Abstract
Background
Primary tumors of the heart are rare even in major cardiac surgery centers. Because of the low case numbers, there is an insufficient evidence base to determine the optimal treatment, particularly for malignant tumors.
Methods
The authors review the pertinent literature retrieved by a selective PubMed search on the terms “cardiac tumor,” “heart tumor,” “cardiac myxoma,” and “cardiac sarcoma.” They also present operative techniques and their own long-term results in 181 patients with cardiac tumors.
Results
Patients with cardiac tumors generally have nonspecific symptoms depending on the site of the tumor and the extent of infiltration into the neighboring tissue. The diagnosis is based on the clinical history, echocardiography (in most cases), and, sometimes, computerized tomography and magnetic resonance imaging. Autopsy studies reveal a 0.02% prevalence of cardiac tumors, of which 75% are benign and 25% malignant. Myxoma is the most common benign tumor (50–70%); angiosarcoma is the most common malignant one (30%), followed by rhabdomyosarcoma (20%). About 10% of all tumor patients develop cardiac metastases, but these are only rarely clinically manifest. From 1989 to 2012, 181 patients underwent surgery for cardiac tumors in the authors’ institution. The 5-year survival rates were 83% for benign tumors (139 patients), 30% for malignant tumors (26 patients), and 26% for cardiac metastases (16 patients).
Conclusion
Patients with cardiac tumors should undergo surgery in a timely fashion in a specialized center. This holds for both malignant and benign tumors, particularly for atrial myxoma, which can cause serious secondary complications by embolization.
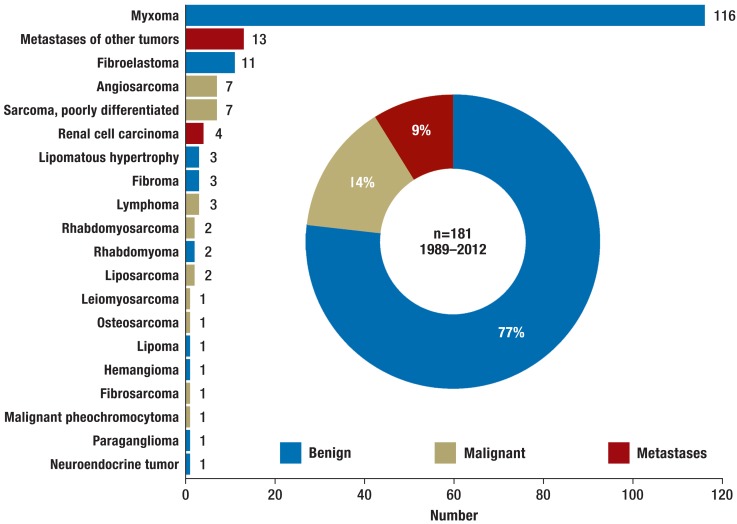
Although the existence of cardiac tumors has been known since the Middle Ages (1), they remain a diagnostic puzzle and a therapeutic challenge. The first successful resection of a cardiac tumor was carried out by Crafoord in 1954, using a heart–lung machine (2), and ensuing advances in cardiosurgical technique enabled resection of malignant tumors. There are no large studies of the best treatment for cardiac tumors, particularly the malignant forms; often, only single case reports are available. With the exception of lymphoma, the treatment of choice is complete resection in combination with chemo-/radiotherapy. Large autopsy series show that the incidence of cardiac tumors is 0.02% (3). Some 75% of the tumors are benign, 25% malignant (3). Metastases are discovered much more frequently than primary neoplasms of the heart, being diagnosed in over 10% of tumor patients at autopsy. Atrial myxoma is the most frequent primary cardiac tumor in adults, while rhabdomyosarcoma predominates in children (3, 4). Our experience over a period of 23 years is summarized in Figure 1.
Figure 1.
Distribution of cardiac tumors diagnosed and treated at the Center for Cardiac Surgery of Münster University Hospital between 1989 and 2012
Clinical presentation
Patients present nonspecific symptoms depending on tumor site and infiltration, regardless of tumor type. Urgent surgical treatment is indicated for all cardiac tumors, malignant or benign, because of the high risk of secondary complications (5– 7).
General symptoms
Cardiac tumors—especially lymphomas—are often manifested by a subfebrile increase in temperature, weight loss, exhaustion, muscle pain, night sweats, coughing, or leukocytosis (7, 8). Malignant tumors are frequently accompanied by hemorrhagic pericardial effusion (6).
Obstruction
Tumors in the region of the atria or atrioventricular valves may restrict the blood flow into the heart, mimicking stenosis of the mitral or tricuspid valve. Mobile, pediculated neoplasms generally lead to paroxysmal heart failure or dyspnea, depending on posture (8– 10). Tumor infiltration into the wall of the heart may produce the symptoms of hypertrophic or restrictive cardiomyopathy. The clinical presentation is dominated by heart failure (6). Expansion into the superior vena cava may result in superior vena cava syndrome.
Arrhythmias
Tumor infiltration of the neural pathways or the myocardium can cause irregular heartbeat and especially AV block. This is particularly true for fibromas. In some cases the first manifestation of a cardiac tumor is sudden cardiac death (5).
Embolisms
Cardiac tumors are often first diagnosed after the patient has suffered a stroke, an embolism of the peripheral vasculature, or a pulmonary artery embolism, caused by detached tumor tissue or mobilization of thrombotic deposits. The possibility of crossed embolisms should also be kept in mind (5). Therefore, all fragments of embolic material retrieved during diagnostic investigations should be subjected to histological analysis. Particularly myxomas tend to cause embolisms because of their gelatinous structure (5, 10).
Diagnosis
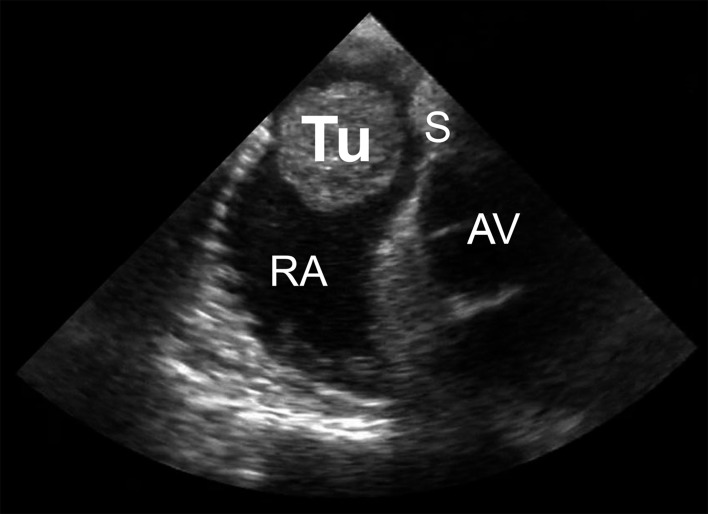
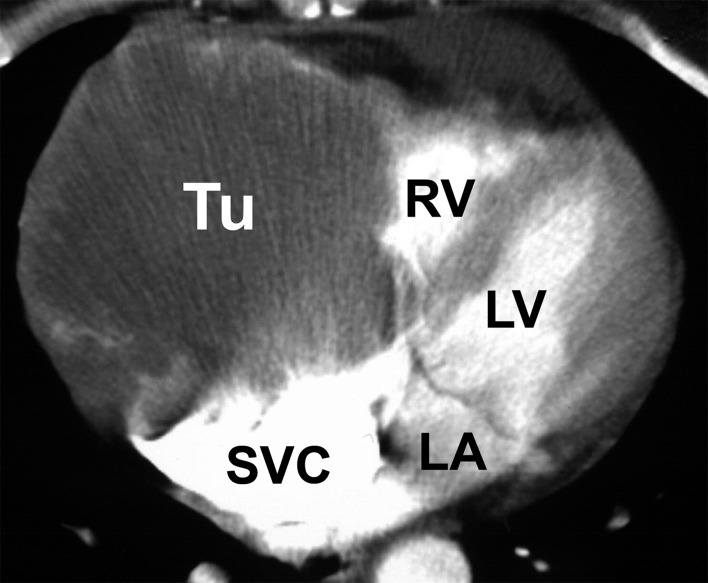
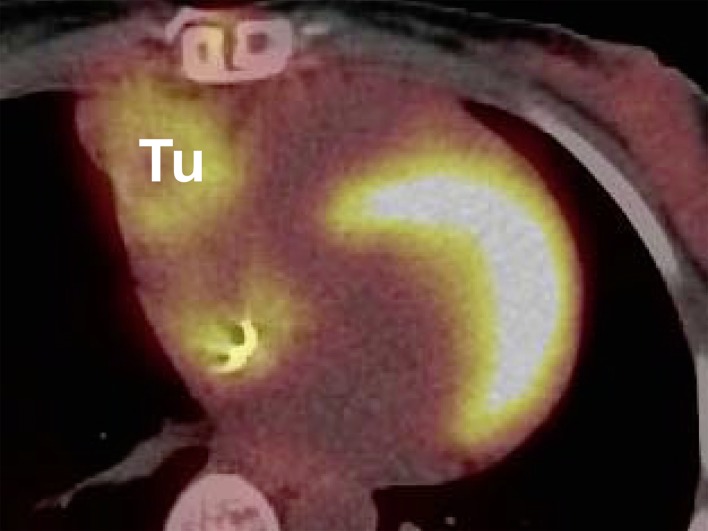
Diagnostic investigation of a suspected cardiac tumor should begin with exclusion of a thrombus or a vegetation. The myxoma syndromes should also be kept in mind when establishing the patient’s previous medical history. After history-taking, echocardiography is the first diagnostic procedure (Figure 2) (11). If a cardiac tumor cannot be confirmed by echocardiography, further imaging procedures such as computed tomography (Figure 3) or magnetic resonance imaging are employed (8, 12). Using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT), we achieved a sensitivity of over 90% in differentiating benign and malignant processes (Figure 4) (13). If these investigations confirm the suspicion of a malignancy, further diagnostic tests are required. In the absence of tumors elsewhere, a primary malignant cardiac tumor must be assumed. Suspicion of a lymphoma should immediately be confirmed by histological examination of a tissue sample obtained by biopsy, followed by chemotherapy and/or radiotherapy (14). In the presence of risk factors for coronary heart disease, additional coronary angiography or CT coronary angiography should be carried out so that any coronary stenoses can be bypassed during cardiac tumor surgery. Angiography is also sometimes helpful to determine the extent of highly vascular tumors, e.g., angiosarcoma. Primary cardiac tumors should be resected in toto as soon as circumstances allow (Figure 5).
Figure 2.
Transesophageal echocardiography (ca. 50°) in a patient with a myxoma of the right atrium (Tu, myxoma; RA, right atrium; AV, aortic valve; S, atrial septum)
Figure 3.
Computed tomography of a sarcoma of the right atrium and ventricle (Tu, tumor; RV, right ventricle; LV, left ventricle; LA, left atrium; SVC, superior vena cava)
Figure 4.
Positron emission tomography/computed tomography (PET/CT) of nodular recurrent sarcoma (Tu, tumor)
Figure 5.
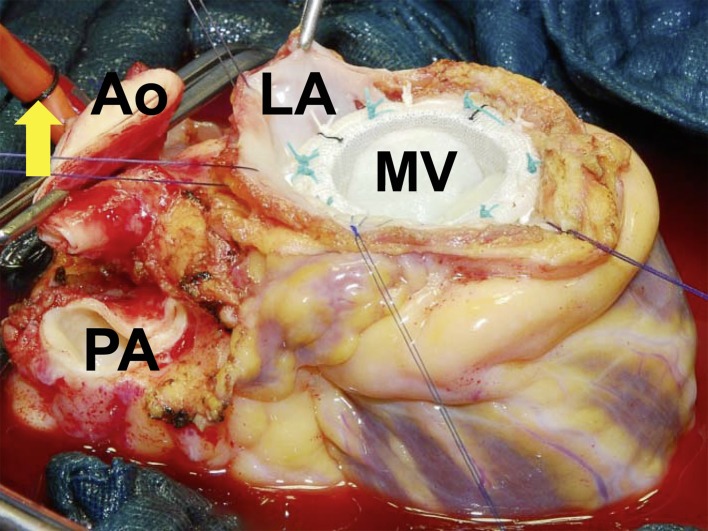
Explanted heart after ex-situ resection of a sarcoma and implantation of a mitral valve (MV) before cardiac reimplantation (LA, resection margin, left atrium; Ao, aorta; PA, pulmonary artery; arrow, cardioplegia catheter)
Benign tumors
Myxoma
Epidemiology—Myxomas make up 50 to 70% of all primary cardiac tumors (Figure 1) (4, 9, 10, 15, 16). They occur mainly in middle age, affecting women more frequently than men. Myxomas are also found in children, although they comprise only 10% of benign tumors in this age group (3, 6).
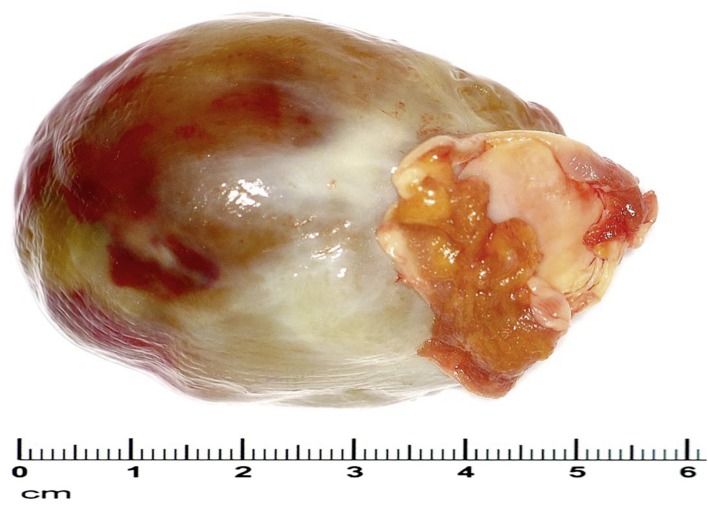
Macropathology—Around 75% of all myxomas arise in the left atrium, 18% in the right atrium. They rarely occur in the ventricles. Myxomas usually grow on pedicles, in the manner of polyps, extending into the affected chamber of the heart. In extreme cases the cavity can be filled by the tumor mass. The tumor may prolapse towards the ventricle during diastole. Myxomas mostly arise in the area of the fossa ovalis, occasionally in the subendocardial tissue of the atrial wall or the cardiac valves. The tumor generally has a soft, gelatinous consistency; the surface is usually completely smooth and often covered with thromboses. Myxomas have an average diameter of 5 to 6 cm (Figure 6), but may grow as large as 15 cm (17).
Figure 6.
Excised myxoma with base
Histopathology—Myxomas are neoplasms of multipotent mesenchymal cells in the subendocardial tissue. The precise histogenetic origin has not been identified (18). Histologically, the so-called myxoma cells predominate. These polygonal, occasionally multinucleate cells have an eosinophilic cytoplasm and are surrounded by a myxoid stroma. Degenerative changes such as cystic formations, hemorrhages, fibroses, and calcifications occur, as well as gland formation (lithomyxoma) (17).
Myxoma syndromes—More than 90% of myxomas are sporadic and seldom reoccur after complete resection (3, 6, 16). The remainder, less than 10%, occur in familial clusters as part of the rare Carney syndrome. This syndrome comprises a combination of cardiac and cutaneous myxomas, endocrine hyperfunction (19) (adrenals, hypophysis, thyroid, Sertoli cells), and cutaneous hyperpigmentation in the form of lentiginosis (20). The acronyms NAME (nevi, atrial myxomas, myxoid fibromas, ephelides) and LAMB (lentiginosis, atrial myxomas, mucocutaneous myxomas, blue nevi) are used to describe combinations of simultaneously occurring findings that are viewed as subforms of Carney syndrome (21, 22). The cause of these syndromes has been demonstrated to be a genetically heterogeneous mutation of the tumor suppressor gene PRKAR1A (protein kinase A regulatory subunit-1-alpha gene) on chromosome 17q22–24 (23). The myxomas typically occur early in life, with peak incidence in the third decade, and tend to recur even after complete resection. Furthermore, the myxomas of Carney syndrome are more frequently multicentric and often show atypical localization.
Other benign tumors
The remaining benign cardiac tumors are all rare and will be described only briefly here.
Papillary fibroelastoma (endocardial papilloma)—Papillary fibroelastoma is a rare (5%) benign tumor of the (valvular) endocardium. It accounts for three fourths of all tumors of the cardiac valves (24%). The average age of the patients affected is 60 years (24). The macroscopic appearance is of a white, gelatinous structure reminiscent of a sea anemone, floating in the blood flowing through the heart. The tumor is usually 1–2 cm in size (6, 17, 24).
Lipomatous hypertrophy (LHIS) and lipoma—Lipomas and LHIS arise from benign neoplastic proliferation of mature adipocytes. The lipomatous hypertrophy affects the interatrial septum. In contrast to LHIS, lipomas are usually enclosed in a capsule (25).
Rhabdomyoma—Rhabdomyomas are the commonest primary cardiac tumor in children, making up 40 to 60% of the total (26), but are rare in adults. Strictly speaking, rhabdomyoma corresponds to a focal hamartomatous accumulation of the striated cardiomyocytes and is not actually a neoplasm (25). Rhabdomyomas occur most frequently in the myocardium of the left ventricle or in the interventricular septum (25). In around half of all cases rhabdomyoma is associated with tuberous sclerosis, and congenital defects of the heart can also be accompanied by rhabdomyomas (26). Macroscopically, the tumor appears as a single or, more often, multiple circumscribed, whitish, generally intramural nodules, usually a few centimeters in size (25). Typically, rhabdomyomas tend to regress spontaneously; this occurs in 50% of patients (26).
Malignant tumors
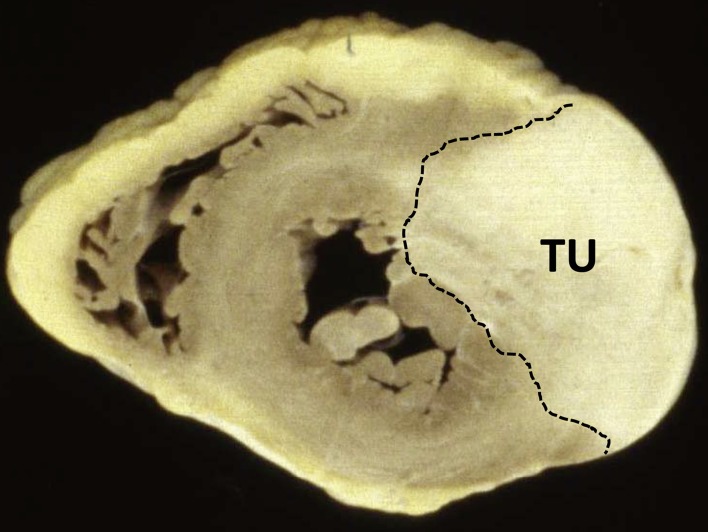
The various types of sarcoma together form 75% of all malignant cardiac tumors. They can occur in any chamber of the heart (Figure 7), but are frequently found in the right atrium. Without treatment, patients have a life expectancy of only a few months (7, 16, 17).
Figure 7.
Explanted heart in cross section, showing a hemangiosarcoma of the left ventricle (TU)
Angiosarcoma
Angiosarcomas (Figure 7) are the most common form of cardiac tumor, accounting for 30% of cardiac malignancies. These infiltrative tumors arise from mesenchymal angioblasts and occur preferentially in middle-aged men (3, 15, 27). Histological examination shows infiltration of the myocardium by spindle cells, which often exhibit hyperchromatic nuclei and mitotic figures in various stages. Giant cells and pronounced necroses may also be found. The malignant endothelial cells form vascular structures (15).
Rhabdomyosarcoma
Rhabdomyosarcomas arise from the striated cardiac musculature. Accounting for 20% of all cardiac tumors, they are the second most common primary malignancy of the heart (3, 17). Rhabdomyosarcomas occur with equal frequency in males and females. They can be found in any chamber of the heart, singly or multiply, and grow invasively. Macroscopically they appear as soft nodes with a central necrosis. They may also infiltrate the cardiac valves (3). This aggressive tumor type may also spread to neighboring organs and infiltrate the pericardium, the pleura, or the mediastinum.
Fibrosarcoma
Fibrosarcomas comprise approximately 10% of all malignant tumors of the heart and arise from the fibroblasts of the cardiac connective tissue. In contrast to fibromas, they occur preferentially in adults and may be multiple (17). Fibrosarcomas are gray, nodular, infiltrative tumors with a firm consistency. They frequently arise in the right atrium and display intracavitary growth, although the pericardium may be infiltrated as well. Histologically, bundled spindle cells are characteristic (27).
Leiomyosarcoma
Leiomyosarcomas, arising from the smooth cardiac muscles, make up 9% of all primary malignant tumors of the heart (28). They can be found in every age group and occur with equal frequency in males and females. The macroscopic appearance of a leiomyosarcoma is of a soft mass extending beyond the borders of the heart. Histological examination reveals elongated, closely spaced spindle cells interspersed with zones populated by only few cells (29).
Li–Fraumeni syndrome
Particularly in young patients (under 45 years) with previous cardiac tumor disease, the congenital Li–Fraumeni syndrome should be considered as a possible differential diagnosis. The cause of this autosomal–dominant condition is now assumed to be a mutation of the TP53 gene on chromosome 17p13.1 (30).
Metastases
Cardiac secondaries of peripheral tumors can occur by means of hematogenous or lymphogenous spread (26, 31). The precise incidence of such metastases is unknown; data is mainly collected from large autopsy studies. With a rate of over 10% in tumor patients, cardiac metastases are diagnosed far more frequently than primary neoplasms of the heart (26, 31). Clinical presentation is rare, because the symptoms of the primary tumor dominate the presentation and determine the prognosis (26). Macroscopically, cardiac metastases are usually small and solid; multiple tumors may be found. Surgical management is generally critical, as the metastases are often neither solitary nor confined to the heart. Nevertheless, resection can by all means be considered in individual cases, provided no other organs are involved and the tumor can be removed in toto (32).
Treatment
As soon as the diagnosis of cardiac tumor is confirmed, the patient should be treated at an interdisciplinary center. It is crucial for oncologists, radiotherapists, and surgeons to cooperate closely.
Cardiac tumors present a particular challenge for heart surgeons, because great versatility is required. The treating cardiac center must have experience over the whole spectrum of heart surgery, including adult, pediatric, and rhythm surgery as well as transplantation and artificial heart implantation. Owing to the small numbers of cases there is no evidential basis for the optimal treatment regime, especially when it comes to malignant tumors.
Simple tumor resection
Simple resection is generally selected for benign tumors such as myxomas. Care must be exercised in connecting the heart–lung machine to avoid dislodging any tumor material. For this reason we open both atria from the right superior pulmonary vein without injuring the tumor or its base. The tumor and its root can then be removed from the septum in toto. We always inspect all the chambers of the heart to exclude the presence of further tumors (16). The defect is closed with patch material. Apart from the usual risks involved in the use of a heart–lung machine this intervention is relatively uncomplicated.
Complex tumor resection
Complex tumor resection is possible provided the mass is confined to the heart and, if malignant, has not infiltrated the adjacent tissues.
In advanced tumors involving the right side of the heart the whole right half of the organ can be resected. Pulmonary blood flow is assured by means of Fontan circulation, well known from pediatric heart surgery (16, 33). Chronic right heart failure may result.
Ex-situ resection
In the event that the tumor involves the posterior wall of the left atrium or the dorsal great vessels, the heart can be removed from the thorax to facilitate complete resection. Attempts at conventional surgery in such circumstances are hampered by an inadequate view of the tumor, leading to incomplete resection and an unfavorable long-term outcome. Lifting the heart out of the body means it can be turned for optimal visualization of all structures. Following tumor resection the cardiac anatomy is restored using artificial materials (prostheses, patches, valves) or biological tissue before the heart is reimplanted (Figure 5) (34– 37). Depending on the amount of cardiac tissue excised, left and/or right heart failure may result as a secondary complication.
Implantation of an artificial heart
If the tumor also involves the left heart, and provided there are no metastases, the implantation of a total artificial heart (TAH) can be considered as a treatment of last resort—especially in younger patients. The principal complications following successful implantation are hemorrhages, thromboses, infections, and, as a result of the necessary anticoagulation, embolisms (38).
Heart transplantation
Heart transplantation (HTx) can occasionally be considered as a final treatment option in individual cases, provided distant metastases can be ruled out (39). We have successfully treated two patients in this way. One significant risk of this procedure is the danger of exacerbation of undetected micrometastases by the necessary immunosuppression.
Results
Our own results and those of others (8) indicate that the results of surgical treatment of benign cardiac tumors are excellent in all age groups, with an extremely low rate of recurrences. In the long term, the tumor disease is seldom the cause of death. For malignant tumors of the heart, however, literature reports show that the prognosis is very poor. The stated duration of survival from the time of diagnosis varies from 7 months to a maximum of 2 years (8, 16, 17, 40). On long-term follow-up, the majority of patients die of distant metastases (16, 35, 40).
The average survival of patients treated purely conservatively with chemotherapy and radiotherapy is just under a year (8, 40).
Simple, incomplete resection (debulking) extends survival by only a few months (8, 40).
In 2002 Überfuhr et al. reported four patients with cardiac tumors who were treated by heart transplantation with pre- or postoperative chemotherapy. This method achieved a mean duration of survival of 18 months, with one patient living for 37 months (39).
Radical resection—combined, if indicated, with postoperative chemotherapy—improves the long-term outcome with a good quality of life for the patients (Table 1) (16).
Table. Experience and results of surgical treatment of cardiac tumors at Münster University Hospital*.
| Benign (n=139) | Malignant (n=26) | Metastasis (n=16) | |
|---|---|---|---|
| Age of patient at time of surgery (median, years) | 60.7+/–17.4 | 54.5+/–19.4 | 51.2+/–18.7 |
| Sex M/F (%) | 35/36 | 46/54 | 63/27 |
| Most frequent sites (%) | L atrium (70) R atrium (14) |
R atrium (39) L atrium (35) |
R atrium (50) R ventricle (50) Septum (37) |
| Postoperative length of stay (median, days) | 7.0+/–7.2 | 13.0+/–9.7 | 7.0+/–8.7 |
| 1-year SR (%) | 95 | 47 | 56 |
| 5-year SR (%) | 83 | 30 | 26 |
| 10-year SR (%) | 75 | 22 | 26 |
*Corresponding to Figure 1, retrospective analysis (as of January 2013). SR, survival rate; L, left; R, right
Key Messages.
Patients with cardiac tumors often suffer nonspecific symptoms such as weight loss, exhaustion, heart failure, arrhythmias, and embolisms.
The incidence of cardiac tumors in autopsy studies is 0.02%.
Around 75% of cardiac tumors are benign, with myxomas the most frequent type; among the 25% that are malignant, angiosarcomas and rhabdomyosarcomas predominate.
A total of 181 patients with cardiac tumors were treated at the Center for Cardiac Surgery of Münster University Hospital between 1989 and 2012.
The 5-year survival rates were 83% for benign tumors, 30% for malignant neoplasms, and 26% for metastases.
Acknowledgments
Translated from the original German by David Roseveare.
This review was supported by a grant from the DFG (HO 4295/2–1).
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Columbus M. Paris: Libri 15 De re anatomica; 1562 pp. [Google Scholar]
- 2.Chitwood WR. Clarence Crafoord and the first successful resection of a cardiac myxoma. Ann Thorac Surg. 1992;54:997–998. doi: 10.1016/0003-4975(92)90676-u. [DOI] [PubMed] [Google Scholar]
- 3.McAllister H, Fenoglio J. Tumors of the cardiovascular system. In: Hartmannn W, Cowan W, editors. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1978. pp. 1–20. [Google Scholar]
- 4.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77 doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe UC, La Rosee K, Beuckelmann DJ, Erdmann E. Herztumoren - Manifestation durch uncharakteristische Symptomatik [Heart tumors—their manifestation through uncharacteristic symptoms] Dtsch Med Wochenschr. 1997;122:551–557. doi: 10.1055/s-2008-1047653. [DOI] [PubMed] [Google Scholar]
- 6.Just S, Schubel B, Minden HH. herzchirurgisches Handbuch. Berlin: Verlag HJB; 2002. Tumoren des Herzens. [Google Scholar]
- 7.Strotmann J. Kardiale Tumoren - Klinik, Diagnostik und Therapie. [Cardiac tumors—clinical symptoms, diagnostic approaches, and therapeutic aspects] Med Klin. 2008;103:175–180. doi: 10.1007/s00063-008-1025-2. [DOI] [PubMed] [Google Scholar]
- 8.Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007;19:748–756. doi: 10.1016/j.clon.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine. 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira R, Branco L, Galrinho A, et al. Cardiac myxoma: a 13-year experience in echocardiographic diagnosis. Rev Port Cardiol. 2010;29:1087–1100. [PubMed] [Google Scholar]
- 12.Baumgartner RA, Das SK, Shea M, LeMire MS, Gross BH. The role of echocardiography and CT in the diagnosis of cardiac tumors. Int J Card Imaging. 1988;3:57–60. doi: 10.1007/BF01801645. [DOI] [PubMed] [Google Scholar]
- 13.Rahbar K, Seifarth H, Schafers M, et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53:856–863. doi: 10.2967/jnumed.111.095364. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmeier A, Semik M, Schmid C, et al. Primäres Burkitt-Lymphom des Herzens - Diagnose und Therapie [Primary Burkitt lymphoma of the heart-diagnosis and therapy] Z Kardiol. 2002;91:347–351. [PubMed] [Google Scholar]
- 15.Heath D. Pathology of cardiac tumors. Am J Cardiol. 1968;21:315–327. doi: 10.1016/0002-9149(68)90136-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmeier A, Schmid C, Deiters S, et al. Neoplastic heart disease—the Muenster experience with 108 patients. Thorac Cardiovasc Surg. 2005;53:1–8. doi: 10.1055/s-2004-830389. [DOI] [PubMed] [Google Scholar]
- 17.McAllister HA. Primary tumors and cysts of the heart and pericardium. Curr Probl Cardiol. 1979;4:1–51. doi: 10.1016/0146-2806(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 18.Rogov KA, Sheremet’eva GF, Nechaenko MA. (Heart myxoma histogenesis in the light of its histological and ultrastructural features) Arkh Patol. 2003;65:20–24. [PubMed] [Google Scholar]
- 19.Raimann A, Wustinger M, Niederle B, Haas O, Häusler G. Cushing, Hypertonie, Sommersprossen. Der Carney-Komplex: Fallbericht eines seltenen Neoplasiesyndroms. J Clin Endocrinol Metab. 2009;3:22–25. [Google Scholar]
- 20.Bertherat J. Carney complex (CNC) Orphanet J Rare Dis. 2006;1 doi: 10.1186/1750-1172-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes AR, Silverman RA, Harrist TJ, Perez-Atayde AR. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the „LAMB“ syndrome. J Am Acad Dermatol. 1984;10:72–82. doi: 10.1016/s0190-9622(84)80047-x. [DOI] [PubMed] [Google Scholar]
- 22.Atherton DJ, Pitcher DW, Wells RS, MacDonald DM. A syndrome of various cutaneous pigmented lesions, myxoid neurofibromata and atrial myxoma: the NAME syndrome. Br J Dermatol. 1980;103:421–429. doi: 10.1111/j.1365-2133.1980.tb07266.x. [DOI] [PubMed] [Google Scholar]
- 23.Vezzosi D, Vignaux O, Dupin N, Bertherat J. Carney complex: Clinical and genetic 2010 update. Ann Endocrinol (Paris) 2010;71:486–493. doi: 10.1016/j.ando.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Mariscalco G, Bruno VD, Borsani P, Dominici C, Sala A. Papillary fibroelastoma: insight to a primary cardiac valve tumor. J Card Surg. 2010;25:198–205. doi: 10.1111/j.1540-8191.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 25.Jain D, Maleszewski JJ, Halushka MK. Benign cardiac tumors and tumorlike conditions. Ann Diagn Pathol. 2010;14:215–230. doi: 10.1016/j.anndiagpath.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Gunther T, Schreiber C, Noebauer C, Eicken A, Lange R. Treatment strategies for pediatric patients with primary cardiac and pericardial tumors: a 30-year review. Pediatr Cardiol. 2008;29:1071–1076. doi: 10.1007/s00246-008-9256-6. [DOI] [PubMed] [Google Scholar]
- 27.Molina JE, Edwards JE, Ward HB. Primary cardiac tumors: experience at the University of Minnesota. Thorac Cardiovasc Surg. 1990;38:183–191. doi: 10.1055/s-2007-1014064. [DOI] [PubMed] [Google Scholar]
- 28.Donsbeck AV, Ranchere D, Coindre JM, et al. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34:295–304. doi: 10.1046/j.1365-2559.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 29.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Schneider SRB, Sindermann JR, Welp H, et al. Herztumor als Manifestation eines Li-Fraumeni-Syndroms - Ein diagnostisches Chamäleon. Z Herz-Thorax-Gefäßchir. 2009;23:23–26. [Google Scholar]
- 31.Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar N, Agarwal S, Ahuja A, et al. Spectrum of cardiac tumors excluding myxoma: Experience of a tertiary center with review of the literature. Pathol Res Pract. 2011;207:769–774. doi: 10.1016/j.prp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmeier A, Deiters S, Schmidt C, et al. Radical resection of cardiac sarcoma. Thorac Cardiovasc Surg. 2004;52:77–81. doi: 10.1055/s-2004-817809. [DOI] [PubMed] [Google Scholar]
- 34.Scheld HH, Nestle HW, Kling D, et al. Resection of a heart tumor using autotransplantation. Thorac Cardiovasc Surg. 1988;36:40–43. doi: 10.1055/s-2007-1020040. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmeier A, Scheld HH, Tjan TDT, et al. Ex situ resection of primary cardiac tumors. Thorac Cardiovasc Surg. 2003;51:99–101. doi: 10.1055/s-2003-38982. [DOI] [PubMed] [Google Scholar]
- 36.Blackmon SH, Patel AR, Bruckner BA, et al. Cardiac autotransplantation for malignant or complex primary left-heart tumors. Tex Heart Inst J. 2008;35:296–300. [PMC free article] [PubMed] [Google Scholar]
- 37.Reardon MJ, Malaisrie SC, Walkes JC, et al. Cardiac autotransplantation for primary cardiac tumors. Ann Thorac Surg. 2006;82:645–650. doi: 10.1016/j.athoracsur.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 38.Ried M, Rupprecht L, Hirt S, et al. Sequential therapy of primary cardiac lymphoma with cardiectomy, total artificial heart support, and cardiac transplantation. J Heart Lung Transplant. 2010;29:707–709. doi: 10.1016/j.healun.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Überfuhr P, Meiser B, Fuchs A, et al. Heart transplantation: an approach to treating primary cardiac sarcoma? J Heart Lung Transplant. 2002;21:1135–1139. doi: 10.1016/s1053-2498(02)00409-6. [DOI] [PubMed] [Google Scholar]
- 40.Brandt RR, Arnold R, Bohle RM, Dill T, Hamm CW. Cardiac angiosarcoma: case report and review of the literature. Z Kardiol. 2005;94:824–828. doi: 10.1007/s00392-005-0296-0. [DOI] [PubMed] [Google Scholar]