Abstract
Background:
Amyloidosis, oxidative stress and inflammation have been strongly implicated in neurodegenerative disorders like Alzheimer's disease. Traditionally, Caesalpinia crista and Centella asiatica leaf extracts are used to treat brain related diseases in India. C. crista is used as a mental relaxant drink as well as to treat inflammatory diseases, whereas C. asiatica is reported to be used to enhance memory and to treat dementia.
Objective:
The present study is aimed to understand the anti-oxidant and anti-inflammatory potential of C. asiatica and C. crista leaf extracts.
Materials and Methods:
Phenolic acid composition of the aqueous extracts of C. crista and C. asiatica were separated on a reverse phase C18 column (4.6 x 250 mm) using HPLC system. Antioxidant properties of the leaf extracts were determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay and the reducing potential assay. The anti-inflammatory activities of aqueous extracts of C. crista and C. asiatica were studied using 5-lipoxygenase assay. Polymorphonuclear leukocytes (PMNLs) were isolated from blood by Ficoll-Histopaque density gradient followed by hypotonic lysis of erythrocytes.
Results:
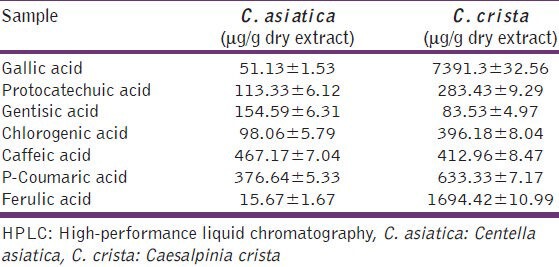
Gallic, protocatechuic, gentisic, chlorogenic, caffeic, p-coumaric and ferulic acids were the phenolic acids identified in C. crista and C. asiatica leaf aqueous extracts. However, gallic acid and ferulic acid contents were much higher in C. crista compared to C. asiatica. Leaf extracts of C. asiatica and C. crista exhibited antioxidant properties and inhibited 5-lipoxygenase (anti-inflammatory) in a dose dependent manner. However, leaf extracts of C. crista had better antioxidant and anti-inflammatory activity compared to that of C. asiatica. The better activity of C. crista is attributed to high gallic acid and ferulic acid compared to C. asiatica.
Conclusions:
Thus, the leaf extract of C. crista can be a potential therapeutic role for Alzheimer's disease.
KEY WORDS: Anti-inflammation, antioxidant, Caesalpinia crista and Centella asiatica leaf extract, lipoxygenase, reversed phase-high performance liquid chromatography
The oxidative stress and inflammation have been implicated in neurodegenerative disorders like Alzheimer's disease (AD) and Parkinson's disease, among others.[1,2] The generation of reactive oxygen species, which are toxic, is a part of normal metabolism in a biological system. The balance between the production of reactive oxygen species and anti-oxidants is essential in a biological system to prevent adverse effects of oxidative stress. The imbalance between reactive oxygen species and antioxidants in the human body leads to oxidative stress.[3] The oxidative stress has detrimental effects on central nervous system.[2] The brain is more prone to oxidative stress because of (i) brain is rich in easily oxidizable unsaturated fatty acids; (ii) brain requires more oxygen per unit weight (20% of total oxygen requirement in human beings); (iii) brain is also rich in iron and ascorbate which are key players in oxidation; and (iv) brain is deficient in antioxidants. The free radicals thus generated are known to attack macromolecules such as deoxyribonucleic acid, proteins, lipids and carbohydrates. This leads to either onset or acceleration of degenerative disorders.[4] Overall, the oxidative modification of biomolecules leads to the imbalance in the metabolic activities in AD.[5] The accumulating evidences on the role of oxidative stress suggest that it may be an early event in the onset of AD.[6] The defense system of the human body against oxidative stress induced impairments includes antioxidant enzymes and non-enzymatic antioxidant proteins.[7] These inbuilt defense system are reduced in AD.[8] The inflammation is another responsible factor in AD and is presumed to be mediated through the cross talk among the amyloid, astrocytes and microglia.[9] These reactions lead to altered neuronal function and the inflammatory injury. The leukotrienes and prostaglandins are the mediators of inflammatory response in the cell.[10] 5-lipoxygenase enzyme is important in the biosynthesis of leukotrienes and its location is confined to polymorphonuclear leukocytes (PMNLs), monocytes, macrophages, mast cells and B-lymphocytes.[11] Since 5-lipoxygenase mediates a key step in the generation of inflammatory molecules, modulation of its activity has therapeutic implications.[12] For screening the compounds for anti-inflammatory actions, 5-lipoxygenase provides a good in vitro model.
There are reports on use of plant extracts as antioxidants in animal models to test their efficacy.[13] Caesalpinia crista Linn (Syn. C. bonducella L. Roxb.) belongs to family Fabaceae, found abundantly in tropical and subtropical regions of Southeast Asia. The kernel of C. crista contains cassane- and norcassane-type diterpenoids.[14] The stem and roots are also known to contain new type of diterpenes.[15] The tribal knowledge base in India insighted that aqueous extract of C. crista is used as stress relaxation health drink by forest dwellers. There are limited studies on anti-diuretic, antibacterial, anti-diabetic and anti-oxidant potential properties of C. crista.[16] The dried leaves of Indian penny wort (botanical name: Centella asiatica) is mixed with milk and consumed to improve memory[17] and this is practiced traditionally in selected regions in India.[18] There are studies on diverse effects of C. asiatica such as acetlylcholine-esterase inhibition, antioxidant, neuroprotection and amyloid load reduction.[19,20,21]
We have reported for the first time that aqueous extracts of C. crista not only inhibited the amyloid fibril formation, but also could dis-aggregate the pre-formed fibrils, while C. asiatica partially inhibited the amyloid fibril formation.[20,22] The current trend in controlling AD is multi-target directed ligands approach. The single molecule can possess multiple activities such as anti-amyloidogenic, anti-oxidant and anti-inflammatory activities. Since we have already reported the anti-amyloidogenic potential of C. crista and C. asiatica[20,22] the present study is undertaken to study the effects of the aqueous extracts of both C. crista and C. asiatica leaves for their antioxidant and anti-inflammatory activities.
Materials and Methods
Chemicals and plant material
1,1-Diphenyl-2-picrylhydrazyl (DPPH), adenosine triphosphate (ATP), dithiothreitol (DTT), arachidonic acid (AA), nordihydroguaiaretic acid (NDGA) from Sigma Chemical Co., MO, USA. All other chemicals and solvents used are of analytical grade.
Preparation of aqueous extracts of C. asiatica and C. crista
C. crista was obtained from Western Ghats of Karnataka, India and the species of the plant was identified by the Botanist (taxonomic deposit number: 417358). The C. asiatica was procured from local vegetable market, Mysore and it was identified by authenticated botanist (Taxonomic deposit number is 9831. The aqueous extract of leaves was prepared. A total of 40 g of dried leaf was washed in triple distilled water. The washed leaf was boiled in steam extractor (2 L of triple distilled water for ~ 3 h until the aqueous content become half). The extract was filtered through Whatman No. 42 filter paper to get the clear solution and lyophilized to powder (yield – 2.5%, w/w).[20,22]
Determination of total phenolics in aqueous extract of C. asiatica and C. crista
Total phenolics in aqueous extract of C. asiatica and C. crista were determined according to the method followed by Swain and Hillis.[23] Gallic acid was used as a standard. The total polyphenols content in the extract was expressed as gallic acid equivalents (GAE).
Identification of phenolic compounds in aqueous extract of C. asiatica and C. crista by high performance liquid chromatography
Polyphenols in the aqueous extracts of C. asiatica and C. crista were separated on a reverse phase C18 column (4.6 mm × 250 mm) using HPLC system (Agilent-Model 1200 series) using diode array detector (operating at 280 nm and 320 nm). A solvent system consisting of water: methanol: acetic acid (83:15:2) was used as mobile phase (isocratic) at a flow rate of 1 ml/min.[24] Known quantities of phenolic acid standards such as caffeic acid, p-coumaric acid, cinnamic acid, ferulic acid, gallic acid, gentisic acid, protocatechuic acid, syringic acid, vanillic acid were used for identification and quantification of phenolic acids present in the extract.
Antioxidant assays
DPPH radical scavenging assay
DPPH is a stable free radical that accepts an electron or hydrogen atom to become a stable 1,1-diphenyl-2-picrylhydrazine molecules. The reduction of DPPH radical was determined by a decrease in the absorbance at 517 nm. The antioxidant activity of leaf aqueous extracts of C. crista and C. asiatica and standard synthetic antioxidant ascorbic acid was measured in terms of hydrogen donating or radical scavenging ability. Briefly, 1 mL of 200 μM methanolic solution of DPPH was incubated with different concentrations of C. crista and C. asiatica extracts and standard ascorbic acid for 20 min at room temperature. At the end of the incubation period, the absorbance was measured using an ultra violet-visible spectrophotometer at 517 nm. The percentage of scavenging or quenching of DPPH radicals (Q) by C. crista, C. asiatica and ascorbic were calculated using the following formula.
Q = 100 (Ao−Ac)/Ao
Where Ao is the absorbance of the control tube and Ac was the absorbance of the tube with ‘c’ concentration of the sample. All the experiments were performed in triplicates.
Reducing potential: Potassium ferricyanide reducing method
The reductive potential of the C. crista and C. asiatica extracts was determined according to the method of Oyaizu[25] Different concentrations of C. crista and C. asiatica extracts in 0.5 mL of water were mixed with equal volumes of 0.2 M phosphate buffer, pH 6.6 and 1% potassium ferricyanide [K3Fe (CN) 6]. The mixture was incubated for 20 min at 50°C. At the end of incubation, an equal volume of 10% trichloroacetic acid was added to the mixture and centrifuged at 3200 g for 10 min. The supernatant was mixed with distilled water and 0.1% ferric chloride at 1:1:0.2 (v/v/v) and the absorbance were measured at 700 nm. An increase in the absorbance of the reaction mixture indicates the potential reducing power of the sample. Ascorbic acid was used as a standard for comparison.
Anti-inflammatory activity: 5-lipoxygenase assay
Isolation of 5-lipoxygenase from human PMNLs
Human peripheral venous blood from healthy persons who have not under any medical prescription was collected in a tube containing ethylenediaminetetraacetic acid. The experimental protocol adopted was approved by the Central Food Technological Research Institute's animal ethical committee. PMNLs were isolated from blood by Ficoll-Histopaque density gradient followed by hypotonic lysis of erythrocytes.[26] All the procedures were performed at 4°C. PMNLs were re-suspended in phosphate buffer saline and sonicated for 20-30 s at 20 kHz to release the cytosolic 5-lipoxygenase enzyme into solution. This solution was centrifuged at 100,000 g for 30 min at 4°C and the supernatant was used as a source of enzyme. The purity and viability of PMNLs were checked by trypan blue and Wright's straining methods, respectively. The purity and viability of PMNLs were found to be >90% and >95%, respectively. The Protein content in human PMNLs was estimated according to Lowry's method.[27]
5-lipoxygenase enzyme assay
5-lipoxygenase enzyme assay was performed using previously reported method.[28] The enzyme reaction mixture contains 100 mM pH 7.4, 50 μM DTT, 200 μM ATP, 300 μM CaCl2, 150 μM AA and 5 μg enzyme. The aqueous leaf extracts of C. crista and C. asiatica were incubated with the enzyme for 2 min prior to the addition of AA. The enzymatic reactions were carried out at room temperature. 5-lipoxygenase activity was measured as 5-hydroxyeicosatetraenoic acid formed at 234 nm using spectrophotometer (Shimadzu).
Results and Discussion
Polyphenol content in C. crista and C. asiatica aqueous extract and identification of phenolic acids
Polyphenols are the major group of compounds that contribute to the antioxidant and anti-inflammatory properties. The total phenolic content in the aqueous leaf extracts of C. crista and C. asiatica was found to be 119.7 ± 3.5 and 73.8 ± 2.8 mg GAE/g, respectively. The polyphenols in the aqueous leaf extracts of C. crista and C. asiatica were separated on reverse phase C18 column on HPLC. Gallic acid, protocatechuic acid, gentisic acid, chlorogenic, caffeic, p-coumaric and ferulic acids were the phenolic acids identified in C. crista and C. asiatica aqueous extracts. Gallic acid content (7391 μg/g) was more followed by ferulic acid (1694 μg/g) and p-coumaric acid (633 μg/g) in C. crista, whereas in C. asiatica, caffeic acid content (467 μg/g) was more followed by p-coumaric acid (376 μg/g) and gentisic acid (154 μg/g). We have analyzed aqueous extracts of C. crista and C. asiatica for phenolic acid composition by HPLC. The results showed that gallic acid and ferulic acid contents were much higher in C. crista compared with C. asiatica, while caffeic acid was present in both the extracts, almost in similar quantities. It should be noted that significantly high contents of gallic acid, ferulic acid and p-coumaric acid were present in C. crista leaf extract compared with that of C. asiatica extract. Gallic acid is a potential antioxidant, prevents memory deficits, neuroprotective agent against oxidative stress,[29,30,31] whereas ferulic acid is a potential antioxidant, it exerts an antidepressant-like effect and it protects brain tissues against cerebral ischemic injury.[32,33,34] On the other hand, p-coumaric acid also is an antioxidant compound and it attenuates apoptosis, inhibits adipogenesis and effective antibacterial compound.[35,36,37]
Anti-oxidant assays
DPPH radical scavenging assay
To study the antioxidant properties of the leaf extracts, the DPPH scavenging potential of C. crista and C. asiatica leaf extracts were determined. We have used ascorbic acid as a positive control, due to its well-known antioxidant properties. Aqueous leaf extracts of C. crista and C. asiatica scavenged DPPH radicals in a dose dependent manner. Between the C. crista and C. asiatica leaf extracts, C. crista leaf extract had lower IC50 value (24.35 μg/mL) compared to C. asiatica (139.5 μg/mL) indicating that C. crista had better DPPH radical scavenging activity [Table 1].
Table 1.
Phenolic content in aqueous extracts of C. asiatica and C. crista as measured by HPLC
The DPPH scavenging assay is used to evaluate the free radical scavenging potential of various plant extracts.[38,39] Our results are in agreement with other studies.[40,41] The effect of antioxidants on DPPH may be due to either hydrogen donating property or electron transfer by polyphenols.[42] The DPPH radical scavenging ability of the aqueous extracts may be attributed to the polyphenols present in the extracts of C. crista and C. asiatica.
Reducing potential of C. crista and C. asiatica leaf extracts
The reducing potentials of the extracts indicate their ability to act as antioxidant. The reducing power of aqueous extracts of C. crista and C. asiatica leaves were assessed. The ascorbic acid was used as a standard. A dose dependent increase in reducing power of C. crista and C. asiatica were observed. C. crista extract was found to be more efficient in reducing ferric to ferrous form of iron compared to that of C. asiatica [Figure 1]. However, the standard ascorbic acid showed relatively higher reducing power compared to C. crista and C. asiatica [Figure 1a and b]. The iron reducing potential of a compound indicates its ability to act as antioxidant. In potassium ferricyanide reducing assay, the reductants in the extracts may reduce ferric to ferrous by donating electron. The amount of ferrous can be monitored by measuring the complex formation with Perl's Prussian blue at 700 nm.[43] Our results showed that C. crista has more reducing ability than C. asiatica. The reducing potential of extracts of C. crista and C. asiatica may be due to the electron donating property of the water-soluble polyphenols present in them.[44] Differences in their phenolic acids may be responsible for their differences in antioxidant properties.
Figure 1.
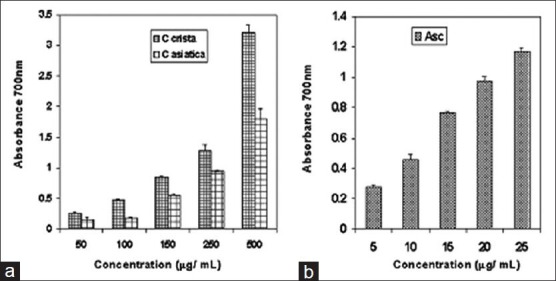
Reducing potential of (a) aqueous extracts of Caesalpinia crista and Centella asiatica and (b) ascorbic acid (standard)
Anti-inflammatory assay: 5-lipoxygenase assay
The anti-inflammatory activities of aqueous extracts of C. crista and C. asiatica were studied by inhibiting 5-lipoxygenase that was isolated from PMNLs assay. The results showed that C. crista showed significant inhibition of 5-Lipoxygenase with IC50 of 23 ± 1.1 μg/mL compared to C. asiatica with IC50 of 250 ± 2.81 μg/mL. The standard, NDGA had the lowest IC50 value of 8.6 ± 0.52 μg/mL [Table 2]. These results indicated that C. crista was effective as anti-inflammatory molecule compared to C. asiatica. The 5-lipoxygense enzymes is a key enzyme in the biosynthesis of leukotriens. 5-lipoxygenase contains non-heme iron in the catalytic site. It catalyzes incorporation of dioxygen into unsaturated fatty acid. It mainly converts AA to biologically active leukotriens. These leukotrienes are implicated in inflammatory and allergic reactions. The harmful effects can be prevented by inhibiting its production. Hence, it can be done by inhibiting the 5-lipoxygenase, which catalyses its production. Few studies have been focused on spices and its effect on 5-lipoxygenase and leukotriene synthesis in the human PMNLs. These studies also shows that kind of inhibition by these natural molecules are reversible in nature and there lies the importance of these natural compounds in fighting against the inflammation without any side-effects, but these studies fail to explain the mechanism of action. Our results demonstrated that C. crista extract had better potential to inhibit 5-lipoxygenase activity compared to that C. asiatica. The IC50 value of C. crista extract was closer to NDGA (standard) compared with that of C. asiatica extract [Table 3]. These effects may be attributed to the polyphenols present in the extracts. Our results are in agreement with other studies where phenols/flavonoid compounds in vegetable/fruits are shown to modulate activities of 5-lipoxygenase and prostaglandin-H synthase pathways of AA.[45]
Table 2.
DPPH scavenging assay for aqueous extracts of C. crista and C. asiatica*

Table 3.
Inhibition of 5-lipoxygenase activity by C. crista and C. asiatica extract

Conclusion
More emphasis on search for novel natural molecules that have multi-target directed ligands such as anti-amyloidogenic, antioxidant, acetylcholine esterase inhibitors, anti-inflammatory molecules etc., is necessary to combat AD. Earlier, we have reported that aqueous extracts of C. crista not only inhibited the amyloid fibril formation but also could dis-aggregate the pre-formed fibrils while C. asiatica partially inhibited the amyloid fibril formation. In the present study, we have found that C. crista leaf extract exhibited better antioxidant and anti-inflammatory activities compared with that of C. asiatica, which may be due to the differences in their polyphenol composition and other bioactive compounds. As aqueous extract of C. crista has shown anti-amyloidogenic, antioxidant and anti-inflammatory activities, C. crista has all the potential to possibly become the drug candidate for AD.
Acknowledgment
R.B.N. is thankful to University Grant Commission (UGC), India, for Fellowship. This work is funded by MEF project (approval no: DIPRENA-DPIP-10866-2013) on Nutritive supplements, Republic Panama. Dr. Rao KS is thankful to National Science System (SNI) of SENACYT, Republic Panama for the financial support.
Footnotes
Source of Support: This work is funded by MEF project (approval no: DIPRENA-DPIP-10866-2013) on Nutritive supplements from Republic Panama. Dr. K. S. Rao is thankful to National science System (SNI) of SENACYT, Republic of Panama for the financial support
Conflict of Interest: None declared.
References
- 1.Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: Stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–67. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 3.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaki T, Kishi Y, Sasaki F, Ameshima S, Nakai T, Miyabo S. Effect of probucol, an oral hypocholesterolaemic agent, on acute tobacco smoke inhalation in rats. Clin Sci (Lond) 1996;90:517–23. doi: 10.1042/cs0900517. [DOI] [PubMed] [Google Scholar]
- 5.Sultana R, Perluigi M, Butterfield DA. Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: Role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–50. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan V, Chauhan A. Oxidative stress in Alzheimer's disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 8.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zotova E, Nicoll JA, Kalaria R, Holmes C, Boche D. Inflammation in Alzheimer's disease: Relevance to pathogenesis and therapy. Alzheimers Res Ther. 2010;2:1. doi: 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–8. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- 11.Raghavenra H, Diwakr BT, Lokesh BR, Naidu KA. Eugenol - The active principle from cloves inhibits 5-lipoxygenase activity and leukotriene-C4 in human PMNL cells. Prostaglandins Leukot Essent Fatty Acids. 2006;74:23–7. doi: 10.1016/j.plefa.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease. J Histochem Cytochem. 2008;56:1065–73. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darvesh AS, Carroll RT, Bishayee A, Geldenhuys WJ, Van der Schyf CJ. Oxidative stress and Alzheimer's disease: Dietary polyphenols as potential therapeutic agents. Expert Rev Neurother. 2010;10:729–45. doi: 10.1586/ern.10.42. [DOI] [PubMed] [Google Scholar]
- 14.Kalauni SK, Awale S, Tezuka Y, Banskota AH, Linn TZ, Kadota S. Methyl migrated cassane-type furanoditerpenes of Caesalpinia crista from Myanmar. Chem Pharm Bull (Tokyo) 2005;53:1300–4. doi: 10.1248/cpb.53.1300. [DOI] [PubMed] [Google Scholar]
- 15.Cheenpracha S, Srisuwan RC, Karalai CP, Chantrapromma S, Chantrapromma K. New diterpenoids from stems and root of Caesalpinia crista. Tetrahedron. 2005;61:8656–62. [Google Scholar]
- 16.Katbamna RV, Rana MG, Dhudhoursejiya AV, Sheth NR. In vitro antioxidant activity of leaves extracts of Caesalpinia bonducella. Pharmacol Online. 2008;3:3665–73. [Google Scholar]
- 17.Manyam BV. Dementia in Ayurveda. J Altern Complement Med. 1999;5:81–8. doi: 10.1089/acm.1999.5.81. [DOI] [PubMed] [Google Scholar]
- 18.Nadkarni AK. I. Bombay: Popular Book Depot; 1954. Indian Materia Medica; pp. 153–5. [Google Scholar]
- 19.Mukherjee PK, Kumar V, Houghton PJ. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res. 2007;21:1142–5. doi: 10.1002/ptr.2224. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh BN, Indi SS, Rao KS. Anti-amyloidogenic property of leaf aqueous extract of Caesalpinia crista. Neurosci Lett. 2010;475:110–4. doi: 10.1016/j.neulet.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 21.Dhanasekaran M, Holcomb LA, Hitt AR, Tharakan B, Porter JW, Young KA, et al. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer's disease animal model. Phytother Res. 2009;23:14–9. doi: 10.1002/ptr.2405. [DOI] [PubMed] [Google Scholar]
- 22.Ramesh BN, Indi SS, Rao KS. Studies to understand the effect of Centella asiatica on Aβ (42) aggregation in vitro. Curr Trends Biotechnol Pharm. 2010;4:716–24. [Google Scholar]
- 23.Swain T, Hillis WE. The phenolic constituents of Prunus domestica. 1. The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–8. [Google Scholar]
- 24.Glowniak K, Zgorka G, Kozyra M. Solid-phase extraction and reversed phase high-performance liquid chromatography of free phenolic acids in some Echinacea species. J Chromatogr. 1996;730:25–9. [Google Scholar]
- 25.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 26.Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;(Suppl 5):9–15. [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 28.Aharony D, Stein RL. Kinetic mechanism of guinea pig neutrophil 5-lipoxygenase. J Biol Chem. 1986;261:11512–9. [PubMed] [Google Scholar]
- 29.Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013;80:274–9. doi: 10.1016/j.jbspin.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Mansouri MT, Farbood Y, Sameri MJ, Sarkaki A, Naghizadeh B, Rafeirad M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013;138:1028–33. doi: 10.1016/j.foodchem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Farbood Y, Sarkaki A, Bavarsad K. Gallic acid prevents memory deficits and oxidative stress induced by intracerebroventricular injection of streptozotocin in rats. Pharmacol Biochem Behav. 2013;111:90–6. doi: 10.1016/j.pbb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Urbaniak A, Szela²g M, Molski M. Theoretical investigation of stereochemistry and solvent influence on antioxidant activity of ferulic acid. Comput Theor Chem. 2013;1012:33–40. [Google Scholar]
- 33.Koh PO. Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett. 2012;507:156–60. doi: 10.1016/j.neulet.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Zeni AL, Zomkowski AD, Maraschin M, Rodrigues AL, Tasca CI. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur J Pharmacol. 2012;679:68–74. doi: 10.1016/j.ejphar.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Stanely Mainzen Prince P, Roy AJ. p-Coumaric acid attenuates apoptosis in isoproterenol-induced myocardial infarcted rats by inhibiting oxidative stress. Int J Cardiol. 2013;168:3259–66. doi: 10.1016/j.ijcard.2013.04.138. [DOI] [PubMed] [Google Scholar]
- 36.Kang SW, Kang SI, Shin HS, Yoon SA, Kim JH, Ko HC, et al. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem Toxicol. 2013;59:380–5. doi: 10.1016/j.fct.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Lou Z, Wang H, Rao S, Sun J, Ma C, Li J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012;25:550–4. [Google Scholar]
- 38.Srivastava A, Greenspan P, Hartle DK, Hargrove JL, Amarowicz R, Pegg RB. Antioxidant and anti-inflammatory activities of polyphenolics from Southeastern U.S. range blackberry cultivars. J Agric Food Chem. 2010;58:6102–9. doi: 10.1021/jf1004836. [DOI] [PubMed] [Google Scholar]
- 39.Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51:7292–5. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 40.Nagai T, Inoueb R, Inoueb H, Suzukia H. Scavenging capacities of pollen extracts from Cistus landaniferus on auto-oxidation, superoxide radicals, hydroxyl radicals and DPPH radicals. Nutr Res. 2002;22:519–26. [Google Scholar]
- 41.Mandal S, Hazra B, Sarkar R, Biswas S, Mandal N. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/nep072. 173768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foti MC, Daquino C, Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH(*) radical in alcoholic solutions. J Org Chem. 2004;69:2309–14. doi: 10.1021/jo035758q. [DOI] [PubMed] [Google Scholar]
- 43.Chung YC, Chang CT, Chao WW, Lin CF, Chou ST. Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem. 2002;50:2454–8. doi: 10.1021/jf011369q. [DOI] [PubMed] [Google Scholar]
- 44.Peñarrieta JM, Alvarado JA, Akesson B, Bergenståhl B. Total antioxidant capacity and content of flavonoids and other phenolic compounds in canihua (Chenopodium pallidicaule): An Andean pseudocereal. Mol Nutr Food Res. 2008;52:708–17. doi: 10.1002/mnfr.200700189. [DOI] [PubMed] [Google Scholar]
- 45.Langlois A, Ferland C, Tremblay GM, Laviolette M. Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. J Allergy Clin Immunol. 2006;118:113–9. doi: 10.1016/j.jaci.2006.03.010. [DOI] [PubMed] [Google Scholar]


