Abstract
Phospholipids of erythrocyte are found as bilayer with choline containing phospholipid like phosphatidyl choline and sphingomylein in the outer layer and amine containing phospholipid like phosphatidyl ethanolamine and phosphatidyl serine in the inner layer. This arrangement is known as phospholipid asymmetry. Lipid asymmetry is maintained throughout the entire life span of red blood cell and is disturbed when cells enter into the stage of apoptosis. We here discuss some of the conditions in which phospholipid asymmetry of erythrocyte is maintained and disturbed and the various detection methods to check the distortion phospholipid asymmetry of it.
KEY WORDS: Erythrocytes, lipid asymmetry, phosphatidyl serine, phospholipids
Cell is the structural and functional unit of all living organism. The membrane that bound the cell and cell organelle is made up of phospholipids. Accordingly we can say our body as a puddle of phospholipids. Most components of cells are water soluble; however phospholipids are water insoluble, which provide integrity to the cell. Apart from phospholipids other lipids such as glycolipids and cholesterol also play an important role in providing integrity to the cell. Phospholipids are found as bilayer with choline containing phospholipid like phosphatidyl choline (PC) and sphingomylein in the outer layer and amine containing phospholipid like phosphatidyl ethanolamine and phosphatidyl serine in the inner layer. These are non-randomly arranged in bilayer in normal cells hence the name lipid asymmetry. The phospholipid bilayer act as semi permeable membrane, thus protecting cells from the external environment, while transport the essential component to the cell for its metabolic activity. Phospholipid contains one hydrophilic head and two hydrophobic tails. There is one more type of asymmetry with respect to lipid head group. The fatty acyl chains of the exterior phospholipids are highly saturated than the acyl chains of the interior phospholipids. This type of lipid asymmetry is known as transverse phospholipid asymmetry. This makes the cells interior more lipophilic. Once, this transverse phospholipid asymmetry is established, it is maintained by the combination of slow transbilayer movement of lipids and the presence of selective lipid transporters. Lipid asymmetry is maintained by the co-operative activities of three transporters namely flippase, floppase and scramblase.[1,2]
Flippase
It is an ATP dependent amino phospholipid – specific translocase, which transports phosphatidl Serine and Phosphatidyl ethanaol amine from the cells outer to the inner leaflet. Flippases are transmembrane lipid transporter enzymes located in the membrane.
Floppase
It is ATP dependent nonspecific translocase, which slowly transport lipid from the cell's inner to the outer leaflet.
Scramblase
Ca2+ dependent non-specific lipid transporter, which allows lipid to move randomly between the leaflets; that means they can transport the negatively charged phospholipids from the inner-leaflet to the outer-leaflet and vice versa. In humans, phospholipid scramblases constitute a family of five homologous proteins, which are hPLSCR1, hPLSCR2, hPLSCR3, hPLSCR4 and hPLSCR5.[2,3]
Role of Spectrin in Phospholipid Asymmetry
Spectrin is a major protein component of human red blood cells (RBC) located in intracellular region. Spectrin forms pentagonal or hexagonal arrangements, forming scaffolding and plays an important role in maintenance of phospholipid asymmetry by interacting with the lipid component. When oxidation takes place in membrane the sulfhydryl group converts to disulfide bond and the cross linking of membrane skeletal protein and aggregation of membrane proteins.[4,5]
Role of Calcium in Lipid Asymmetry
Increased cytoplasmic Ca2+ concentration disrupt lipid asymmetry by activating the scramblase and inhibiting the Mg2+ ATPase amino phospholipid translocase. Elevation of Ca2+ leads to calpalin induced degradation of variety of membrane protein.[6]
Condition in Which Phospholipid Asymmetry is Maintained and or Disturbed
Phospholipid asymmetry is maintained throughout the life span of the cell. Once the cell enters into apoptosis stage, this phospholipid asymmetry gets distorted. When lipid asymmetry is distorted, amine containing phosphatidy Serine will exposed, phosphatidyl Serine act as a self-marker formacrophages and removed from the body by engulfing them[7] [Figure 1].
Figure 1.
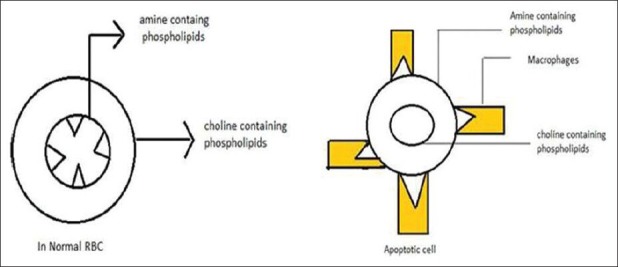
Lipid asymmetry in normal cells and exposure of phosphatidyl serine in apoptotic cell
We here described some of the physiological condition and pathologic conditions in which phospholipid asymmetry is maintained and or distorted [Figures 2 and 3] and various methods to detect the phospholipid asymmetry.
Figure 2.
Conditions in which phospholipid asymmetry maintained
Figure 3.
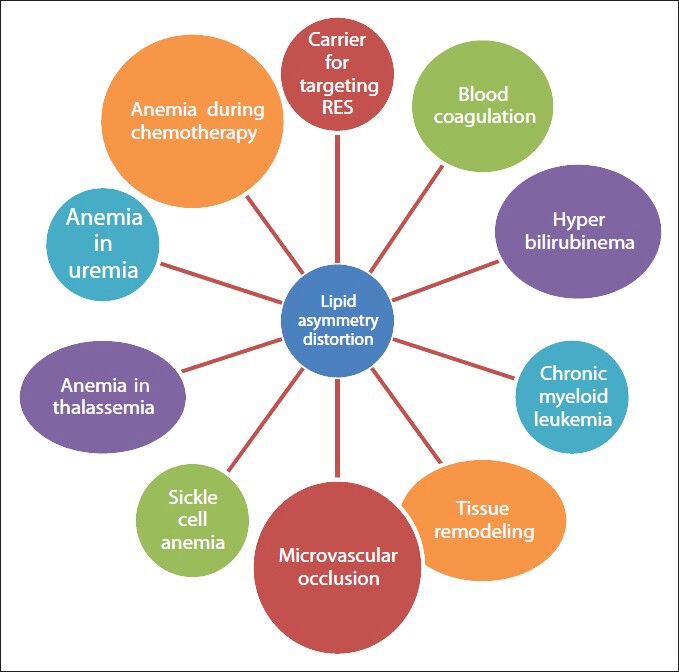
Conditions in which lipid asymmetry distorted
In uremic patients
In patients with the chronic kidney disease, anemia occurs due to interaction of various factors such as uremic toxins, free radical and elevated Ca2+ concentration with erythrocyte membrane accompanied by loss of asymmetry.[8,9]
Erythrocytes as drug carrier
The relationship between phospholipid asymmetry and erythrocyte as drug carrier can be studied under two heading
As slow release depot formulation.
For targeting mononuclear phagocyte system.
Slow release depot formulation
Erythrocytes can be used as drug carrier for slow release depot formulation provided that there should not any exposure of phosphatidyl Serine during the loading procedure. Exposure of phosphatidyl Serine will leads to rapid recognition of erythrocytes by mononuclear phagocytic system.
For targeting disease affecting reticuloendothelial system/mononuclear phagocytic system
Exposure of phosphatidyl Serine is preferred if we are supposed to target RES system because phosphatidyl Serine is a self-marker for macrophages. Thus we can able to target diseases like liver tumor, certain parasitic diseases in liver, disease affecting spleen etc.[10,11,12]
Microvascular occlusion
The loss of phospholipid asymmetry of erythrocytes activate coagulation factors like platelet activating factor (PAF) so that blood cell coagulate and this will adhere to endothelial cells, micro vascular occlusions happen and ultimately lead to cardiovascular complications such as shock, myocardial infarction etc.[13]
In sickle cell anemia
In sickle cell anemia, erythrocyte loss their lipid asymmetry as a consequence of rapid transbilayer diffusion of PC so that phospholipids will exposed. It will lead to the activating of factors responsible for clotting (like PAF) leading to micro vascular occlusion, clinically manifested most often as an acute painful episode or “crisis,” but with many other acute and chronic complications. Hence microvascular occlusion can be considered as secondary complications of loss of phospholipid asymmetry in much pathological condition.[13,14,15]
In blood coagulation
Activation of procoagulant cascade is not very significant in erythrocyte phosphatidyl Serine exposure. But is significant when comes in the case of platelet's phosphatidyl Serine exposure. However the exposure of phosphatidyl Serine s of erythrocyte in diabetic mellitus, sickle cell anemia and the resulting blood coagulation and microvasular occlusion is a matter to be considered.[16,17,18]
During inflammation or tissue remodeling
During tissue remodeling or inflammation it is important to remove apoptotic cells from body in order to restore normal tissue structure and function. Loss of phospholipid asymmetry makes the inflamed cell recognized by the macrophages. So that rapid clearance from the body will happen.[19]
Chronic myeloid leukemia
Chronic myeloid leukemia is a clonal disorder common to granulocyte.
Platelet and erythrocyte precursors due to phosphatidyl Serine exposure the spectrin cross linking occur because of the formation of disulfide between its two subunits.[20,21]
Hyperbilirubinema
High secretion or low excretion of bilirubin results in hyperbilirubinema in infants. When excess of bilirubin accumulate in body it binds with erythrocyte membrane and dissolves majority of glycerophospholipids like PC and cholesterol and expose the phosphatidyl Serines on the outer layer.[22]
During chemotherapy
Mature RBC the lipid asymmetry is disturbed during chemotherapy resulting in anemia; this condition may sometimes fatal.[23]
β thalassemia
Thalassemia is an inherited disease, clinically manifested as chronic anemia, which is partially due to ineffective erythropoiesis and decreased survival of erythrocyte in peripheral blood because of loss of lipid asymmetry.[24]
Detection of Phospholipid Asymmetry
By binding with fluorescently labeled Annexin V
Annexin V is a 35-36 kDa Ca2+ dependent phospholipid-binding protein that has a high affinity for phosphatidyl Serine and binds to cells with exposed phosphatidyl Serine. The binding assay result will be provided by flow cytometry in a four quadrant table [Figure 4].
Figure 4.
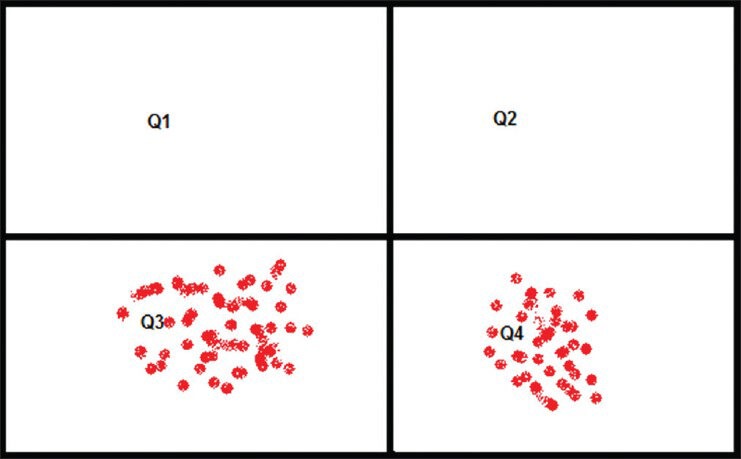
Annexin V binding assay test four quadrant table
Quadrant 3 (Q3): This quadrant shows the no. of cells that do not have affinity for annexin, which means all cells are live and no exposure of phosphatidyl Serine.
Quadrant 4 (Q4): Annexin V positive cell is quantified in this table. That means it gives information about the apoptotic cell or phosphatidyl Serine exposed erythrocytes.
Q1 and Q2 have no role in determining the phospholipid asymmetry of RBC as these quadrants are meant for nucleus containing cells. This quadrants give positive results for propidium iodide dye which stains the nucleus.[25]
In vitro macrophages uptake studies
Damaged/senescent cell are removed from the circulation by macrophages. Macrophages cellular uptake studies give valuable information about the lipid asymmetry. Phosphatidyl Serine s exposure will make erythrocytes easily recognizable by these macrophagal cell lines.
By confocal microscopy
Confocal microscopy is used for quantifying cellular uptake of erythrocyte. Phosphatidyl Serine exposed erythrocytes are internally localized in live macrophages cells.
Limitation
One of the most important limitations of confocal microscopy is the cost of instrument. Statistical problem also arise because only small number of cells can be monitored by it. Moreover, expertise needed to perform the experiment.
Procedure
Cells were grown to about 80% confluence in a 35 mm gloss bottom dishes. Fluorescently labeled erythrocytes is added to culture medium and incubated at 37°C. The cells are washed with phosphate-buffered saline after incubating and visualized under confocal microscope.[26]
Fluorescent assisted cell sorting
Erythrocytes are coupled with a flourophore and then added to cultured macrophages cell line and the fluorescent intensity of cells is taken by FACS.[27,28]
Limitation
It gives false positive result if the erythrocytes bind to the surface of macrophages cells.
Even though the detection of phospholipid asymmetry uses the sophisticated techniques, the uses of these techniques are limited to biomedical research.
Detection of phospholipid asymmetry is not only a diagnosis test for majority of diseases discussed above but also an important stability test parameter of storing RBC for transfusion. Erythrocytes which maintain lipid asymmetry throughout the entire storage period and storage condition is used for transfusion. Increase in storage period will lead to lipid asymmetry distortion. Proper storage is also a criteria for maintain phospholipid asymmetry.[29] Detection of phospholipid asymmetry can be considered an important recently updated evaluation parameter of resealed erythrocytes used as carrier for parenteral controlled release of drugs.
Conclusion
Maintenance of phospholipid asymmetry of erythrocyte membrane is essential for retaining the normal lifespan of the cell. Whenever distortion in lipid asymmetry occurs, the cells undergo apoptosis and are then removed from circulation by the mono nuclear phagocytic system. Many patho physiological conditions are attributed due to distortion in lipid asymmetry. Even though the lipid asymmetry seems to a minor phenomenon, a balance in maintenance and disturbance in lipid asymmetry is essential for a better life processes. This nature's own phenomenon of this lipid asymmetry of erythrocyte is used by man to make drug carrier for slow release depot formulation and also for targeting reticulo endothelial system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Antony GL. Structure and function of cell membranes. In: John BT, editor. Comprehensive Medicinal Chemistry. Delhi: Elsevier Publication; 2005. pp. 13–43. [Google Scholar]
- 2.Daleke DL. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 2008;15:191–5. doi: 10.1097/MOH.0b013e3282f97af7. [DOI] [PubMed] [Google Scholar]
- 3.Sugihara T, Sugihara K, Hebbel RP. Phospholipid asymmetry during erythrocyte deformation: Maintenance of the unit membrane. Biochim Biophys Acta. 1992;1103:303–6. doi: 10.1016/0005-2736(92)90100-z. [DOI] [PubMed] [Google Scholar]
- 4.Bitbol M, Fellmann P, Zachowski A, Devaux PF. Ion regulation of phosphatidylserine and phosphatidylethanolamine outside-inside translocation in human erythrocytes. Biochim Biophys Acta. 1987;904:268–82. doi: 10.1016/0005-2736(87)90376-2. [DOI] [PubMed] [Google Scholar]
- 5.Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci USA. 2002;99:1943–8. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder F. Role of membrane lipid asymmetry in aging. Neurobiol Aging. 1984;5:323–33. doi: 10.1016/0197-4580(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 7.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–7. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 8.Kong QY, Wu X, Li J, Peng WX, Ye R, Lindholm B, et al. Loss of phospholipids asymmetry in red blood cells contributes to anemia in uremic patients. Adv Perit Dial. 2001;17:58–60. [PubMed] [Google Scholar]
- 9.Bonomini M, Sirolli V, Settefrati N, Dottori S, Di Liberato L, Arduini A. Increased erythrocyte phosphatidylserine exposure in chronic renal failure. J Am Soc Nephrol. 1999;10:1982–90. doi: 10.1681/ASN.V1091982. [DOI] [PubMed] [Google Scholar]
- 10.Williamson P, Algarin L, Bateman J, Choe HR, Schlegel RA. Phospholipid asymmetry in human erythrocyte ghosts. J Cell Physiol. 1985;123:209–14. doi: 10.1002/jcp.1041230209. [DOI] [PubMed] [Google Scholar]
- 11.Connor J, Gillum K, Schroit AJ. Maintenance of lipid asymmetry in red blood cells and ghosts: Effect of divalent cations and serum albumin on the transbilayer distribution of phosphatidylserine. Biochim Biophys Acta. 1990;1025:82–6. doi: 10.1016/0005-2736(90)90193-r. [DOI] [PubMed] [Google Scholar]
- 12.Mehrdad H, Mahshid F, Abdolhossein Z, Ahmadreza M. Erythrocytes: From oxygen delivery to drug delivery. Control Release J. 2012;1:1–33. [Google Scholar]
- 13.Setty BN, Betal SG. Microvascular endothelial cells express a phosphatidylserine receptor: A functionally active receptor for phosphatidylserine-positive erythrocytes. Blood. 2008;111:905–14. doi: 10.1182/blood-2007-07-099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubin B, Chiu D, Bastacky J, Roelofsen B, Van Deenen LL. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981;67:1643–9. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenfeld N, Zachowski A, Galacteros F, Beuzard Y, Devaux PF. Transmembrane mobility of phospholipids in sickle erythrocytes: Effect of deoxygenation on diffusion and asymmetry. Blood. 1991;77:849–54. [PubMed] [Google Scholar]
- 16.Larson MC, Woodliff JE, Hillery CA, Kearl TJ, Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochim Biophys Acta. 2012;1821:1501–7. doi: 10.1016/j.bbalip.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim KM, Kim S, Noh JY, Kim K, Jang WH, Bae ON, et al. Low-level mercury can enhance procoagulant activity of erythrocytes: A new contributing factor for mercury-related thrombotic disease. Environ Health Perspect. 2010;118:928–35. doi: 10.1289/ehp.0901473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setty BN, Kulkarni S, Rao AK, Stuart MJ. Fetal hemoglobin in sickle cell disease: Relationship to erythrocyte phosphatidylserine exposure and coagulation activation. Blood. 2000;96:1119–24. [PubMed] [Google Scholar]
- 19.Robert AS, Susan K, Patrick W. Functional and pathological significance of phospholipid asymmetry in erythrocyte membranes. J Biosci. 1990;15:187–91. [Google Scholar]
- 20.Kumar A, Daniel S, Agarwal SS, Gupta CM. Abnormal erythrocyte membrane phospholipid organization in chronic myeloid leukemia. J Biosci. 1987;11:543–8. [Google Scholar]
- 21.Reed JA, Kough RH, Williamson PL, Schlegel RA. Merocyanine 540 recognizes membrane abnormalities of erythrocytes in chronic myelogenous leukemia. Cell Biol Int Rep. 1985;9:43–9. doi: 10.1016/0309-1651(85)90140-7. [DOI] [PubMed] [Google Scholar]
- 22.Brito MA, Silva RF, Brites D. Bilirubin induces loss of membrane lipids and exposure of phosphatidylserine in human erythrocytes. Cell Biol Toxicol. 2002;18:181–92. doi: 10.1023/a:1015563704551. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Inukai T, Hirose K, Akahane K, Nemoto A, Takahashi K, et al. Induction of impaired membrane phospholipid asymmetry in mature erythrocytes after chemotherapy. Int J Hematol. 2005;82:132–6. doi: 10.1532/IJH97.05009. [DOI] [PubMed] [Google Scholar]
- 24.Kuypers FA, Yuan J, Lewis RA, Snyder LM, Kiefer CR, Bunyaratvej A, et al. Membrane phospholipid asymmetry in human thalassemia. Blood. 1998;91:3044–51. [PubMed] [Google Scholar]
- 25.Lizarbe MA, Barrasa JI, Olmo N, Gavilanes F, Turnay J. Annexin-phospholipid interactions. Functional implications. Int J Mol Sci. 2013;14:2652–83. doi: 10.3390/ijms14022652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Bai J, Jiang X, Nienhaus GU. Cellular uptake of nanoparticles by membrane penetration: A study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano. 2012;6:1251–9. doi: 10.1021/nn203892h. [DOI] [PubMed] [Google Scholar]
- 27.Fatemeh M, Staffan L, Ulo L, Shiroh F, Astrid G. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011. 2011 doi: 10.1155/2011/414729. 414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 29.van der Meer PF, Cancelas JA, Cardigan R, Devine DV, Gulliksson H, Sparrow RL, et al. Evaluation of overnight hold of whole blood at room temperature before component processing: Effect of red blood cell (RBC) additive solutions on in vitro RBC measures. Transfusion. 2011;51(Suppl 1):15S–24. doi: 10.1111/j.1537-2995.2010.02959.x. [DOI] [PubMed] [Google Scholar]


