P. Catalano, C. Milluzzi, B. Slivers, C. Santori
University of Chicago — A. Moawad, J. Hibbard, P. Jones, M. Ramos-Brinson, M. Moran, D. Scott
Eunice Kennedy Shriver National Institute of Child Health and Human Development —D. McNellis, K. Howell, S. Tolivaisa
MFMU Steering Committee Chair (Vanderbilt University Medical Center) - S. Gabbe
Source of financial support: The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) [HD21410, HD21414, HD27860, HD27861, HD27869, HD27905, HD27915, HD27917, HD34116, HD34122, HD34136, HD34208, HD34210, HD40500, HD40485, HD40544, HD40545, HD40560, HD40512, and HD36801] and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NICHD or the NIH.
Footnotes
Other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are listed in the Acknowledgments.
None of the authors have a conflict of interest.
Presented at the 33rd Annual Meeting of the Society for Maternal-Fetal Medicine. February 11-16, 2013. San Francisco, CA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDorman M, Declerq E, Menacker F. Recent trends and patterns in cesarean birth after cesarean (VBAC) deliveries in the United States. ClinPerinatol. 2011;38:179–92. doi: 10.1016/j.clp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Ventura SJ, et al. National vital statistics reports. 60 no1. Hyattsville, MD: National Center for Health Statistics; 2011. Births: Final data for 2009. [PubMed] [Google Scholar]
- 3.Guise JM, Eden K, Emeis C, et al. Evidence/report technology assessment no 191. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2010. Vaginal birth after cesarean: new insights. Prepared by the Oregon Health & Science University Evidence-based Practice Center under contract no. 290-2007-10057-I. AHRQ publication no. 10-E003. [Google Scholar]
- 4.Chung A, Macario A, El-Sayed YY, et al. Cost-effectiveness of a trial of labor after previous cesarean. Obstet Gynecol. 2001;97:932–41. doi: 10.1016/s0029-7844(01)01355-2. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert SA, Grobman WA, Landon MB, et al. Cost-effectiveness of trial of labor after previous cesarean in a minimally biased cohort. Am J Perinatol. 2013;30:11–20. doi: 10.1055/s-0032-1333206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fawsitt CG, Bourke J, Greene RA, et al. At what price? A cost-effectiveness analysis comparing trial of labour after previous caesarean versus elective repeat caesarean delivery. PLoS ONE. 2013;8:e58577. doi: 10.1371/journal.pone.0058577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grobman WA, Peaceman AM, Socol ML. Cost-effectiveness of elective cesarean delivery after one prior low transverse cesarean. Obstet Gynecol. 2000;95:745–51. doi: 10.1016/s0029-7844(00)00783-3. [DOI] [PubMed] [Google Scholar]
- 8.Pare E, Quinones JN, Macones GA. Vaginal birth after caesarean section versus elective repeat caesarean section: assessment of maternal downstream health outcomes. BJOG: An Int J Obst & Gynaecol. 2006;113:75–85. doi: 10.1111/j.1471-0528.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 9.Gold MR, Siegel JE, Russell LB, et al. for the Panel on Cost-Effectiveness in Health and Medicine. Cost-Effectiveness in Health and Medicine. New York [NY]: Oxford University Press; 1996. p. 6. [Google Scholar]
- 10.Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 11.Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351:2581–9. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 12.Spong CY, Landon MB, Gilbert S, et al. Risk of uterine rupture and adverse perinatal outcome at term after cesarean delivery. Obstet Gynecol. 2007;110:801–7. doi: 10.1097/01.AOG.0000284622.71222.b2. [DOI] [PubMed] [Google Scholar]
- 13.Cesarean delivery on maternal request. ACOG Committee Opinion No.394. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2007;110:1501–14. doi: 10.1097/01.AOG.0000291577.01569.4c. [DOI] [PubMed] [Google Scholar]
- 14.ACOG Practice Bulletin No 107. Vol. 114. American College of Obstetricians and Gynecologists; 2009. Induction of labor; pp. 386–97. [DOI] [PubMed] [Google Scholar]
- 15.Grobman WA, Gilbert S, Landon MB, et al. Outcomes of induction of labor after one prior cesarean. Obstet Gynecol. 2007;109:262–69. doi: 10.1097/01.AOG.0000254169.49346.e9. [DOI] [PubMed] [Google Scholar]
- 16.Martinez G, Daniels K, Chandra A. National health statistics reports. no. 51. Hyattsville, MD: National Center for Health Statistics; 2012. Fertility of men and women aged 15-44 years in the United States: National Survey of Family Growth 2006-2010. [PubMed] [Google Scholar]
- 17.Arias E. National vital statistics reports. 59 no 9. Hyattsville, MD: National Center for Health Statistics; 2011. United States life tables, 2007. [PubMed] [Google Scholar]
- 18.Casella G. Refining binomial confidence intervals. Can J Stat. 1986;14:113–29. [Google Scholar]
- 19.Simpson EL, Lawrenson RA, Nightingale AL, et al. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors form a London perinatal database. BJOG: Int J Obst & Gynaecol. 2001;108:56–60. doi: 10.1111/j.1471-0528.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 20.Press JZ, Klein MC, Kaczorowski J, et al. Does cesarean section reduce postpartum urinary incontinence? A systematic review. Birth. 2007;34:228–37. doi: 10.1111/j.1523-536X.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- 21.Guise JM, McDonagh M, Hashima J, et al. Evidence Report/Technology Assessment No 71. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2003. Vaginal Birth After Cesarean (VBAC) (Prepared by the Oregon Health & Science University Evidence-based Practice Center under Contract No 290-97-0018). AHRQ Publication No. 03-E018. [PMC free article] [PubMed] [Google Scholar]
- 22.Baldawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47:293–8. doi: 10.1017/s0012162205000575. [DOI] [PubMed] [Google Scholar]
- 23.HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality; Rockville, MD: [Accessed July 7, 2011]. Available from: http://hcupnet.ahrq.gov/ [Google Scholar]
- 24.American Medical Association. [Accessed September 28, 2010]; Available from: https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp.
- 25.United States Department of Labor, Bureau of labor Statistics. [Accessed July 7, 2011]; Available from: http://www.bls.gov/cps/cpswktabs.htm.
- 26.CDC. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment-United States, 2003. MMWR. 2004;53:57–9. [PubMed] [Google Scholar]
- 27.Katz RT. Life expectancy for children with cerebral palsy and mental retardation: implications for life care planning. Neurorehabilitation. 2003;18:261–70. [PubMed] [Google Scholar]
- 28. [Accessed October 25, 2009];Cerebral Palsy Source. Available from: http://www.cerebralpalsysource.com/About_CP/life_cp/index.html.
- 29.Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 30.United States Department of Labor, Bureau of labor Statistics. [Accessed July 7, 2011]; Available from: http://www.bls.gov/cpi/
- 31.Haddox AC, Teutsch SM, Corso PS. Prevention Effectiveness a Guide to Decision Analysis and Economic Evaluation. New York [NY]: Oxford University Press; 2003. [Google Scholar]
- 32.Harris RA, Washington AE, Nease RF, et al. Cost utility of prenatal diagnosis and the risk-based threshold. Lancet. 2004;363:276–82. doi: 10.1016/S0140-6736(03)15385-8. [DOI] [PubMed] [Google Scholar]
- 33.Manca A, Sculpher MJ, Ward K, et al. A cost-utility analysis of tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence. BJOG: Int J Obst & Gynaecol. 2003;110:255–62. [PubMed] [Google Scholar]
- 34.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(Suppl.):S287–S95. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 35.Gold MR, Siegel JE, Russell LB, et al. for the Panel on Cost-Effectiveness in Health and Medicine. Cost-Effectiveness in Health and Medicine. New York [NY]: Oxford University Press; 1996. p. 233. [Google Scholar]
- 36.O'Shea MT, Klebanoff MA, Signore C. Delivery after a previous cesarean: long-term outcomes in the child. Semin Perinatol. 2010;34:281–92. doi: 10.1053/j.semperi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health Consensus Development Conference Statement: Vaginal Birth After Cesarean: New Insights, March 8-10, 2010. Obstet Gynecol. 2010;115:1279–95. doi: 10.1097/AOG.0b013e3181e459e5. [DOI] [PubMed] [Google Scholar]
- 38.Lewis RM, McKoy JN, Andrews JC, et al. Future Research Needs Paper No 22. Rockville, MD: Agency for Healthcare Research and Quality; Oct, 2012. Future research needs to reduce cesarean birth in low-risk women. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No.290-2207-1065-I.) AHRQ Publication No. 12(13)-EHC131-EF. [Google Scholar]