Abstract
Multiscale asymptotic methods developed previously to study macromechanical wave propagation in cochlear models are generalized here to include active control of a cochlear partition having three subpartitions, the basilar membrane, the reticular lamina, and the tectorial membrane. Activation of outer hair cells by stereocilia displacement and/or by lateral wall stretching result in a frequency-dependent force acting between the reticular lamina and basilar membrane. Wavelength-dependent fluid loads are estimated by using the unsteady Stokes' equations, except in the narrow gap between the tectorial membrane and reticular lamina, where lubrication theory is appropriate. The local wavenumber and subpartition amplitude ratios are determined from the zeroth order equations of motion. A solvability relation for the first order equations of motion determines the subpartition amplitudes. The main findings are as follows: The reticular lamina and tectorial membrane move in unison with essentially no squeezing of the gap; an active force level consistent with measurements on isolated outer hair cells can provide a 35-dB amplification and sharpening of subpartition waveforms by delaying dissipation and allowing a greater structural resonance to occur before the wave is cut off; however, previously postulated activity mechanisms for single partition models cannot achieve sharp enough tuning in subpartitioned models.
Full text
PDF
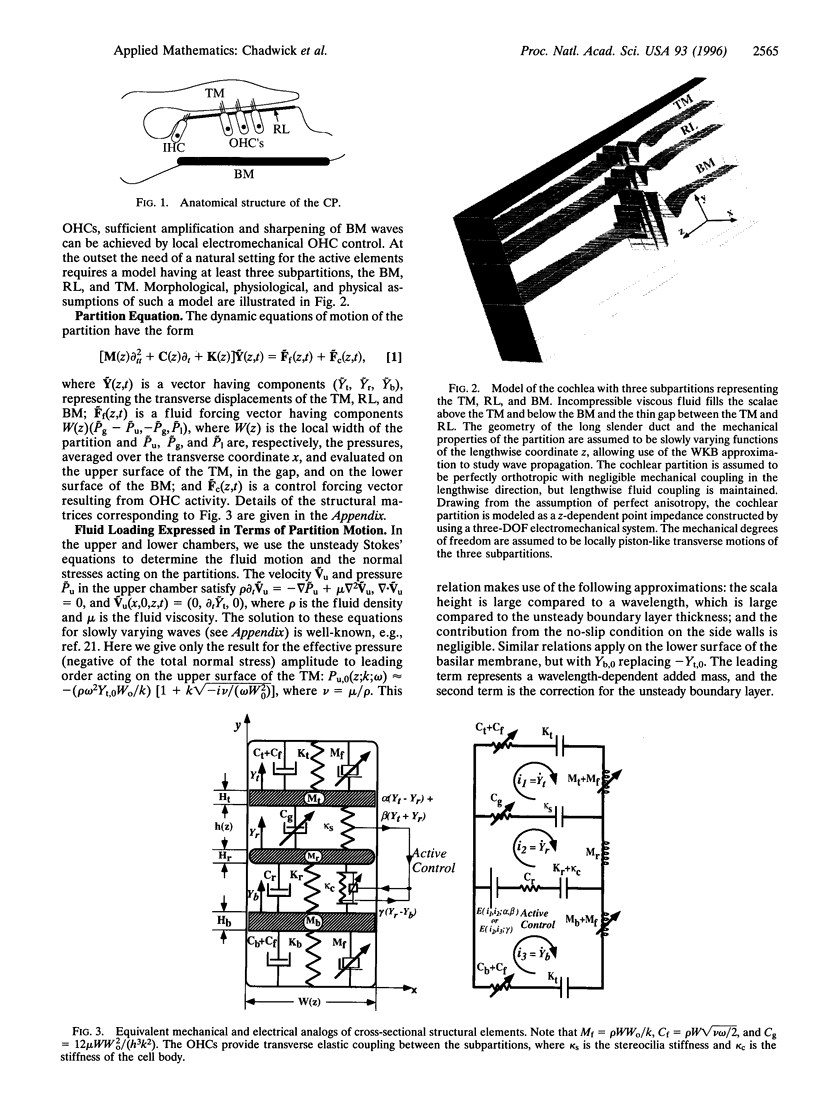
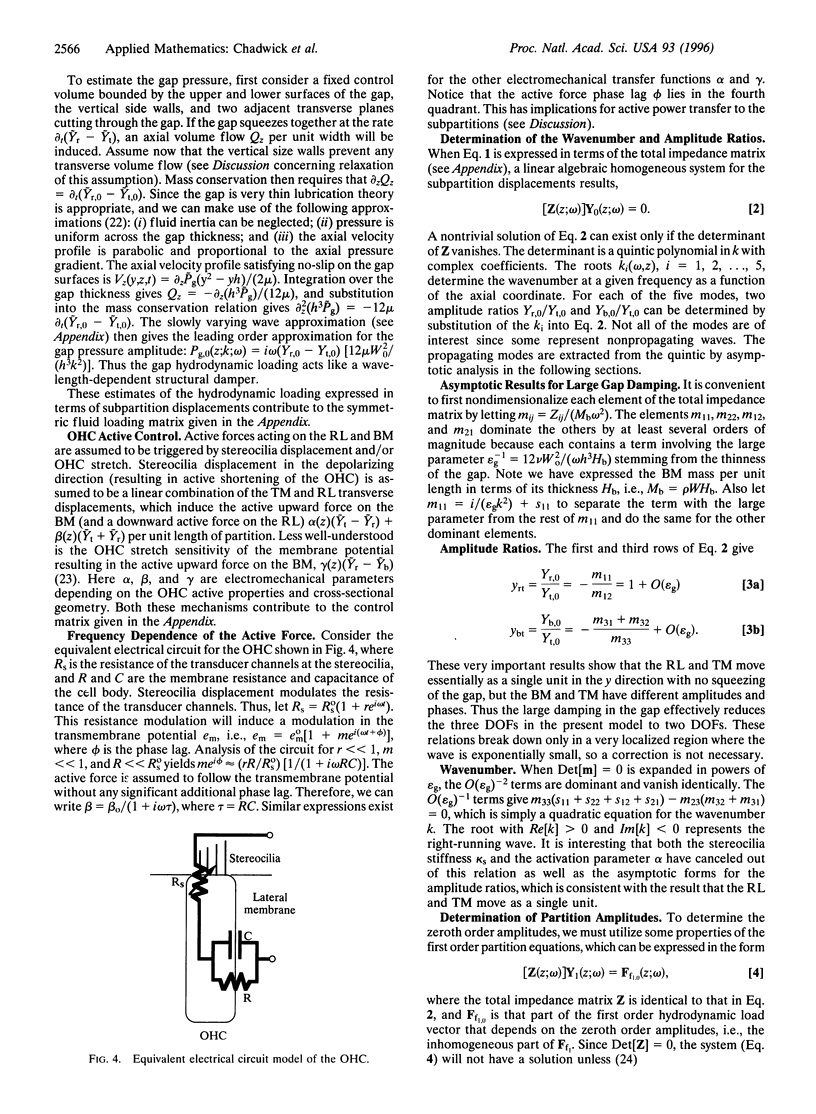
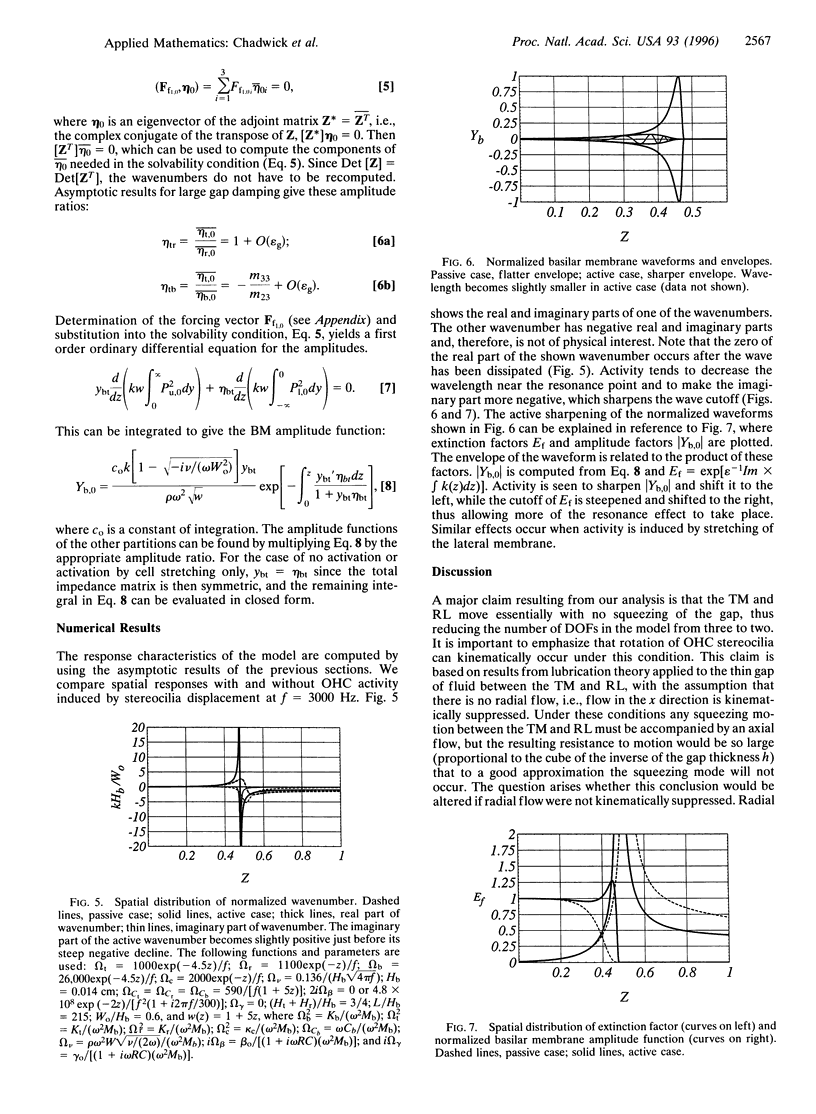


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B. Cochlear micromechanics--a physical model of transduction. J Acoust Soc Am. 1980 Dec;68(6):1660–1670. doi: 10.1121/1.385198. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F. Forward and reverse transduction in the mammalian cochlea. Neurosci Res Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Dallos P., Evans B. N. High-frequency outer hair cell motility: corrections and addendum. Science. 1995 Jun 9;268(5216):1420–1421. [PubMed] [Google Scholar]
- Holmes M. H., Cole J. D. Cochlear mechanics: analysis for a pure tone. J Acoust Soc Am. 1984 Sep;76(3):767–778. doi: 10.1121/1.391300. [DOI] [PubMed] [Google Scholar]
- Hubbard A. A traveling-wave amplifier model of the cochlea. Science. 1993 Jan 1;259(5091):68–71. doi: 10.1126/science.8418496. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Chadwick R. S. Elasticity and active force generation of cochlear outer hair cells. J Acoust Soc Am. 1992 Dec;92(6):3169–3173. doi: 10.1121/1.404194. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993 Jul;65(1):492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K. H., Li M. X., Jia M., Kachar B. Stretch sensitivity of the lateral wall of the auditory outer hair cell from the guinea pig. Neurosci Lett. 1991 Dec 9;133(2):171–174. doi: 10.1016/0304-3940(91)90562-8. [DOI] [PubMed] [Google Scholar]
- Mammano F., Nobili R. Biophysics of the cochlea: linear approximation. J Acoust Soc Am. 1993 Jun;93(6):3320–3332. doi: 10.1121/1.405716. [DOI] [PubMed] [Google Scholar]
- Neely S. T. A model of cochlear mechanics with outer hair cell motility. J Acoust Soc Am. 1993 Jul;94(1):137–146. doi: 10.1121/1.407091. [DOI] [PubMed] [Google Scholar]
- Neely S. T., Kim D. O. A model for active elements in cochlear biomechanics. J Acoust Soc Am. 1986 May;79(5):1472–1480. doi: 10.1121/1.393674. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Harmonics of outer hair cell motility. Biophys J. 1993 Nov;65(5):2217–2227. doi: 10.1016/S0006-3495(93)81247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. R., Taber L. A. Three-dimensional model calculations for guinea pig cochlea. J Acoust Soc Am. 1981 Apr;69(4):1107–1111. doi: 10.1121/1.385679. [DOI] [PubMed] [Google Scholar]
- Zweig G. Finding the impedance of the organ of Corti. J Acoust Soc Am. 1991 Mar;89(3):1229–1254. doi: 10.1121/1.400653. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. J., Kletsky E. J. Tectorial membrane: a possible effect on frequency analysis in the cochlea. Science. 1979 May 11;204(4393):639–641. doi: 10.1126/science.432671. [DOI] [PubMed] [Google Scholar]