Abstract
Although many laboratories currently use small molecule inhibitors of the BMP (Dorsomorphin/DM) and TGF-β (SB431542/SB) signaling pathways in protocols to generate midbrain dopamine (mDA) neurons from hES and hiPS cells, until now, these substances have not been thought to play a role in the mDA differentiation process. We report here that the transient inhibition of constitutive BMP (pSMADs 1, 5, 8) signaling, either alone or in combination with TGF-β inhibition (pSMADs 2, 3), is critically important in the upstream regulation of Wnt1-Lmx1a signaling in mDA progenitors. We postulate that the mechanism via which DM or DM/SB mediates these effects involves the up-regulation in SMAD-interacting protein 1 (SIP1), which results in greater repression of the Wnt antagonist, secreted frizzled related protein 1 (Sfrp1) in stem cells. Accordingly, knockdown of SIP1 reverses the inductive effects of DM/SB on mDA differentiation while Sfrp1 knockdown/inhibition mimics DM/SB. The rise in Wnt1-Lmx1a levels in SMAD-inhibited cultures is, however, accompanied by a reciprocal down-regulation in SHH-Foxa2 levels leading to the generation of few TH+ neurons that co-express Foxa2. If however, exogenous SHH/FGF8 is added along with SMAD inhibitors, equilibrium in these two important pathways is achieved such that authentic (Lmx1a+Foxa2+TH+) mDA neuron differentiation is promoted while alternate cell fates are suppressed in stem cell cultures. These data indicate that activators/inhibitors of BMP and TGF-β signaling play a critical upstream regulatory role in the mDA differentiation process in human pluripotent stem cells.
Keywords: Human pluripotent stem cells, Midbrain dopaminergic differentiation, BMP, TGF-beta, SMAD, Wnt, SHH
Introduction
Cell replacement therapy remains a potentially important treatment strategy to replace the dead or dying midbrain dopamine (mDA) neurons that underlie Parkinson’s Disease (PD). The success of this approach, however, greatly depends upon the discovery of an abundant source of cells capable of mDAergic function in the brain. Currently, pluripotent stem cells, either human embryonic stem cells (hES cells) or human induced pluripotent stem cells (hiPS cells) remain the most promising source of cells capable of differentiating into mDA neurons (Kim et al., 2002; Ben-Hur et al., 2004; Yang et al., 2004; Arenas, 2005; Hedlund et al., 2008; Cai et al., 2009, 2010; Friling et al., 2009; Lee et al., 2010). Understanding the mechanism underlying dopaminergic differentiation from pluripotent stem cells is key to successfully obtaining large numbers of transplantable cells for PD cell replacement therapy.
This endeavor has been greatly facilitated by studies examining similar mDA differentiation processes in the developing mouse midbrain (Ye et al., 1998; Arenas, 2002; Simon and Bhatt, 2003; Andersson et al., 2006; Prakash and Wurst, 2006; Prakash et al., 2006; Pollard et al., 2008; Joksimovic et al., 2009; Nakatani et al., 2010; Zhang and Zhang, 2010). In brief, development of mouse mDA neurons depends upon spatial and temporal differentiation cues derived from two key brain centers, the mid-hindbrain isthmus and the midbrain floor plate (Roussa and Krieglstein, 2004). The glycoprotein Sonic hedgehog (SHH) which is secreted by floor plate cells is thought to regulate dorsal–ventral patterning (Ye et al., 1998; Blaess et al., 2006) along with FGF8 while positioning along the anterior–posterior axis is mediated by the proto-oncoprotein Wnt1 derived from isthmus cells (Prakash and Wurst, 2006; Prakash et al., 2006). These secreted factors act by inducing expression of complex interrelated transcriptional cascades which are thought to specify an mDA fate in midbrain neuroepithelial cells (Chung et al., 2009; Lin et al., 2009). Key among these is the gene for LIM homeobox transcription factor 1 alpha (Lmx1a) which lies downstream of Wnt (Andersson et al., 2006; Cai et al., 2009, 2010; Chung et al., 2009; Friling et al., 2009). The transcriptional repressor or homeobox protein Msx1 and bicoid-like protein Otx2, promoting neuronal differentiation (via transcription factor Ngn2) and directly regulating the mDA transcription factors Nurr1 and Pitx3 while suppressing alternative cell fates (Andersson et al., 2006; Kittappa et al., 2007). Working coordinately with the floor plate forkhead transcription factors (Foxa1/2) which lie downstream of SHH, Lmx1 is thought to commit mouse floor plate cells to an mDA fate (Kittappa et al., 2007; Chung et al., 2009; Lin et al., 2009; Lee et al., 2010; Nakatani et al., 2010).
Over the last decade, significant strides have been made in developing tissue culture protocols that recapitulate the mDA differentiation process in hES and hiPS cell cultures (Cai et al., 2009, 2010; Chung et al., 2009; Friling et al., 2009; Cooper et al., 2010; Fasano et al., 2010; Nakatani et al., 2010). Most of these employ a 5-stage protocol that moves cells from the undifferentiated state, through pseudo-gastrulation in the embryoid body (EB) to mDA committed neural progenitors (hNPs) and finally into mDA neurons. However, recently, many labs studying hES and hiPS cells have moved away from EBs to monolayer cultures which use small molecule inhibitors of BMP/TGF-β signaling in their media formulations to enhance generation of neural progenitors and neurons by inhibiting mesenchymal differentiation (Chambers et al., 2009; Denham and Dottori, 2009). While a number of labs report the differentiation of mDA neurons in these monolayer cultures (Jaeger et al., 2011; Kim et al., 2011; Kriks et al., 2011; Vogt et al., 2011; Lipchina et al., 2012; Nefzger et al., 2012; Xi et al., 2012), there have been no systematic studies of the effects of BMP/TGF-β inhibitors specifically on the mDA differentiation process.
In general, the superfamily of TGF-β ligands (BMPs, GDFs, activin, nodal, etc.) are thought to mediate their effects by binding specific receptors which phosphorylate SMADs and co-SMADs to form complexes that move to the nucleus where they bind transcription factor promoters. Inhibitors such as DM bind BMP type I receptors ALK2, ALK3 and ALK6 to block phosphorylation of SMADs 1,5,8 (Yu et al., 2008). SB inhibits the activin type I receptor ALK5, the TGFβR1 receptor ALK4 and the nodal type I receptor ALK7 which phosphorylate SMADs 2 and 3 (Inman and Hill, 2002). Whether SMAD inhibition by small molecule BMP and/or TGF-β inhibitors alters mDA differentiation and whether it does so by affecting specific transcription factors etc. remains to be established.
In this paper, we will show evidence that transient inhibition of the constitutive BMP pathway, either alone or in combination with TGF-β inhibition, is critical to the upstream regulation of the SMAD-interacting protein 1 (SIP1) and its downstream effector secreted frizzled related protein 1 (Sfrp1) and their reciprocal regulation of Wnt1-Lmx1a and Shh-FoxA2 signaling during mDA differentiation in stem cell cultures. However, generating authentic mDA neurons is achieved only when a proper balance between Wnt and SHH pathways is attained which requires both SMAD inhibition and exogenous SHH/FGF8.
Results
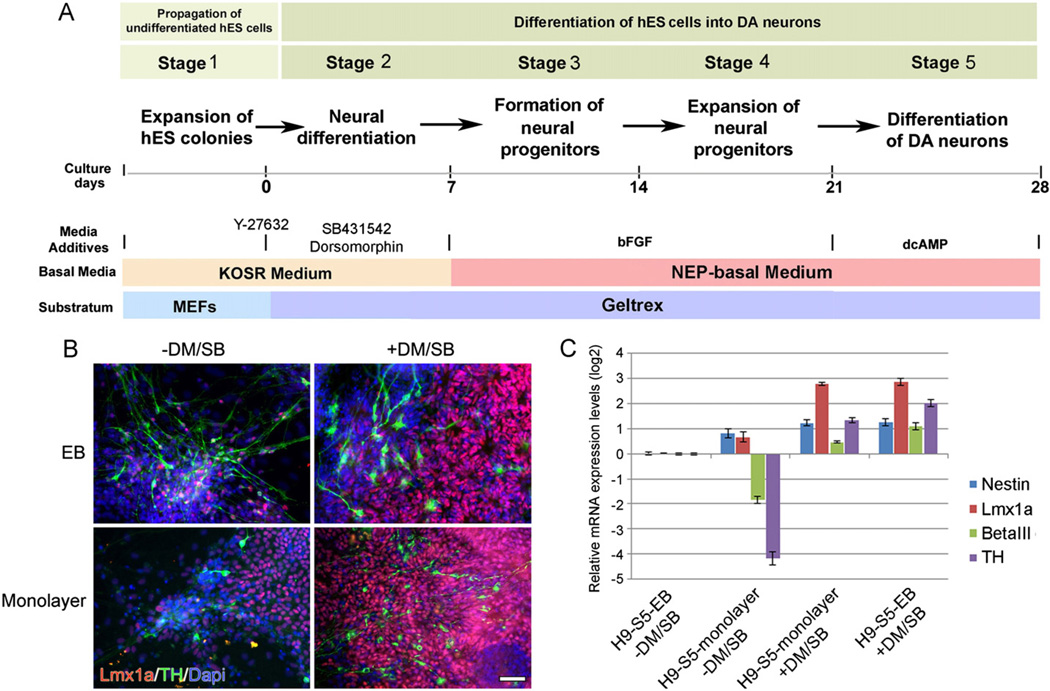
In the last year, in addition to our usual EB method of differentiating mDA neurons from hES/hiPS cells, cells were also differentiated using a simplified monolayer method (Fig. 1A). This was in part made possible by the discovery that the rho-associated kinase (ROCK) inhibitor, Y-27632, markedly diminishes dissociation-induced apoptosis in stem cells (Watanabe et al., 2007), allowing us to proceed directly from undifferentiated cell colonies to mDA differentiation while omitting the EB step.
Fig. 1.
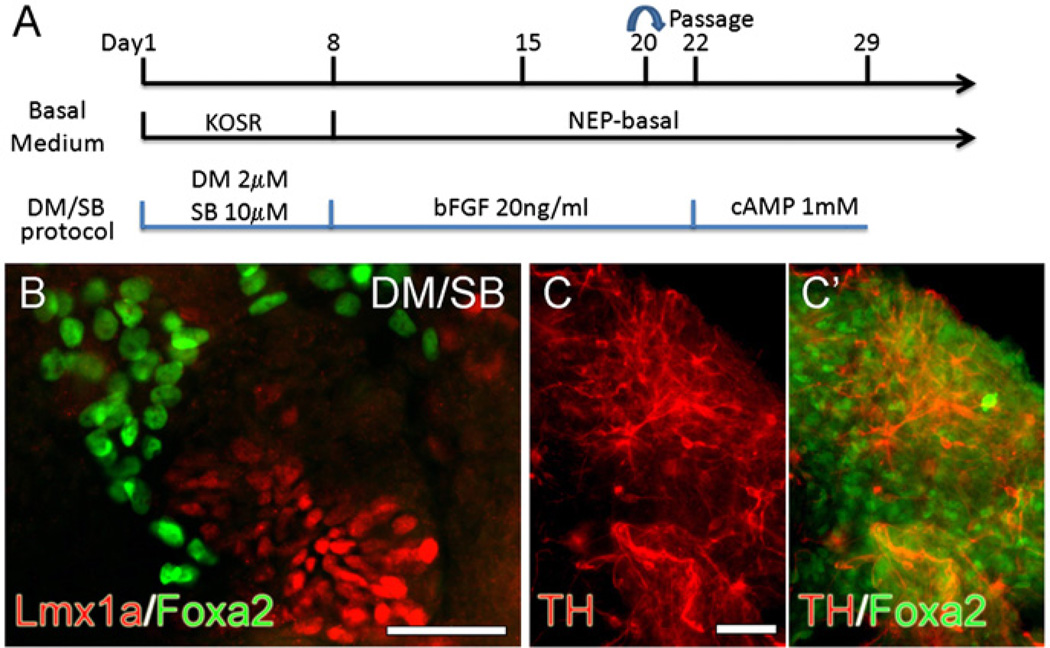
(A) Depiction of the various stages and treatments used to directly differentiate H9 cells into DA neurons in monolayer culture (modified from Iacovitti et al., 2007). (B, C) Expression levels of Lmx1a and TH are greatly increased by the treatment of stem cells with DM/SB. At stage 2, cultures were incubated with or without DM/SB then carried through the subsequent stages, (B) Stage 5 monolayer or EB-derived cultures stained for Lmx1a, TH and counter-stained with Dapi. Scale bar=50 µm, and (C) mRNA levels were quantified by qPCR compared to cells grown using the EB method in the absence of DM/SB treatment.
Curiously, when hNPs were generated in monolayer cultures, we found that some hNPs expressed Lmx1a but rarely went on to develop into tyrosine hydroxylase (TH)-expressing neurons at stage 5. Instead, they appeared to be ‘trapped’ at the mDA specification step (Fig. 1B). However, when both the BMP inhibitor, Dorsomorphin (DM) and the TGF-β inhibitor, SB431542 (SB) were added, we found a dramatic rise in Lmx1a expression in NPs as well as a marked amplification in the number of TH+ mDA neurons, disproportionate to the increase in nestin+ progenitors and β-III tubulin+ (β-III tub) neurons observed in the same cultures (Fig. 1B,C). Likewise, when EB cultures were treated with DM/SB, there was a significant increase in Lmx1a and TH over nestin and β-III tub expression (Fig. 1B,C). Taken together, these results suggested that while DM/SB modestly increases NP and neuron production in monolayer cultures, it greatly increases the proportion of those cells that are mDA-specified and that go on to become TH+ neurons.
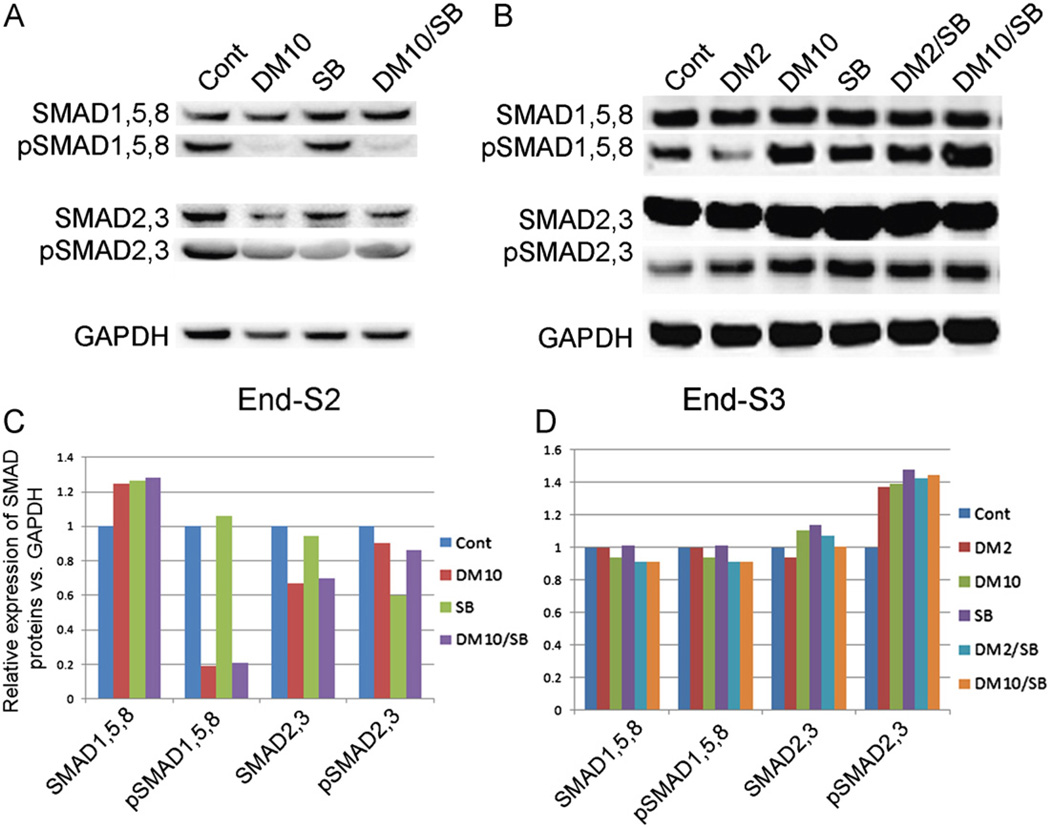
We next investigated the mechanism via which BMP/TGF-β inhibitors of specific receptor SMADs exerted their effects on mDA differentiation. Western analysis of hES cells maintained in basal growth media (control cultures) exhibited moderate levels of pSMADs 1, 5, 8 and pSMADs 2, 3 (Fig. 2A, C). However, constitutive BMP signaling was nearly totally blocked after treatment (stage 2) with highly specific BMP pathway inhibitor, DM (Fig. 2A, C). In contrast to DM, 10 µM SB was a relatively ineffectual inhibitor of the TGF-β pathway, only partially blocking the formation of pSMADs 2, 3 in stage 2 (Fig. 2A, C). After removal of SMAD inhibitors, phosphorylation of all SMADs was restored to near normal levels in stage 3.
Fig. 2.
Western blot detection of protein levels of SMADs and pSMADs expressed at the end of Stg2 or Stg3 in cells treated with DM, SB or DM/SB (A, B). All cell cultures exhibit similar levels of SMADs 1, 5, 8 and SMADs 2, 3. DM and DM/SB dramatically down-regulated pSMADs 1, 5, 8 while SB only partially lowered the levels of pSMADs 2, 3. (C, D) Quantification of western blot results shown in panel A, B.
To identify potential downstream molecular targets of BMP/TGF-β inhibitors, we used human PCR arrays (Qiagen PAHS-047Z — stem cell signaling) or (Qiagen PAHS-035Z — BMP/TGF-β signaling pathway) to compare control and DM/SB-treated monolayer cultures. While a number of genes were induced by DM/SB treatment, only those that were increased at least 5-fold upon treatment were verified by qPCR (Suppl. Fig. 1). Of that group, we found that inhibition of SMAD signaling in both EB and monolayer cultures caused a dramatic rise in the levels of the transcription factor, SMAD-interacting protein 1 (SIP1, also known as Zinc finger E-box-binding homeobox 2 or ZEB2). Interestingly, SIP1 levels were also elevated in untreated EB cultures compared to untreated monolayers, suggesting that the same factors may have been involved in mediating mDA differentiation in EB cultures even in the absence of DM/SB supplementation, possibly as a result of endogenous BMP/TGF-β inhibitors (ie. noggin) (Chambers et al., 2009; Krause et al., 2011).
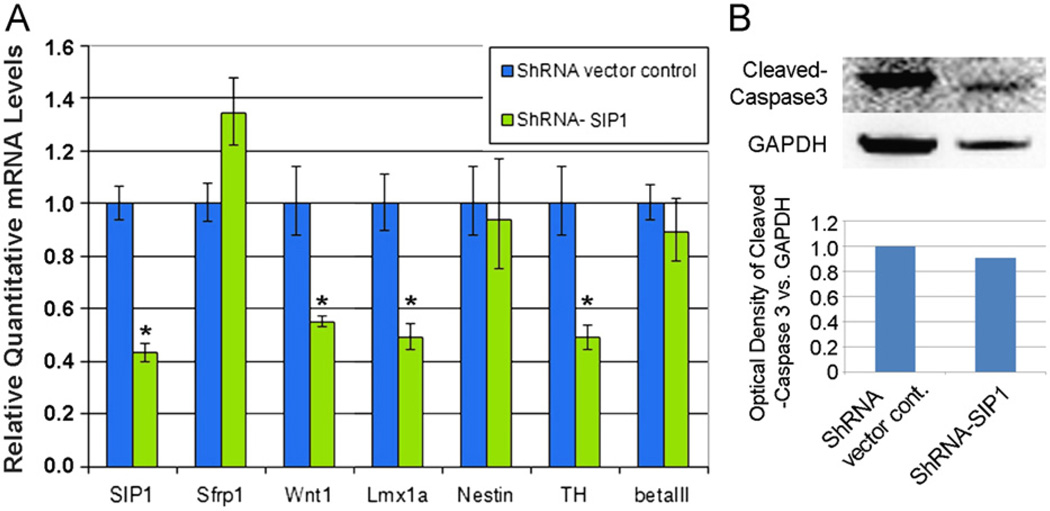
An important confirmation of SIP1’s role in mDA specification and differentiation was provided by SIP1 knockdown experiments. In these studies, SIP1 shRNA and control (empty and scramble) vectors were transfected into undifferentiated stem cells. Following puromycin selection and subsequent differentiation, qPCR analysis revealed significant knockdown in SIP1 transcripts, and importantly, a reduction in Lmx1a in stage 4 hNPs and TH in stage 4/5 neurons, without a change in nestin or β-III tub expression (Fig. 3A). Cleaved caspase 3 protein was not increased in SIP1 knockdown cultures (Fig. 3B), indicating that the decrease in Lmx1a and TH was not due to enhanced toxicity/cell death from genetic engineering. These data demonstrate that SIP1 knockdown results in decreased mDA specification and differentiation without altering neurogenesis, suggesting that the two developmental processes are likely mediated by different pathways acting downstream of DM/SB. Moreover, these data further suggest that constitutive SIP1 levels normally hold in check Wnt1-Lmx1a-TH expression in stem cells, and that by increasing SIP1 with DM/SB treatment, the internal brakes on the mDA differentiation process can be released. Interestingly, sustained treatment with DM or DM/SB through stage 3 in culture resulted in reduced rather than enhanced mDA differentiation (data not shown), suggesting that the resumption of SMAD signaling after transient SMAD inhibition is also important in mDA differentiation.
Fig. 3.
(A) Gene expression analysis of markers of differentiation after SIP1 knockdown at Stg1. Wnt1, Lmx1a, and TH were down-regulated at the end of stage 4 while nestin and β-III tub show no significant changes (unpaired t test): *P < 0.05. (B) Western blot detection of similar cleaved Caspase3 expression in SIP1 knockdown samples as vector control.
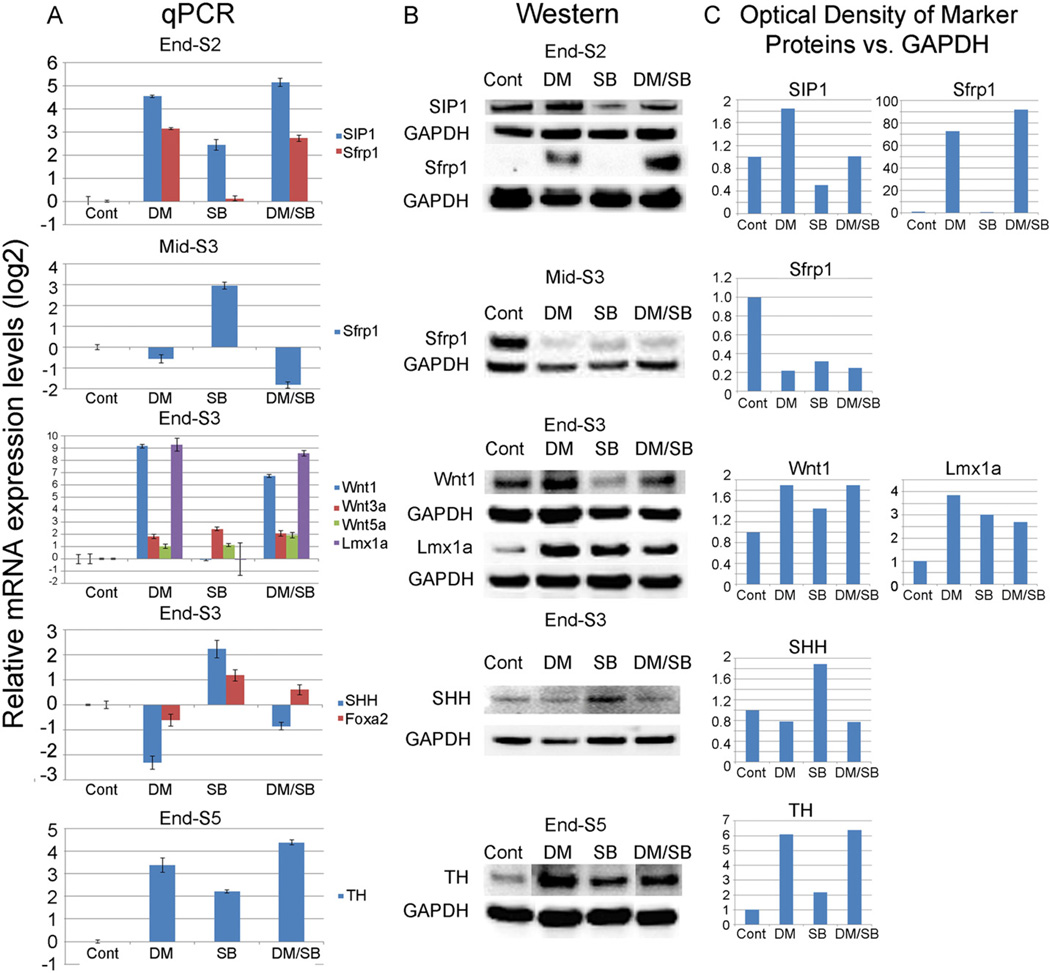
We next sought to identify the molecular mediator via which SIP1 regulates mDA differentiation in stem cells. As Wnt signaling is critical for mDA differentiation, it was of particular interest that SIP1 can directly repress the promoter of the Wnt antagonist, Secreted frizzled receptor protein 1 (Sfrp1) (Miquelajauregui et al., 2007). According to this mechanism, a rise in SIP1 would result in a decrease in Sfrp1 and its ability to bind Wnt ligands and their frizzled receptors, resulting in an up-regulation in Wnt signaling and mDA differentiation in our system. To test this possibility, SIP1 and Sfrp1 levels were measured by qPCR and Western in stem cells at various time points after treatment with BMP inhibitors (DM or LDN-193189), TGF-β inhibitors (SB or LY-364947), or a combination of BMP/TGF-β inhibitors (DM/SB). We found that, by the end of stage 2, cultures treated with BMP inhibitors expressed greatly amplified levels of SIP1 which were accompanied by a spike in Sfrp1 expression (snapshot view at relevant stage shown in Fig. 4 detailed time courses shown in Suppl. Figs. 2 and 3). In contrast, expression was only somewhat changed by TGF-β inhibitors; while combined DM/SB produced levels more closely resembling DM alone (Fig. 4; Suppl. Figs. 2 and 3). These changes were correlated with a profound rise in Wnt1, and to a lesser extent Wnt3a and Wnt5a expression and an upsurge in Lmx1a expression by the end of stage 3. In contrast, no induction in Wnt1 and Lmx1a was observed in SB only cultures (Fig. 4). Taken together, these results suggest that while TGF-β inhibition somewhat modifies SIP1/Sfrp1, these changes affect Wnt1–Lmx1a signaling only when coupled with BMP inhibition-induced changes in stem cells.
Fig. 4.
mRNA levels (A) and protein levels (B) of mDA markers examined at different stages after treatment of hES (H9 line) cells with DM, SB or DM/SB. At the end of Stg2, both SIP1 and Sfrp1 expression levels were increased after DM and DM/SB treatment. By mid-Stg3, Sfrp1 expression levels fell dramatically with DM and DM/SB treatment. At the end of Stg3, DM and DM/SB treatment greatly increased the expression of Wnt1 and Lmx1a (and somewhat increased Wnt3a and Wnt5a) while SHH expression decreased. At the end of Stg5, TH expression levels were increased with DM, SB and DM/SB treatment. (C) Quantification of Western blot results shown in panel B.
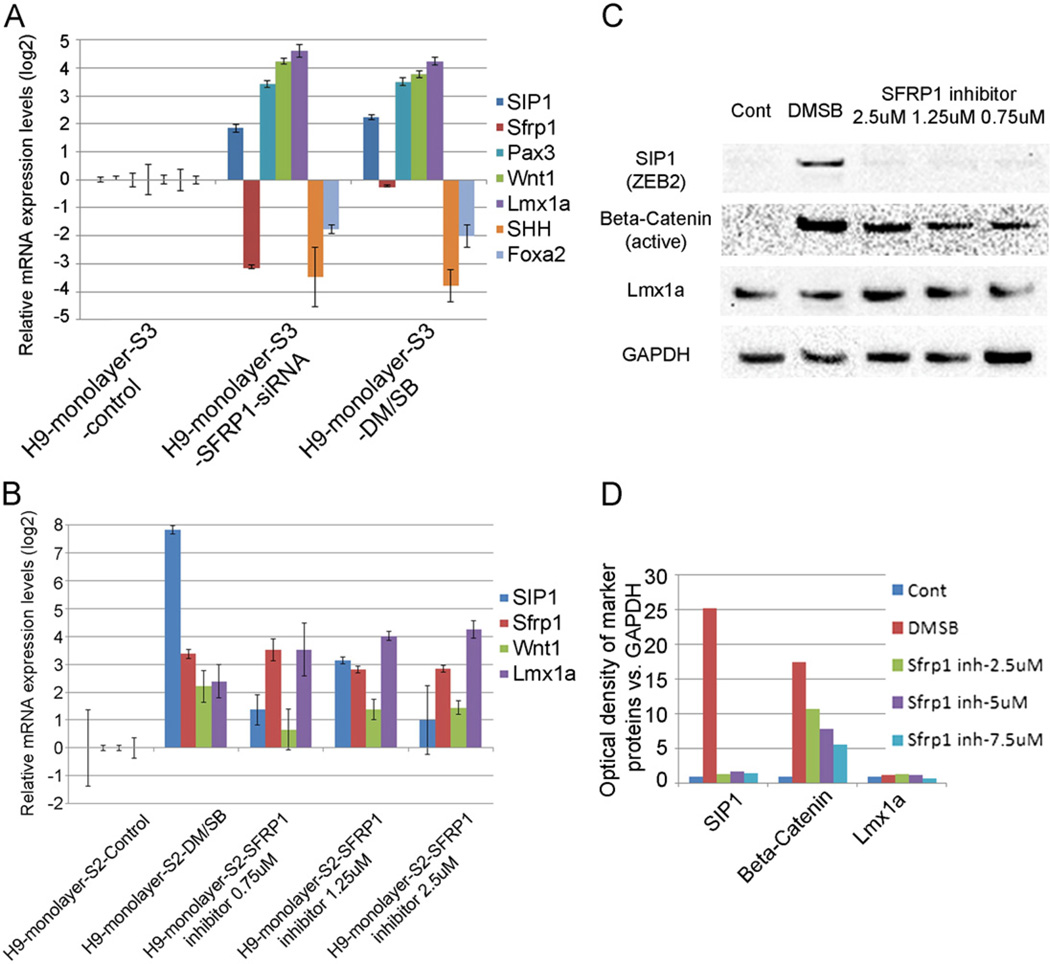
To further confirm the putative role of Sfrp1 in the regulation of Wnt1 signaling, stage 3 cultures were transiently transfected with siRNA for Sfrp1, which resulted in a significant knockdown of Sfrp1 expression and consequent up-regulation in Wnt1 signaling (as evidenced by an increase in Pax3 and Wnt1) (Fig. 5A). Interestingly, there was an unexpected and simultaneous increase in the presumptive upstream mediator, SIP1, possibly as a compensatory feedback consequence of Sfrp1 down-regulation, as has been seen previously (Gauger et al., 2011). Importantly, the effects of Sfrp1 knockdown on mDA differentiation markers mirrored those produced by DM/SB treatment, suggesting that the increased Wnt signaling seen after inhibition of BMP/TGF-β signaling was similarly dependent on the down-regulation of Sfrp1 in cells. Supporting this putative mechanism, we further showed that treating cells with pharmacological inhibitors (EMD Millipore 344300; N-(3-(Dimethylamino) propyl)-2-ethyl-5-(phenylsulfonyl)benzenesulfonamide) which bind Sfrp1 (but do not decrease Sfrp1 levels), also markedly increased active Wnt signaling (non-phosphorylated β-catenin) and Lmx1a expression, similar to DM/SB treatment (Fig. 5B–D). Conversely, the addition of exogenous human recombinant Sfrp1 did not significantly change Wnt1–Lmx1a signaling, although a small rise in expression was noted at 100 ng/ml (data not shown), as in other studies (Kele et al., 2012; Schwartz et al., 2012).
Fig. 5.
(A) Gene expression analysis of markers of differentiation 2 days after knockdown of Sfrp1 on Day 2 of Stg3. Sfrp1 knockdown produced similar changes in its downstream genes, Pax3, Wnt1, Lmx1a, SHH and Foxa2 as DM/SB treatment at Stg2. (B) Treatment with EMD Millipore 344300, a chemical inhibitor of Sfrp1, at Stg2 increased mRNA expression levels of mDA markers SIP1, Sfrp1, Wnt1 and Lmx1a as DM/SB treatment at Stg2. (C) Sfrp1 inhibitor, like DM/SB, increased Wnt signaling as indicated by the rise in active β-catenin on Western blot analysis. As Sfrp1 inhibition occurs downstream of Sip1, no change in Sip 1 was noted as with DM/SB treatment. (D) Quantification of Western blot results shown in panel C.
Of further significance, the up-regulation in Wnt1 signaling seen after DM or DM/SB treatment or after Sfrp1 knockdown/inhibition was accompanied by a striking concomitant reduction in SHH and Foxa2 levels (Fig. 4; Fig. 5A). These data support the widely held belief that Wnt and SHH signaling pathways work in a coordinated but opposing fashion (Chung et al., 2009; Joksimovic et al., 2009) and further indicate that BMP/TGF-β modulators can act upstream of these pathways to critically regulate the mDA differentiation process in stem cells.
In an attempt to further characterize the cellular phenotypes being generated in BMP and/or TGF-β-inhibited cultures (Fig. 6A), we evaluated not only levels of mDA markers but also markers of other cell types, including dorsal forebrain (EMX2, LHX2, PAX6, HES5) (Monuki et al., 2001; Theil et al., 2002), roof plate (BMP2) (Monuki et al., 2001), hypothalamic (SIX3, SIX6, RAX) (VanDunk et al., 2011), cortical hem (p73) (Cabrera-Socorro et al., 2006) and glutamatergic/GABAergic (Nkx2.2, GAD67) neurons (Nakatani et al., 2007). We found that by the end of stage 2, there was a rise particularly in forebrain and hypothalamic neuronal markers in all SMAD-inhibited cultures. However, following the removal of BMP or TGF-β inhibitors from the media, expression of these markers fell to near control levels (with the exception of EMX2) as mDA phenotypic markers (Wnt1, Lmx1a) increased dramatically in stage 3 cultures (Suppl. Fig. 4). Indeed, when sister cultures were immunocytochemically stained, we found many Lmx1a+ NPs in DM and DM/SB-treated stage 3 cultures as compared to control or SB cultures (data not shown). Importantly, however, these Lmx1a+ NPs did not co-label with Foxa2 although the culture did contain many brightly fluorescent Foxa2+ cells (Fig. 6B).
Fig. 6.
(A) Experiment paradigm of DM/SB protocol used to directly differentiate H9 cells into DA neurons in monolayer culture. (B, C) Immunocytochemical analysis of differentiated DA NPs and neurons. At the end of Stg3, many Lmx1a+ NPs were found in DM/SB-treated cultures. These Lmx1a+ NPs did not overlap with brightly fluorescent Foxa2+ cells (B). At the end of Stg5, mature DA neurons were detected as TH+ cells, most of which were Foxa2− in DM/SB-treated cultures (C, C′). Scale bars=50 µm.
At the end of differentiation (stage 5), all cultures were stained immunocytochemically for TH. Somewhat unexpectedly, we observed flattened neurite-free TH+ cells in control cultures which increased in number after SB treatment (Suppl. Fig. 5A). These TH+ cells did not stain for nestin or β-III tub and did not incorporate BrdU (Suppl. Fig. 5B–D), indicating that they were not dividing neural progenitors or postmitotic neurons. Importantly, this non-neural TH+ cell type was not routinely seen in DM or DM/SB-treated cultures where TH staining was observed only in process-bearing cells that co-labeled for β-III tub (data not shown). However, despite their mature appearance, these neurons did not co-label for Foxa2 (although many Foxa2+ cells were present) (Fig. 6C). These data, taken together with the qPCR and Western results (Fig. 4), suggest that TGF-β-inhibition alone yields a non-neural TH+ cell type in culture. In contrast, cultures treated with BMP inhibitors or combined BMP/TGF-β inhibitors are initially induced to become dorsal forebrain and hypothalamic neurons. Upon removal of these inhibitors from the media, NPs lose expression of these phenotypic markers and partially differentiate down the mDA pathway to express the mDA fate gene Lmx1a. However, their continued lack of Foxa2 expression brings into question their authenticity as bona fide mDA neurons.
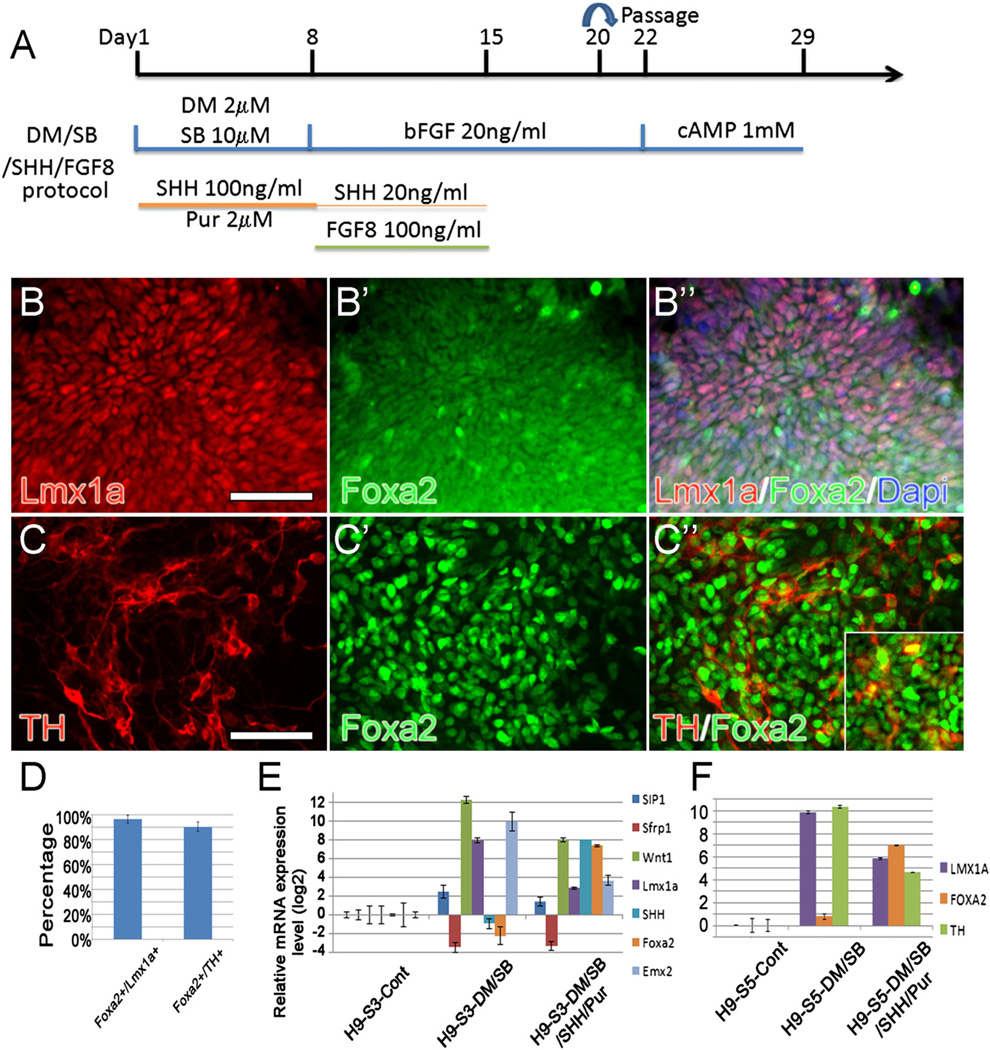
During the course of these studies, several other reports appeared emphasizing the importance of increasing downstream Wnt1 signaling (via the GSK3β inhibitor CHIR99021; [CHIR]) (Kriks et al., 2011; Xi et al., 2012) during the mDA differentiation process. In addition, these studies further stressed the need for high levels of early SHH and later FGF8 supplementation, mimicking more closely conditions in the ventral midbrain floor plate (Jaeger et al., 2011; Kriks et al., 2011; Xi et al., 2012). We therefore initiated a study in which cultures were simultaneously treated with BMP inhibitors and/or CHIR and/or activators of SHH and FGF8 (dose and treatment schedules shown in (Suppl. Fig. 6A)). We found no additivity/synergy in expression of mDA markers when downstream (CHIR) and upstream (DM/SB) Wnt inducers were combined. Like DM/SB only cultures (Suppl. Fig. 2), DM/SB/CHIR cultures (Suppl. Fig. 6B) also expressed high levels of Wnt1 and Lmx1a but low levels of SHH and Foxa2. Importantly however, by adding activators of SHH signaling (100ng/ml C24II+2 µM of the Smoothened receptor agonist Purmorphamine [Pur]; 8 days) early on (during neuroectodermal specification) to DM/SB (Fig. 7A) or DM/SB/CHIR (Suppl. Fig. 6B), expression of Wnt1/Lmx1a at stage 3 (Fig. 7 B–B″, E) and TH at stage 5 (Fig. 7C–C″, F) declined while SHH/Foxa2 rose dramatically (Fig. 7E,F). This critical change in the equilibrium between Wnt and SHH signaling lead to the co-expression of Foxa2 in 96.6%+3.1% mDA-specified Lmx1a+ NPs and 90.5+3.9% of authentic TH+ mDA neurons in cell aggregates (Fig. 7D). Concomitant with the increased production of authentic mDA neurons was the significant down-regulation of markers of other neuronal types in these cultures, including the dorsal forebrain marker EMX2.
Fig. 7.
(A) Experiment paradigm of DM/SB/SHH/FGF8 protocol used to directly differentiate H9 cells into DA neurons in monolayer culture. (B–C) The vast majority of authentic Lmx1a+/Foxa2+ mDA NPs (B–B″) and TH+/Foxa2+ mDA neurons (C–C″) were found in DM/SB/SHH/FGF8 treated cultures. (inset in C″ shows Lmx1a+/TH+ neurons). Scale bars=50 µm. (D) Quantification of Foxa2+/Lmx1a+ and Foxa2+/TH+ cells found in the cell aggregates. (E, F) Gene expression analysis of mDA markers examined at stages 3 (E) and 5 (F) after treatment of cells with DM/SB with and without added 100 ng/ml SHH/2 µM Pur. Note that in DM/SB only cultures, expression of Wnt1/Lmx1a (S3) and TH (S5) is high but SHH/Foxa2 is low while in cultures treated with both DM/SB plus SHH/Pur, expression of Wnt1/Lmx1a/TH is lower but SHH/Foxa2 is higher. Note that other cell type markers like EMX2 also declined. All cultures also contain FGF8 as described above.
Discussion
While others have previously maintained that the Wnt1–Lmx1a pathway works in a cooperative (Chung et al., 2009) but antagonistic (Joksimovic et al., 2009) fashion with SHH-Foxa2 to promote mDA differentiation, the upstream regulators of this complex equilibrium have remained elusive. The studies presented here suggest that the transient inhibition of constitutive BMP (pSMADs 1, 5, 8) signaling, either alone or in combination with TGF-β inhibition (pSMADs 2, 3), may play an important role in the upstream regulation of the Wnt1–Lmx1a and SHH-Foxa2 signaling pathways in stem cells. Thus, in control monolayer cultures where there is no significant mDA differentiation, we observe little Wnt1–Lmx1a signaling but robust SHH-Foxa2 signaling. However, this equilibrium is reversed when cells are transiently exposed to BMP inhibitors or BMP/TFG-β inhibitors early on in differentiation, leading to a marked amplification in Wnt1, Lmx1a and TH expression at subsequent stages and a concomitant decline in SHH and Foxa2. Gene knockdown experiments further implicate the SMAD-interacting transcription factor SIP1 and its downstream target gene Sfrp1 as important mediators of these effects, linking upstream BMP/TGF-β pathways and downstream Wnt1–Lmx1a and SHH-Foxa2 pathways. Together these results have led us to postulate a novel putative pathway for regulation of this complex signaling during mDA differentiation in stem cells (Fig. 8).
Fig. 8.
Diagram of putative mDA differentiation pathway based on this and other studies.
According to this pathway, pSMADs together with SIP1 act to co-repress the Wnt antagonist Sfrp1 in stem cells as in other cell systems (Postigo et al., 2003; Miquelajauregui et al., 2007). With less Sfrp1 available to compete for frizzled receptors (Molenaar et al., 1996; Peifer, 1997; Van de Wetering et al., 1997; Uren et al., 2000), Wnt ligands are able to promote Lmx1a expression and mDA differentiation. This then begs the question of how SMAD inhibition enhances this process. We would postulate that this is achieved through a series of critical molecular steps. Under normal basal culture conditions, stem cells express Sfrp1 despite constitutive SMAD signaling, possibly as a result of low levels of SIP1 co-repressors. With the addition of BMP inhibitors (DM, LDN) or combined BMP/TGF-β inhibitors (DM/SB) that block pSMADs 1, 5, 8 and/or pSMADs 2, 3, SIP1-mediated repression of Sfrp1 is even further diminished, resulting in a spike in Sfrp1 levels during stage 2. These elevated levels of Sfrp1 further antagonize Wnt signaling, working against the differentiation of an mDA phenotype in stem cells and in favor of alternate cell fates. As such, we find an induction in dorsal forebrain and hypothalamic markers LHX2, EMX2, SIX3, etc. in stage 2 after SMAD inhibition. Consistent with these results, other studies have also reported that dorsal forebrain markers LHX2 (Monuki et al., 2001) and EMX2 (Theil et al., 2002) are highly expressed with low (but not high) BMP signaling in stem cells.
However, another major consequence of BMP or BMP/TGF-β inhibition in stem cells is the dramatic rise in SIP1 levels during stage 2–3, possibly as a rebound response to the early upsurge in Sfrp1 levels. We posit that it is this elevation in SIP1 that allows Sfrp1 expression to be dramatically repressed once DM/SB is removed from the media and SMAD signaling is restored. Thus, both the rise in SIP1 co-repressors during transient BMP/TGF-β inhibition and the subsequent restoration of SMAD co-repressors after cessation of treatment may be necessary steps in ultimately driving down Sfrp1 levels and driving forward mDA differentiation. The importance of SMAD/SIP1 regulation in CNS development is not limited to the mDA differentiation process but is thought to also be involved in SVZ gliogenesis and myelination (Nityanandam et al., 2012; Weng et al., 2012).
Concomitant with the reduction in Sfrp1 in NPs is a shift in the equilibrium towards Wnt signaling, as evidenced by an increase in Wnt1/Pax3/β-catenin, and to a lesser extent Wnt3a and Wnt5a. Although in rare instances, low concentrations of Sfrp1 have been shown to increase rather than decrease mDA differentiation in stem cell cultures (Kele et al., 2012; Schwartz et al., 2012), our results after treatment with human recombinant Sfrp1, Sfrp1 antagonists or Sfrp1 siRNA, suggests that it is the decline, not the spike, in Sfrp1 which induces Wnt signaling in hES cell cultures. As a result of the rise in Wnt signaling in DM or DM/SB-treated stage 3 cultures, the vast majority of NPs go on to express Lmx1a while expression of other forebrain markers declines.
Of particular significance is the fact that increased Wnt1 signaling in DM and DM/SB-treated cultures results in a reciprocal reduction in SHH and Foxa2 levels. Precisely how downstream mediators of SMAD inhibition regulate SHH-Foxa2 signaling remains unclear. In the literature, no direct modulatory effect of Sfrp1 on the SHH promoter has been reported, although the converse has been widely observed (Ingram et al., 2002; He et al., 2006; Yauch et al., 2008; Katoh and Katoh, 2009; Shahi et al., 2011). Thus, the regulation of SHH-Foxa2 by Sfrp1 may occur through an indirect compensatory feedback pathway. Supporting this possibility is the previous demonstration in other systems that SHH, working through TGF-β signaling, can alter SIP1 and Sfrp1 levels to inhibit the Wnt pathway (He et al., 2006; Katoh and Katoh, 2009). Taken together, these data raise the possibility that the widely recognized cross talk that occurs between the SHH and Wnt signaling pathways during mDA differentiation (Chung et al., 2009; Joksimovic et al., 2009) may transpire at points (ie. SIP1 and Sfrp1) further upstream than formerly appreciated.
At the completion of differentiation when the phenotype of cells treated with SMAD inhibitors was assessed, we were surprised to find TH staining in cells with vastly different morphologies. Thus, control monolayer cultures contained non-dividing non-neural TH+ cells which increased in number with SB treatment. The identity of these cells remains unknown. However, it is a well-established fact that Alk4 receptor signaling promotes the formation of mesoendoderm over ectoderm (Chng et al., 2010). Since in control cultures, and even after 10 µM SB, Alk4/SMAD 2, 3 signaling remains high (> 70% of control), it is possible that TH+ non-neural cells are mesoendodermal in origin (Kunisada et al., 2012). Alternatively, SB has also been used to differentiate trophectoderm in hES cell cultures (Chambers et al., 2009) and therefore derivatives of this germ layer may also be present in our cultures. Distinguishing between these possibilities will require continued investigation. Presumably, however, it is this critical difference in cellular composition that accounts for the fact that SB-induced changes in SIP1 and Sfrp1 do not amplify Wnt1–Lmx1a signaling or mDA differentiation in these cultures.
Taken together, these data raise questions as to the benefits of routinely adding SB to monolayer cultures as is now done in many labs (He et al., 2011; Kim et al., 2011; Kriks et al., 2011; Vogt et al., 2011; Lipchina et al., 2012; Nefzger et al., 2012). Arguing in favor of the continued use of SB, we found that when combined with DM, SB induced changes in SIP1/Sfrp1 further enhanced Wnt1–Lmx1a signaling in NPs. Also, other labs report the efficient production of mDA neurons in monolayer cultures treated with combined BMP/TGF-β inhibitors (Kim et al., 2011; Kriks et al., 2011; Vogt et al., 2011; Lipchina et al., 2012; Nefzger et al., 2012), although their critical role in the mda differentiation process was not previously appreciated. In fact, in these studies, increased mDA differentiation was not attributed to the presence of SMAD inhibitors but to the addition of the GSK3β inhibitor CHIR99021 (downstream Wnt activator). We would speculate that the use of CHIR was required in these studies in order to boost Wnt1–Lmx1a signaling to detectable levels because of the low concentration of BMP inhibitor employed (ie. 100nM LDN compared to 2 µM, 10 µM DM used in our studies). The finding that Wnt1 and Lmx1a reached the same high level of expression after DM/SB or DM/SB/CHIR treatment of our cultures suggests that additivity/synergy between upstream and downstream Wnt activators is in fact unnecessary if BMP inhibitors are used at sufficiently high concentration.
Despite the enhanced production of Lmx1a+ NPs in DM/SB and DM/SB/CHIR-treated cultures, unexpectedly, these cells did not express the floor plate marker Foxa2. Moreover, at later stages, these progenitors did not give rise to TH+ neurons that co-expressed Foxa2, bringing into question their authenticity as mDA neurons. Thus, increasing Wnt signaling via BMP/TGF-β/GSK3β inhibition, while desirable, is not sufficient to fully drive mDA differentiation in stem cells. This is likely a result of the concomitant decline in SHH signaling and the forkhead floor plate marker Foxa2 that accompanies increased Wnt signaling in cells. Since authentic mDA neurons derive from NPs that express both Lmx1a and Foxa2 in vivo, it seems likely that it is necessary to drive both SHH and Wnt signaling in stem cell cultures in order to strike the proper balance in these opposing mDA differentiation pathways. Indeed, when potent activators of SHH signaling (C24II+Smoothened agonist Purmorphamine) were added along with Wnt activators DM/SB/CHIR and were continued at low dose for an extended period along with FGF8, we found a dramatic rise in SHH signaling and in the number of mDA-specified Lmx1a+ NPs and TH+ neurons which co-labeled for Foxa2, similar to the results reported previously using variations of the same protocol (Jaeger et al., 2011; Kriks et al., 2011; Xi et al., 2012). Consistent with the enhancement in mDA differentiation in these cultures, markers of other neuronal phenotypes, such as, hypothalamic (SIX3, SIX6, RAX and Nkx2.1), dorsal forebrain (HES5, EMX2, PAX6), roof plate (BMP2) and cortical (GABA) neurons were down-regulated. We conclude that the development of bona fide mDA neurons from stem cells requires sufficiently high SHH–Foxa2 signaling (from potent SHH agonists and FGF8) to balance the robust Wnt signaling seen after treatment with SMAD inhibitors.
Whether upstream (BMP/TGFβ) and downstream (Wnt/SHH) regulatory events occur in the same or different cell populations in our heterogeneous hES cell cultures remains unclear. Since Sfrp1 is a secreted molecule, it is indeed possible that changes in SMAD signaling/SIP1/Sfrp1 in one group of cells (ie. non-mDA specified NPs) could impact, in a paracrine fashion, Wnt/SHH signaling in another stem cell group (ie. prospective mDA-specified NPs). Alternatively, secreted Sfrp1 could act in autocrine fashion to drive mDA differentiation in those NPs which produce it. Drawing these distinctions will depend upon the future development of reliable antibodies able to detect small quantitative changes in SIP1 and Sfrp1, thus allowing for single cell analysis by immuncytochemistry. Regardless of cellular location, however, our data indicate that changes in BMP/TGFβ signaling critically influence Wnt/SHH levels and ultimately the degree of mDA differentiation observed in stem cell cultures. Whether SMADs similarly regulate the Wnt-Lmx1a and SHH-Foxa2 pathways during the development of mDA neurons in vivo remains to be investigated.
In summary, the findings of this paper indicate that the transient inhibition of the constitutive BMP pathway is needed to increase SIP1 such that Sfrp1 can be co-repressed by pSMADS 1, 5, 8, after BMP inhibitor removal. This decline in the Wnt antagonist Sfrp1 results in an up-regulation in Wnt1 signaling and Lmx1a expression in mDA specified NPs. Importantly, the addition of TGF-β inhibitors of SMADs 2, 3 can further amplify this effect but only when combined with BMP inhibitors which have already neuralized stem cells. However, a major consequence of increased Wnt signaling in these cells is the simultaneous and reciprocal down-regulation in SHH-Foxa2 signaling, resulting in the generation of Lmx1a+ NPs and TH+ neurons which lack Foxa2 expression, an impediment which is rectified by co-treating SMAD-inhibited cultures with SHH and FGF8 early on during cell specification. We conclude that inhibitors of BMP and TGF-β signaling play a critical upstream regulatory role in the mDA differentiation process, driving Wnt1–Lmx1a signaling in stem cells but that the generation of authentic mDA neurons requires additional factors (SHH, FGF8) to properly balance the equilibrium between Wnt–Lmx1a and SHH-Foxa2 mDA pathways. We would further postulate that it is the regulation of these critical mDA pathways by SMAD inhibitors which is responsible for the high efficiency production of authentic mDA neurons seen in the present study and in studies published previously (Kriks et al., 2011; Xi et al., 2012). Establishing the mechanisms via which authentic mDA neurons are produced in large quantity from human embryonic stem cells and human induced pluripotent stem cells (our unpublished data, Mak et al., 2012) is key to the successful translation of this technology for PD cell replacement therapy.
Materials and methods
Tissue culture
hES cells (H9 cells, Passage 35–50) were purchased from Wicell Research Institute and maintained according to the supplier’s instructions. Briefly, cells were grown on a monolayer of primary mouse fibroblasts (MEFs; Millipore) in DMEM/F12 media (invitrogen) supplemented with 20% Knockout Serum Replacer™ (KOSR; invitrogen), 1% Non-Essential Amino Acids (invitrogen), 1 mM l-glutamine (invitrogen), 0.1 mM 2-mercaptoethanol, and 4 ng/ml bFGF (R&D systems). Cell propagation was achieved through manual dissection and transfer of cell colonies once per week. The differentiation process was initiated by passaging them on Geltrex (Invitrogen 1:100)-coated tissue culture plates with two TGF/BMP inhibitors SB431542 (SB, Tocris, 10 µM) and Dorsomorphin (DM, Tocris, 2 µM) for 1 week. Additional small chemicals and growth factors, such as LDN-193189 (LDN, Stemgent, 2 µM), LY-364947 (LY, Tocris, 10 µM), CHIR 99021 (CHIR, Tocris, 0.4 µM), SHH (C24II) (SHH, R&D systems, 100 ng/ml), Purmorphamine (Pur, Stemgent, 2 µM), FGF8 (R&D systems, 100 ng/ml) and Sfrp1 inhibitor (Millipore) were also used at Stage 2. Then neural progenitors (NPs) were generated in N2/B27 NEP-basal medium. Rosettes were then expanded in NEP-basal medium supplemented with 20 ng/ml bFGF (R&D system) every other day. For further differentiation down the DA pathway, cells were incubating for 1 week in NEP-basal medium supplemented with 1 mM dibutyryl cAMP (dbcAMP, Sigma) (Fig.1A).
Immunocytochemistry
Cultures were fixed with 4% paraformaldehyde for 30 min at 4 °C and stained with primary antibodies (Suppl. Table 1) at 4 °C overnight. All secondary antibodies were Alexa Fluor antibodies from Invitrogen used at 1:200 for 30 min at room temperature. Cultures were also counter stained with Hoechst 33258 (Invitrogen) at 1:1000. Slides were covered with ProLong Gold antifade reagent (Invitrogen). Single and double labeled cells were counted in all fields of ES cell aggregates in triplicate cultures and averaged+SEM using an Olympus IX81 Image Analysis System.
RNA isolation and cDNA synthesis
Total RNA was isolated directly from freshly collected different treatments and different stages of H9 cells with TRIzol (Invitrogen), a modification of the guanidine isothiocyanate–phenol–chloroform extraction method. cDNA was synthesized by using 1 µg total RNA in a 20 µL reaction with Superscript III (Invitrogen) and oligo (dT)12–18 (Invitrogen). One microliter of RNase H (Invitrogen) was added to each reaction tube, and the tubes were incubated for 20 min at 37 °C before proceeding to PCR.
Real-time PCR analysis
Real-time PCR was carried out by 7500 Real-Time PCR System using SYBR green PCR master mix (both from Applied Biosystems). GAPDH was used as an internal control. All PCR products were checked by running an agarose gel for the first time and by doing dissociation assay every time to exclude the possibility of multiple products. PCR analyses were conducted in triplicate for each sample. Primers are listed as Suppl. Table 2. Statistical analysis was performed using the unpaired t test.
RT2Profiler™ qPCR array
TGF-beta/BMP signaling and stem cell signaling qPCR arrays were obtained from Qiagen. Five µg of total RNA was reverse transcribed in a final reaction mix of 20 µL using RT2 First Strand Kit (Qiagen) according to the manufacturer’s instructions. cDNA was diluted by adding Nuclease-free water (Ambion). The PCR was carried out using the 7500 Real-Time PCR System (Applied Biosystem) according to the protocol provided by Qiagen. For one 96-well-plate of the PCR array, 2550 µL of PCR master mix containing 2× SuperArray RT2 qPCR Master Mix and 102 µL of diluted cDNA was mixed and aliquots of 25 µL were added to each well. Universal cycling conditions (10 min at 95 °C, 15 s at 95 °C, 1 min 60 °C for 40 cycles) were used. After the PCR is done, raw data was saved and submitted to Qiagen’s website http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php for fold change calculating.
shRNA and siRNA
ShRNA vectors of Sip1 and siRNA oligos of Sfrp1 were purchased from Origene. Sip1 shRNA vectors and scrambled or empty control vectors were nucleofected into feeder-free H9 cells on a nucleofector device (Lonza). Two days after transfection, cells were selected with Puromycin (1 mg/ml) for about 14 days when single cell colonies appeared. Then cells were further differentiated before collection at the end of Stg4 and processed with ICC and Real-time PCR. Three pairs of verified siRNAs for human Sfrp1 and scrambled negative control siRNA were transfected into H9 dissociated Day2–Stg3 cells using Lipofectamine 2000 (Invitrogen). Transfection efficiency was monitored by co-transfecting a Trilencer-27 Fluorescent-labeled transfection control siRNA duplex (Origene). Total RNA was isolated two days later and further analyzed by real-time RT-PCR.
Western blot
Cultured cells were rinsed quickly with PBS and lysed in 1 ml Trizol (Invitrogen). The protein phase was collected and rinsed, and dissolved in 1% SDS containing protease and phosphatase inhibitors (Roche). All cell lysates were stored at −20 °C. Prior to electrophoresis, proteins were quantified to ensure equal gel loading using the Lowry Assay. Samples were prepared for western blotting with LDS sample buffer and DTT (Invitrogen), heated at 100 °C for 15 min, and loaded into individual wells of a 4–12% Bis-Tris gel (20 µl/well, Invitrogen), resolved for 60 min at 120 mA. Gels were transferred to nitrocellulose membrane for blocking in either 5% milk/0.1% PBST (Phosphate-Buffered Saline/0.1% Tween-20) or 1% BSA/0.1% PBST, and blots labeled overnight with primary antibody at 4 °C in blocking solution. Primary antibodies were listed in Suppl. Table 3. Blots were washed with PBST for 15 min and labeled for one hour at room temperature with goat-α-rabbit-HRP secondary antibody (Santa Cruz Biotechnology, 1:5000 in blocking solution). Blots were washed again for 15 min with 0.1% PBST, and exposed to peroxide substrate (Pierce) for chemiluminescent imaging. Protein signals on western blots were measured and quantified using densitometry algorithms by Image J software (NIH.gov).
Supplementary Material
Acknowledgments
This work was generously supported by NIH NS075839, the Parkinson’s Council and The Newell DeValpine Foundation.
Footnotes
Appendix A. Supporting information
Supplementary information associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2013.01.012
References
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Arenas E. Stem cells in the treatment of Parkinson’s disease. Brain Res. Bull. 2002;57:795–808. doi: 10.1016/s0361-9230(01)00772-9. [DOI] [PubMed] [Google Scholar]
- Arenas E. Engineering a dopaminergic phenotype in stem/precursor cells: role of Nurr1, glia-derived signals, and Wnts. Ann. N Y Acad. Sci. 2005;1049:51–66. doi: 10.1196/annals.1334.007. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- Cabrera-Socorro A, Pueyo Morlans M, Suarez Sola ML, Gonzalez Delgado FJ, Castañeyra-Perdomo A, Marin MC, Meyer G. Multiple isoforms of the tumor protein p73 are expressed in the adult human telencephalon and choroid plexus and present in the cerebrospinal fluid. Eur. J. Neurosci. 2006;23:2109–2118. doi: 10.1111/j.1460-9568.2006.04750.x. [DOI] [PubMed] [Google Scholar]
- Cai J, Donaldson A, Yang M, German MS, Enikolopov G, Iacovitti L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson’s disease model. Stem Cells. 2009;27:220–229. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010;19:1017–1023. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng Z, Teo A, Pedersen RA, Vallier L. SIP1 mediates cell-fate decisions between neuroectoderm and mesendoderm in human pluripotent stem cells. Cell Stem Cell. 2010;6:59–70. doi: 10.1016/j.stem.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Chung S, Leung A, Han B-S, Chang M-Y, Moon J-I, Kim C-H, Hong S, Pruszak J, Isacson O, Kim K-S. Wnt1-lmx1a forms a novel auto-regulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5:646–658. doi: 10.1016/j.stem.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper O, Hargus G, Deleidi M, Blak A, Osborn T, Marlow E, Lee K, Levy A, Perez-Torres E, Yow A, Isacson O. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol. Cell. Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham M, Dottori M. Signals involved in neural differentiation of human embryonic stem cells. Neurosignals. 2009;17:234–241. doi: 10.1159/000231890. [DOI] [PubMed] [Google Scholar]
- Fasano C, Kortleven C, Trudeau L-E. Chronic activation of the D2 autoreceptor inhibits both glutamate and dopamine synapse formation and alters the intrinsic properties of mesencephalic dopamine neurons in vitro. Eur. J. Neurosci. 2010;32:1433–1441. doi: 10.1111/j.1460-9568.2010.07397.x. [DOI] [PubMed] [Google Scholar]
- Friling S, Andersson E, Thompson LH, Jönsson ME, Hebsgaard JB, Nanou E, Alekseenko Z, Marklund U, Kjellander S, Volakakis N, Hovatta O, El Manira A, Björklund A, Perlmann T, Ericson J. Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2009;106:7613–7618. doi: 10.1073/pnas.0902396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger KJ, Chenausky KL, Murray ME, Schneider SS. SFRP1 reduction results in an increased sensitivity to TGF-β signaling. BMC Cancer. 2011;11:59. doi: 10.1186/1471-2407-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, Luxon BA, Xie J. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J. Biol. Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- He X-B, Yi S-H, Rhee Y-H, Kim H, Han Y-M, Lee S-HS-H, Lee H, Park C-H, Lee Y-S, Richardson E, Kim B-W. Prolonged membrane depolarization enhances midbrain dopamine neuron differentiation via epigenetic histone modifications. Stem Cells. 2011;29:1861–1873. doi: 10.1002/stem.739. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Lardaro T, Ludwig W, Viñuela A, Kim K-S, Isacson O. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson’s disease. Stem Cells. 2008;26:1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: studies in vitro and in vivo. Brain Res. 2007;1127:19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–8205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Hill CS. Stoichiometry of active smad-transcription factor complexes on DNA. Journal Biol. Chem. 2002;277:51008–51016. doi: 10.1074/jbc.M208532200. [DOI] [PubMed] [Google Scholar]
- Jaeger I, Arber C, Risner-Janiczek JR, Kuechler J, Pritzsche D, Chen I-C, Naveenan T, Ungless MA, Li M. Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development. 2011;4374:4363–4374. doi: 10.1242/dev.066746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RDG, Awatramani RB. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat. Neurosci. 2009;12:125–131. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Integrative genomic analyses of ZEB2: transcriptional regulation of ZEB2 based on SMADs, ETS1, HIF1alpha, POU/OCT, and NF-kappaB. Int. J. Oncol. 2009;34:1737–1742. doi: 10.3892/ijo_00000304. [DOI] [PubMed] [Google Scholar]
- Kele J, Andersson ER, Villaescusa JC, Cajanek L, Parish CL, Bonilla S, Toledo EM, Bryja V, Rubin JS, Shimono A, Arenas E. SFRP1 and 2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cells. 2012;30:865–875. doi: 10.1002/stem.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee G, Ganat Y, Papapetrou EP, Lipchina I, Socci ND, Sadelain M, Studer L. miR-371-3 expression predicts neural differentiation propensity in human pluripotent stem cells. Cell Stem Cell. 2011;8:695–706. doi: 10.1016/j.stem.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee S-H, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kittappa R, Chang WW, Awatramani RB, McKay RDG. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007;5:e325. doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, Guzman A, Knaus P. Noggin. Int. J. Biochem. Cell Biol. 2011;43:478–481. doi: 10.1016/j.biocel.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8:274–284. doi: 10.1016/j.scr.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Lee H-S, Bae E-J, Yi S-H, Shim J-W, Jo A-Y, Kang J-S, Yoon E-H, Rhee Y-H, Park C-H, Koh H-C, Kim H-J, Choi H-S, Han J-W, Lee Y-S, Kim J, Li J-Y, Brundin P, Lee S-H. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28:501–512. doi: 10.1002/stem.294. [DOI] [PubMed] [Google Scholar]
- Lin W, Metzakopian E, Mavromatakis YE, Gao N, Balaskas N, Sasaki H, Briscoe J, Whitsett JA, Goulding M, Kaestner KH, Ang S-L. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev. Biol. 2009;333:386–396. doi: 10.1016/j.ydbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Lipchina I, Studer L, Betel D. The expanding role of miR-302–367 in pluripotency and reprogramming. Cell Cycle. 2012;11:1517–1523. doi: 10.4161/cc.19846. [DOI] [PubMed] [Google Scholar]
- Mak SK, Huang YA, Iranmanesh S, Vangipuram M, Sundararajan R, Nguyen L, Langston JW, Schüle B. Small molecules greatly improve conversion of human-induced pluripotent stem cells to the neuronal lineage. Stem Cells Int. 2012;2012:140427. doi: 10.1155/2012/140427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquelajauregui A, Van de Putte T, Polyakov A, Nityanandam A, Boppana S, Seuntjens E, Karabinos A, Higashi Y, Huylebroeck D, Tarabykin V. Smad-interacting protein-1 (Zfhx1b) acts upstream of Wnt signaling in the mouse hippocampus and controls its formation. Proc. Natl. Acad. Sci. USA. 2007;104:12919–12924. doi: 10.1073/pnas.0609863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, Van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destré O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Kumai M, Mizuhara E, Minaki Y, Ono Y. Lmx1a and Lmx1b cooperate with Foxa2 to coordinate the specification of dopaminergic neurons and control of floor plate cell differentiation in the developing mesencephalon. Dev. Biol. 2010;339:101–113. doi: 10.1016/j.ydbio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Minaki Y, Kumai M, Ono Y. Helt determines GABAergic over glutamatergic neuronal fate by repressing Ngn genes in the developing mesencephalon. Development. 2007;134:2783–2793. doi: 10.1242/dev.02870. [DOI] [PubMed] [Google Scholar]
- Nefzger CM, Su CT, Fabb SA, Hartley BJ, Beh SJ, Zeng WR, Haynes JM, Pouton CW. Lmx1a allows context-specific isolation of progenitors of GABAergic or dopaminergic neurons during neural differentiation of embryonic stem cells. Stem Cells. 2012;30:1349–1361. doi: 10.1002/stem.1105. [DOI] [PubMed] [Google Scholar]
- Nityanandam A, Parthasarathy S, Tarabykin V. Postnatal subventricular zone of the neocortex contributes GFAP+ cells to the rostral migratory stream under the control of Sip1. Dev. Biol. 2012;366:341–356. doi: 10.1016/j.ydbio.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Peifer M. Beta-catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- Pollard SM, Wallbank R, Tomlinson S, Grotewold L, Smith A. Fibroblast growth factor induces a neural stem cell phenotype in foetal forebrain progenitors and during embryonic stem cell differentiation. Mol. Cell. Neurosci. 2008;38:393–403. doi: 10.1016/j.mcn.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N, Brodski C, Naserke T, Puelles E, Gogoi R, Hall A, Panhuysen M, Echevarria D, Sussel L, Weisenhorn DMV, Martinez S, Arenas E, Simeone A, Wurst W. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133:89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell. Mol. Life Sci.: CMLS. 2006;63:187–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussa E, Krieglstein K. Induction and specification of midbrain dopaminergic cells: focus on SHH, FGF8, and TGF-beta. Cell Tissue Res. 2004;318:23–33. doi: 10.1007/s00441-004-0916-4. [DOI] [PubMed] [Google Scholar]
- Schwartz CM, Tavakoli T, Jamias C, Park S-S, Maudsley S, Martin B, Phillips TM, Yao PJ, Itoh K, Ma W, Rao MS, Arenas E, Mattson MP. Stromal factors SDF1α, sFRP1, and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J. Neurosci. Res. 2012;90:1367–1381. doi: 10.1002/jnr.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi MH, Schiapparelli P, Afzal M, Sinha S, Rey JA, Castresana JS. Expression and epigenetic modulation of sonic hedgehog-GLI1 pathway genes in neuroblastoma cell lines and tumors. Tumour Biol.: J. Int. Soc. Oncodev. Biol. Med. 2011;32:113–127. doi: 10.1007/s13277-010-0105-x. [DOI] [PubMed] [Google Scholar]
- Simon HH, Bhatt L, Gherbassi D, Sgadó P, Alberí L. Midbrain dopaminergic neurons: determination of their developmental fate by transcription factors. Ann. N. Y. Acad. Sci. 2003;991:36–47. [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Rüther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- Van de Wetering M, Cavallo R, Dooijes D, Van Beest M, Van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- VanDunk C, Hunter LA, Gray PA. Development, maturation, and necessity of transcription factors in the mouse suprachiasmatic nucleus. J. Neurosci.: Off. J. Soc. Neurosci. 2011;31:6457–6467. doi: 10.1523/JNEUROSCI.5385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFβ and BMP pathways. Cell. Signal. 2011;23:1831–1842. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Weng Q, Chen Y, Wang H, Xu X, Yang B, He Q, Shou W, Chen Y, Higashi Y, Van den Berghe V, Seuntjens E, Kernie SG, Bukshpun P, Sherr EH, Huylebroeck QR, Lu QR. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron. 2012;73:713–728. doi: 10.1016/j.neuron.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang S-C. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Donaldson AE, Marshall CE, Shen J, Iacovitti L. Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant. 2004;13:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, De Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-Q, Zhang S-C. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol. Biol. 2010;584:355–366. doi: 10.1007/978-1-60761-369-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.