Abstract
Background:
Neuroimmune factors have been considered as contributors to the pathogenesis of depression. Beside other therapeutic effects, Valeriana officinalis L., have been suggested to have anti-inflammatory effects. In the present study, the effects of V. officinalis L. hydro alcoholic extract was investigated on depression like behavior in ovalbumin sensitized rats.
Materials and Methods:
A total of 50 Wistar rats were divided into five groups: Group 1 (control group) received saline instead of Valeriana officinalis L. extract. The animals in group 2 (sensitized) were treated by saline instead of the extract and were sensitized using the ovalbumin. Groups 3-5 (Sent - Ext 50), (Sent - Ext 100) and (Sent - Ext 200) were treated by 50, 100 and 200 mg/kg of V. officinalis L. hydro-alcoholic extract respectively, during the sensitization protocol. Forced swimming test was performed for all groups and immobility time was recorded. Finally, the animals were placed in the open-field apparatus and the crossing number on peripheral and central areas was observed.
Results:
The immobility time in the sensitized group was higher than that in the control group (P < 0.01). The animals in Sent-Ext 100 and Sent-Ext 200 groups had lower immobility times in comparison with sensitized group (P < 0.05 and P < 0.01). In the open field test, the crossed number in peripheral by the sensitized group was higher than that of the control one (P < 0.01) while, the animals of Sent-Ext 50, Sent-Ext 100 and Sent-Ext 200 groups had lower crossing number in peripheral compared with the sensitized group (P < 0.05 and P < 0.01 respectively). Furthermore, in the sensitized group, the central crossing number was lower than that of the control group (P < 0.001). In the animals treated by 200 mg/kg of the extract, the central crossing number was higher than that of the sensitized group (P < 0. 05).
Conclusions:
The results of the present study showed that the hydro-alcoholic extract of V. officinalis prevents depression like behavior in ovalbumin sensitized rats. These results support the traditional belief on the about beneficial effects of V. officinalis in the nervous system. Moreover, further investigations are required in order to better understand this protective effect.
KEY WORDS: Depression, forced swimming test, open-field, ovalbumin, sensitized, Valeriana officinalis
Depression is the second most common chronic disease, which is widely expanding in the world. It has been reported that about half of the patients are unaware of their disease or their disease has been diagnosed else.[1,2] The patients show a combination of states of sadness, loneliness, irritability, absurdity, despair, confusion and shame and sometimes they reveal physical symptoms.[1] Treatment by drugs or psychotherapeutic methods has been reported unsuccessful in 20% and 35% of patients.[3,4]
Neuroimmune factors have been suggested to have an important role in depression.[5,6] The increased levels of the inflammatory markers such as C-reactive protein, interleukin-6 (IL-6) and tumor necrosis factor alpha in depression may be a good evidence for this idea.[7,8] It has also been shown that antidepressants affect cytokine levels, which the later affect the treatment outcome in depression.[9,10] The glial cell activation and their response during neuroinflammation has also been shown to have an important role in depression.[11] It was also shown that antidepressant agents have anti-neuroinflammatory properties as well.[11] The studies using animal models as well in vitro studies on rodent glial cells, have confirmed that different types of antidepressants affect the expression of inflammatory mediators, such as cytokines, microglials and astroglials in the nervous system.[11]
Ovalbumin sensitization and challenge causes an inflammatory response in the airways and therefore, it has been frequently used as an animal model of asthma.[12] This model is accompanied with acute inflammatory events including infiltration of inflammatory cells such as eosinophils, mast cells, neutrophils and lymphocytes.[13,14] In sensitized animals, both CD4+ and CD8+ cells are activated which they then express Th2 cytokines.[15] The Th2 responses typically lead to an increase in IL-4, IL-5 and IL-13.[16] It was also shown that that the serum levels of interferon (IFN)-γ was increased in sensitized animals compared with the control.[17] There is also a close link between peripheral and brain inflammation due to ovalbumin sensitization.[18]
Valeriana officinalis L. (Valerian) is a plant native to different temperate regions of America, Europe and Asia.[19] Several therapeutic effects including hypnotic, sedative, anxiolytic, anticonvulsant and antidepressant has been suggested for Valerian.[20,21,22] The rhizomes of Valerian contain several compounds including the essential oil and its sesquiterpenoids (valerenic acid), epoxy iridoid esters (valepotriates, valtrate, didravaltrate), amino acids (arginine, γ-aminobutyric acid [GABA], glutamine, tyrosine) and alkaloids.[23] It has also been reported that the ethanolic and aqueous extracts of valerian root inhibited GABA reuptake.[24] In traditional medicine, a variety of herbal preparations from this plant has been advised to use for treatment of hypertension, angina, palpitation, asthma, hepatic colic and menstrual cramps.[25,26,27,28] Antidepressant activity of sesquiterpenes which were fractioned from the roots of Valeriana fauriei Briq, the other genus of Valerianaceae, has also been reported.[29]
Based on the properties of V. officinalis L. which has been reported in traditional medicine and in experimental studies, the present study was designed in order to evaluate the possible effects of hydro-alcoholic extract of V. officinalis L. on depression like behavior in ovalbumin sensitized rats.
Materials and Methods
Preparing the hydroalcoholic plant extract
The plant was identified by the botanists in Ferdowsi University, Mashhad (No: 295-2215-1). To prepare the hydroalcoholic extract, the powdered rhizomes (100 g) of V. officinalis were extracted in a Soxhlet extractor with ethanol (70%). The resulting extract was concentrated under reduced pressure. Five grams extract was yielded from 100 g of the powdered rhizomes. The extract was kept at −20°C until being used. The extract was dissolved in saline.[30,31,32]
Animals and the experimental protocol
A total of 50 male Wistar rats (8 weeks old and weighted 230 ± 20 g) were kept at 22 ± 2°C and 12 h light/dark cycle at 7:00 am. They were randomly divided to five groups and treated according to the experimental protocol for 32 days. All measurements were performed between 10.00 and 14.00.
Rats in group 1 (control group) received saline instead of both ovalbumin and the extract. The animals in group 2 (sensitized; Sent) were injected by saline instead of the extract. The animals in this group were sensitized based on the protocol. Groups 3 (sensitized – extract 50; Sent-Ext 50), 4 (sensitized – Extract 100; Sent-Ext100) and 5 (sensitized – extract 200; Sent-Ext 200) were treated by 50, 100 and 200 mg/kg of the extract respectively from the day before conducting the sensitization and during the sensitization. The doses were chosen from previous studies.[33,34]
Sensitization of the animals to ovalbumin was performed using the method described previously.[12,35,36] Briefly, the rats were sensitized to ovalbumin (Sigma Chemical Ltd., UK) by i.p. injection of 10 mg ovalbumin and 100 mg Al(OH)3 dissolved in 1 ml saline on day 1. At 1 week later they were given 2 mg ovalbumin and 100 mg Al(OH)3 dissolved in 1 ml saline i.p. as a booster dose. From day 14, the sensitized animals were exposed to an aerosol of 4% ovalbumin for 18-19 days, 5 min daily. The aerosol was administered in a closed cylinder, dimensions 19 cm diagonal and 16 cm high and 3 L volume using a nebulizer (Cx3, Omron Healthcare Europe B.V., Netherlands). The animals were placed in the cylinder separately. Control animals were treated similarly but saline was used instead of ovalbumin solution. The study was approved by the ethical committee of Mashhad University of Medical Sciences (NO: AC-34o-1390).
Behavioral procedures
Forced swimming test
17 day after aerosol administration, the animals were compromised to the test room environment for 1 h before the beginning of the experiment. During the FST, the animals were placed in a glass cylindrical tank with 60 cm height and 38 cm width which was filled with water (24 ± 1°C) to the depth of 40 cm. The water was changed between each animal. In the 1st day (day 17 of aerosol administration), the rats were placed inside the water cylinder for 15 min (pre-test). In the next day (day 18 of aerosol administration), the animals were placed individually inside the water cylinder for 5 min.[37] The time of floating (immobility) during the FST was recorded for 5 min.[38,39] Rats were considered immobile when they floated in the water; they only performed movements that enabled them to keep their head above the water. The movements of the animals were recorded by a camera and it was then evaluated by an experimenter who was blind to the animals. After the FST, the rats were dried in a heated cage and then they were returned to their home cages.[40,41]
Open-field
Open-field test was used for studying the depression-like behaviors of animals.[41,42] It was done on day 19 of aerosol administration. In the present study, the open-field measurement was done by a wooden apparatus with an area of 100 cm × 100 cm and height of 40 cm. Inside the apparatus was divided to 16 equal squares using a black line. In addition, within the apparatus was divided to two zones called peripheral and central zones.[43,44,45] All the rats were familiarized with the test environment by being placing in the room for 1-h before beginning the experiment. During the experiment a low-level light was used to reduce anxiety.[40] Each animal was placed in the central zone and its movement was recorded by a digital camera for 5 min[43,44,45] and the following criteria were calculated: (1) The crossing number in the central zone, (2) the crossing number in the peripheral zone and (3) rearing counts: The number of vertical movement when the rat stood vertically on its hind paws on the floor and forepaws on the wall.[40,41] Both behavioral testes were carried out 2 h after aerosol administration.
Statistical analysis
The data were expressed as mean ± standard error of the mean the two-way ANOVA (factor 1: Treatment; factor 2: Sensitization) was run followed by tukey post hoc comparisons test. The criterion for the statistical significance was P < 0.05.
Results
The results of the FST showed that the animals in the sensitized group had 102.66 ± 9.99 s immobility times while; the immobility time in the animals of sensitized group was 63.33 ± 7.03 s. Two-way ANOVA test showed that both sensitization and treatment affected the immobility time (P < 0.01 for sensitization and P < 0.05 for treatment). In the Sent-Ext 100 and Sent-Ext 200 groups, the immobility times were 71.57 ± 5.14 and 54.28 ± 7.47 sec, respectively. Tukey post hoc analysis showed that they were significantly lower than that of sensitized animals (P < 0.05 and P < 0.01). There were also no significant differences between the Sent-Ext 100 and Sent-Ext 200 groups compared with the control group in immobility times. Treatment of the sensitized rats by 50 mg/kg of the extract didn't show significant effects [Figure 1].
Figure 1.
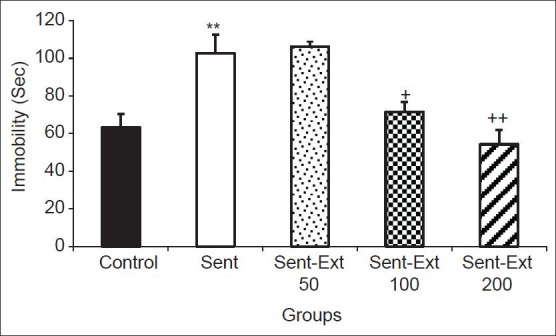
Comparison of immobility times in the forced swimming test between five groups. Data are expressed as mean ± standard error of the mean (n = 10 in each group). **P < 0.01 comparison of sensitized group with control group, +P < 0.05 and ++P < 0.01 comparison of sensitized animals treated by 100 and 200 mg/kg of the extract respectively groups with non treated sensitized group. The two ANOVA followed by Tukey post hoc test. The criterion for the statistical significance was P < 0.05
The results of the open-field test showed that the number of crossing in the peripheral zone by the animals of sensitized group was 71.83 ± 11.45 while in the control group it was 39.62 ± 3.55. Two-way ANOVA test showed that both sensitization and treatment affected the immobility time (P < 0.01 for sensitization and P < 0.001 for treatment). As shown in Figure 2, the crossing number in the peripheral zone by the animals of Sent-Ext 50, Sent-Ext 100 and Sent-Ext 200 groups were 39 ± 4.4, 32.75 ± 7.59 and 22.25 ± 7.74 respectively. Tukey post hoc analysis showed that they were significantly lower than that in the sensitized group (P < 0.05 and P < 0.01) [Figure 2]. There was no significant difference between three doses of the extract.
Figure 2.
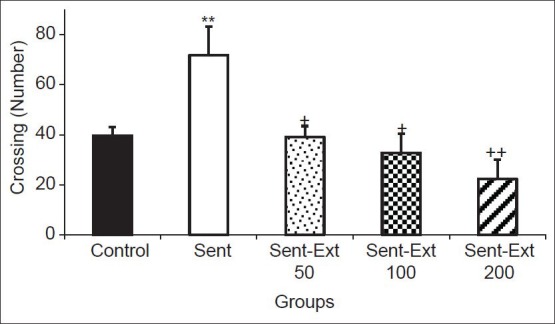
Comparison of the number of crossing in the peripheral zone in the open-field test. Data are expressed as mean ± standard error of the mean (n = 10 in each group). **P < 0.01 comparison of sensitized group with control group, +P < 0.05 and ++P < 0.01 comparison of sensitized animals treated by 50, 100 and 200 mg/kg of the extract respectively, with non-treated sensitized group. The two ANOVA followed by Tukey post hoc test. The criterion for the statistical significance was P < 0.05
The results also showed that the number of crossing in the central zone by the sensitized and control groups were 3 ± 0.68 and 8.83 ± 0.6 respectively. Two-way ANOVA test showed that both sensitization and treatment affected the immobility time (P < 0.001 for sensitization and P < 0.05 for treatment). The crossing number in the central zone by the animals of Sent-Ext 200 group was 7.33 ± 0.98; it was significantly higher than that of the sensitized group (P < 0.05) [Figure 3]. Treatment the sensitized rats by 50 and 100 mg/kg didn't show a significant effect on the crossing number in the central zone. There were no significant differences between groups in rearing number [Figure 4].
Figure 3.
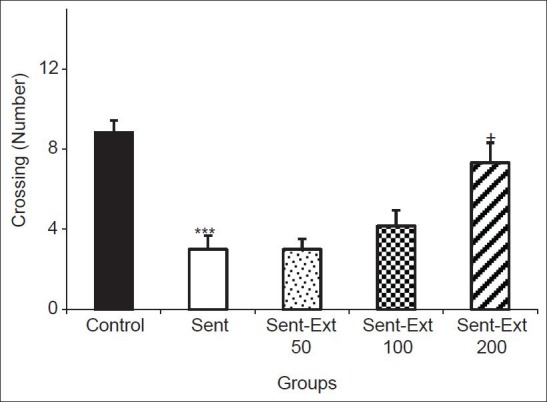
Comparison of the number of crossing in the central zone in the open-field test. Data are expressed as mean ± standard error of the mean (n = 10 in each group). ***P < 0.001 comparison of sensitized group with control group, +P < 0.05 comparison of sensitized animals treated by 200 mg/kg of the extract with non-treated sensitized group. The two ANOVA followed by Tukey post hoc test. The criterion for the statistical significance was P < 0.05
Figure 4.
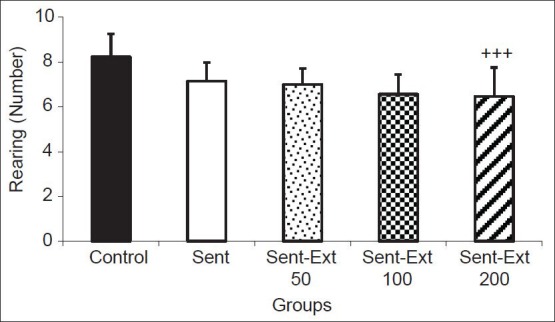
Comparison of the number of rearing in the open-field test. Data are expressed as mean ± standard error of the mean (n = 10 in each group). The two ANOVA followed by Tukey post hoc test. The criterion for the statistical significance was P < 0.05
Discussion
The results of the present study showed that sensitizing of the animals by ovalbumin induces a depression-like behavior in rats. This animal's model is well-known as an experimental model of asthma.[12,35,36] The main pathological feature of asthma is airway inflammation, which is accompanied with increasing in the airway responsiveness.[46] It has also been shown that the increased serum concentrations of IgE and IgG1 is accompanied with airway inflammation and asthma.[47] It has been previously shown that the secretion of cytokines such as IL-4, IL-5, IL-13 levels were increased in ovalbumin sensitized animals.[48] On the other hand we have previously showed that injection of lipopolysacharide was resulted in depression like behavior in rats.[41] Lipopolysaccharide is a fragment from the cell wall of Gram-negative bacteria[49] which triggers the production of the cytokines.[50,51] Both systemic and central administrations of LPS increase the levels of the related pro-inflammatory cytokines in several areas of the brain including the hippocampus, hypothalamus and diencephalon structures.[52,53] A large number of evidence has confirmed the role of inflammation in depression. For example treatment with IL-2 or IFN-γ in patients with cancer, leads to high depression rates.[54,55] In the present study, the corresponding depressive symptoms were observed in ovalbumin sensitizes rats.[56,57] The results of the present study showed that immobility times in the sensitized group were higher than those of the control group in the FST. The results also showed that the crossing number in the central area of the open field by the animals of the sensitized group was lower than that of the control group. It is suggested that various anti-inflammatory manipulations have antidepressant effects in experimental animals and humans. For example, genetic knockout of IL-6 in mice reduces depressive-like behavior in the forced swim, tail suspension, learned helplessness and sucrose preference tests.[58] Knockout of the IL-1 receptor and administration of IL-1 receptor antagonist also blocked stress-induced depressive-like behavior in the sucrose preference, social exploration tests, escape deficits, anhedonia and reduces social behavior.[59,60,61,62] Consistent with the pathophysiology of depression, the cytokine-induced behavioral changes are associated with alterations in the metabolism of serotonin, norepinephrine and dopamine in the brain regions which are essential for the regulation of emotion, including the limbic system (amygdala, hippocampus and nucleus accumbens), as well as the regulation of psychomotor function and reward, including the basal ganglia.[63,64] On the other hand, the reduced number of crossings which was observed in the open field could be related to the sickness behavior which is also repeatedly reported in rodents during neuroinflammation.[65,66] Furthermore, the decreased the number of entries into the central zone has been considered as an anxiety-like behavior[67] therefore, it may also be suggested from the results of the present study due to sensitization.
The correlation between asthma and depression has also been suggested. Clinical reports indicate that patients with asthma commonly have also the symptoms of depression.[68] It has been shown that among the asthma patients, there is a large number of those who have symptoms of anxiety and depression.[69] The results of another study also showed that approximately 43% of 230 asthmatic patients had the symptoms of depression.[68] A study by Lv et al. also found a significant difference in depression among patients with a history of asthma compared with patients who did not have such a history.[70] The patients with depression were also found to exhibit all the cardinal features of inflammation.[54]
The inhibition of allergic lung inflammation and airway responsiveness by the precursor of serotonin, 5-hydroxytryptophan, is a good evidence for the relationship between asthma and depression.[71] 5-hydroxytryptophan also reduced the leukocyte recruitment and allergic inflammation.[71]
The extracts of V. officinalis L. has been proposed as a natural remedy for a number of illnesses due to its pharmacological properties such as anxiolytic, antidepressant, hypnotic, mitogenic, cytoprotective and hypotensive.[33,34,72,73,74] It has been reported that an aqueous extract of valerian root improved sleep quality in human without any side effect.[75] Antidepressant activity of sesquiterpenes which were fractioned from the roots of Valeriana fauriei Briq the other genus of Valerianaceae has also been reported.[29,75]
Many potential mechanisms for the pharmacological action of V. officinalis L. have been proposed based on their agonistic effects through GABA, adenosine, barbiturate and benzodiazepine receptors.[76,77,78,79] It has been sown that valerian increases the serotonin concentrations in the brain.[80] In the present study, the exact mechanism(s) or the component(s) responsible for the anti-depression like behavior wasn't evaluated. Valeric acid as an important component of V. officinalis L., has been shown to inhibit the breakdown of GABA in the central nervous system.[81] Furthermore, the pharmacological properties of some components of V. officinalis L. are believed to be due to their antioxidant activities.[82] The alcoholic extracts of valerian root have been shown to have a good effect against lipid peroxidation.[74] On the other hand, it has been shown that oxidative stress has a role in depression and anxiety[83] which it also increases in asthmatic patients as well in ovalbumin sensitized animals.[84,85] Therefore, it might be suggested as a possible mechanism in the present study. An interaction between valerian and NMDA receptors has been suggested[24] and it should not be ignored as another possible mechanism.
Valerian contains over 150 chemical compounds with physiological activity including: Cadmium, cobalt, chromium, copper, iron, lithium, lipid compounds, nitrogen-containing compounds, amino acids, phenolic and terpenoids compounds.[86] Each of these components may probably have a role in the anti-depression like behavior which was seen in the present study. The analgesics and sedative effects of the plant have been attributed to valeric acid however, it is chemically unstable and quickly decomposes.[81] Alkaloids found in this plant are found only in small quantities.[87] These alkaloids have a cholinesterase activity which may have a role in the anti-depressive like effects of the extract in the present study. However, it needs more studies to be done using other animal models of depression to confirm the beneficial effects of the plant on depression. Furthermore, further studies are recommended to characterize the component(s) and the mechanism(s) responsible for anti-depression effects of the extract in the future.
Conclusion
The results of the present study showed that the hydro-alcoholic extract of V. officinalis L. prevented depression like behavior in ovalbumin sensitized rats. These results support the traditional belief on about the beneficial effect of V. officinalis L. on the nervous system. Further studies are required for determining (confirming) the protective effect of V. officinalis L.
Acknowledgments
This paper is a part of M.Sc. thesis that performed in Islamic Azad University, Fars Science and Research Branch. The authors would like to thank all personnel of Department of Biology, Fars Science and Research Branch and Mashhad Branch who helped to carry out this research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001–8. [PubMed] [Google Scholar]
- 2.Spitzer RL, Kroenke K, Linzer M, Hahn SR, Williams JB, deGruy FV, 3rd, et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. JAMA. 1995;274:1511–7. [PubMed] [Google Scholar]
- 3.Coulehan JL, Schulberg HC, Block MR, Madonia MJ, Rodriguez E. Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med. 1997;157:1113–20. [PubMed] [Google Scholar]
- 4.Fava M, Davidson KG. Definition and epidemiology of treatment- resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation- associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory and neurodegenerative (I and ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 7.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 8.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol. 2010;25:201–15. doi: 10.1002/hup.1103. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashioka S. Antidepressants and neuroinflammation: Can antidepressants calm glial rage down? Mini Rev Med Chem. 2011;11:555–64. doi: 10.2174/138955711795906888. [DOI] [PubMed] [Google Scholar]
- 12.Boskabady MH, Adel-Kardan S. Increased muscarinic receptor blockade by atropine in tracheal chains of ovalbumin-sensitized guinea pigs. Pharmacology. 1999;58:300–8. doi: 10.1159/000028295. [DOI] [PubMed] [Google Scholar]
- 13.Kucharewicz I, Kasacka I, Pawlak D, Tankiewicz-Kwedlo A, Mroczko B, Buczko W, et al. The concentration of kynurenine in rat model of asthma. Folia Histochem Cytobiol. 2008;46:199–203. doi: 10.2478/v10042-008-0030-7. [DOI] [PubMed] [Google Scholar]
- 14.Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy. 2007;37:973–88. doi: 10.1111/j.1365-2222.2007.02740.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee SC, Jaffar ZH, Wan KS, Holgate ST, Roberts K. Regulation of pulmonary T cell responses to inhaled antigen: Role in Th1- and Th2-mediated inflammation. J Immunol. 1999;162:6867–79. [PubMed] [Google Scholar]
- 16.Kucharewicz I, Bodzenta-Łukaszyk A, Buczko W. Experimental asthma in rats. Pharmacol Rep. 2008;60:783–8. [PubMed] [Google Scholar]
- 17.Keyhanmanesh R, Boskabady MH, Eslamizadeh MJ, Khamneh S, Ebrahimi MA. The effect of thymoquinone, the main constituent of Nigella sativa on tracheal responsiveness and white blood cell count in lung lavage of sensitized guinea pigs. Planta Med. 2010;76:218–22. doi: 10.1055/s-0029-1186054. [DOI] [PubMed] [Google Scholar]
- 18.Costa-Pinto FA, Basso AS, Britto LR, Malucelli BE, Russo M. Avoidance behavior and neural correlates of allergen exposure in a murine model of asthma. Brain Behav Immun. 2005;19:52–60. doi: 10.1016/j.bbi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Hadley S, Petry JJ. Valerian. Am Fam Physician. 2003;67:1755–8. [PubMed] [Google Scholar]
- 20.Wagner H, Jurcic K, Schaette R. Comparative studies on the sedative action of Valeriana extracts, valepotriates and their degradation products (author's transl) Planta Med. 1980;39:358–65. doi: 10.1055/s-2008-1074930. [DOI] [PubMed] [Google Scholar]
- 21.Andreatini R, Sartori VA, Seabra ML, Leite JR. Effect of valepotriates (valerian extract) in generalized anxiety disorder: A randomized placebo-controlled pilot study. Phytother Res. 2002;16:650–4. doi: 10.1002/ptr.1027. [DOI] [PubMed] [Google Scholar]
- 22.Oshima Y, Matsuoka S, Ohizumi Y. Antidepressant principles of Valeriana fauriei roots. Chem Pharm Bull (Tokyo) 1995;43:169–70. doi: 10.1248/cpb.43.169. [DOI] [PubMed] [Google Scholar]
- 23.Hendriks H, Bos R, Allersma DP, Malingré TM, Koster AS. Pharmacological screening of valerenal and some other components of essential oil of Valeriana officinalis. Planta Med. 1981;42:62–8. doi: 10.1055/s-2007-971547. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz JG, Nieves-Natal J, Chavez P. Effects of Valeriana officinalis extracts on [3H] flunitrazepam binding, synaptosomal [3H] GABA uptake, and hippocampal [3H] GABA release. Neurochem Res. 1999;24:1373–8. doi: 10.1023/a:1022576405534. [DOI] [PubMed] [Google Scholar]
- 25.Klich R. Behavior disorders in childhood and their therapy. Med Welt. 1975;26:1251–4. [PubMed] [Google Scholar]
- 26.Hoffmann D. The Complete Illustrated Holistic Herbal: A Safe and Practical Guide to Making and Using Herbal Remedies. Shaftesbury, Dorset: Element Books. 1996:256. [Google Scholar]
- 27.Peirce A. New York: William Morrow; 1999. The American Pharmaceutical Association Practical Guide to Natural Medicines. [Google Scholar]
- 28.Circosta C, De Pasquale R, Samperi S, Pino A, Occhiuto F. Biological and analytical characterization of two extracts from Valeriana officinalis. J Ethnopharmacol. 2007;112:361–7. doi: 10.1016/j.jep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Liu XG, Gao PY, Wang GS, Song SJ, Li LZ, Li X, et al. In vivo antidepressant activity of sesquiterpenes from the roots of Valeriana fauriei Briq. Fitoterapia. 2012;83:599–603. doi: 10.1016/j.fitote.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Rakhshandah H, Hosseini M. Potentiation of pentobarbital hypnosis by Rosa damascena in mice. Indian J Exp Biol. 2006;44:910–2. [PubMed] [Google Scholar]
- 31.Hosseini M, Ghasemzadeh Rahbardar M, Sadeghnia HR, Rakhshandeh H. Effects of different extracts of Rosa damascena on pentylenetetrazol-induced seizures in mice. Zhong Xi Yi Jie He Xue Bao. 2011;9:1118–24. doi: 10.3736/jcim20111013. [DOI] [PubMed] [Google Scholar]
- 32.Ebrahimzadeh Bideskan AR, Hosseini M, Mohammadpour T, Karami R, Khodamoradi M, Nemati Karimooy H, et al. Effects of soy extract on pentylenetetrazol-induced seizures in ovariectomized rats. Zhong Xi Yi Jie He Xue Bao. 2011;9:611–8. doi: 10.3736/jcim20110606. [DOI] [PubMed] [Google Scholar]
- 33.Hattesohl M, Feistel B, Sievers H, Lehnfeld R, Hegger M, Winterhoff H. Extracts of Valeriana officinalis L.s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine. 2008;15:2–15. doi: 10.1016/j.phymed.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Hiller KO, Zetler G. Neuropharmacological studies on ethanol extracts of Valeriana officinalis L.: Behavioural and anticonvulsant properties. Phytother Res. 1996;10:145–51. [Google Scholar]
- 35.Boskabady MH, Tabatabaee A, Byrami G. The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized guinea-pigs. Phytomedicine. 2012;19:904–11. doi: 10.1016/j.phymed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Palmans E, Kips JC, Pauwels RA. Prolonged allergen exposure induces structural airway changes in sensitized rats. Am J Respir Crit Care Med. 2000;161:627–35. doi: 10.1164/ajrccm.161.2.9902094. [DOI] [PubMed] [Google Scholar]
- 37.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 38.Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–57. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 39.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–32. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Pitychoutis PM, Nakamura K, Tsonis PA, Papadopoulou-Daifoti Z. Neurochemical and behavioral alterations in an inflammatory model of depression: Sex differences exposed. Neuroscience. 2009;159:1216–32. doi: 10.1016/j.neuroscience.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 41.Hosseini M, Zakeri S, Khoshdast S, Yousefian FT, Rastegar M, Vafaee F, et al. The effects of Nigella sativa hydro-alcoholic extract and thymoquinone on lipopolysaccharide-induced depression like behavior in rats. J Pharm Bioallied Sci. 2012;4:219–25. doi: 10.4103/0975-7406.99052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–51. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 43.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–74. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 44.Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–47. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- 45.Engeland CG, Kavaliers M, Ossenkopp KP. Influence of the estrous cycle on tolerance development to LPS-induced sickness behaviors in rats. Psychoneuroendocrinology. 2006;31:510–25. doi: 10.1016/j.psyneuen.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–83. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubois A, Deruytter N, Adams B, Kanda A, Delbauve S, Fleury S, et al. Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+T lymphocytes in early life. J Immunol. 2010;185:884–91. doi: 10.4049/jimmunol.0903287. [DOI] [PubMed] [Google Scholar]
- 48.Keyhanmanesh R, Boskabady MH, Khamneh S, Doostar Y. Effect of thymoquinone on the lung pathology and cytokine levels of ovalbumin-sensitized guinea pigs. Pharmacol Rep. 2010;62:910–6. doi: 10.1016/s1734-1140(10)70351-0. [DOI] [PubMed] [Google Scholar]
- 49.Kozak W, Conn CA, Kluger MJ. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;266:R125–35. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 50.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 51.Kant GJ, Meyerhoff JL, Jarrard LE. Biochemical indices of reactivity and habituation in rats with hippocampal lesions. Pharmacol Biochem Behav. 1984;20:793–7. doi: 10.1016/0091-3057(84)90201-6. [DOI] [PubMed] [Google Scholar]
- 52.Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49:125–34. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 53.Takao T, Culp SG, De Souza EB. Reciprocal modulation of interleukin-1 beta (IL-1 beta) and IL-1 receptors by lipopolysaccharide (endotoxin) treatment in the mouse brain-endocrine-immune axis. Endocrinology. 1993;132:1497–504. doi: 10.1210/endo.132.4.8462448. [DOI] [PubMed] [Google Scholar]
- 54.Miller AH. Depression and immunity: A role for T cells? Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piser TM. Linking the cytokine and neurocircuitry hypotheses of depression: A translational framework for discovery and development of novel anti-depressants. Brain Behav Immun. 2010;24:515–24. doi: 10.1016/j.bbi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Abdulla D, Renton KW. Beta-adrenergic receptor modulation of the LPS-mediated depression in CYP1A activity in astrocytes. Biochem Pharmacol. 2005;69:741–50. doi: 10.1016/j.bcp.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–94. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–82. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- 60.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–28. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 61.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arakawa H, Blandino P, Jr, Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiol Behav. 2009;98:139–46. doi: 10.1016/j.physbeh.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson's disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 64.Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–27. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- 65.Ribeiro DE, Maiolini VM, Soncini R, Antunes-Rodrigues J, Elias LL, Vilela FC, et al. Inhibition of nitric oxide synthase accentuates endotoxin-induced sickness behavior in mice. Pharmacol Biochem Behav. 2013;103:535–40. doi: 10.1016/j.pbb.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Bassi GS, Kanashiro A, Santin FM, de Souza GE, Nobre MJ, Coimbra NC. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin Pharmacol Toxicol. 2012;110:359–69. doi: 10.1111/j.1742-7843.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 67.Ma XC, Jiang D, Jiang WH, Wang F, Jia M, Wu J, et al. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PLoS One. 2011;6:e20955. doi: 10.1371/journal.pone.0020955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xi L, Zhang Y, Han D, Zhang L. Effect of asthma, aeroallergen category, and gender on the psychological status of patients with allergic rhinitis. J Investig Allergol Clin Immunol. 2012;22:264–9. [PubMed] [Google Scholar]
- 69.Labor S, Labor M, Jurić I, Vuksić Z. The prevalence and pulmonary consequences of anxiety and depressive disorders in patients with asthma. Coll Antropol. 2012;36:473–81. [PubMed] [Google Scholar]
- 70.Lv X, Xi L, Han D, Zhang L. Evaluation of the psychological status in seasonal allergic rhinitis patients. ORL J Otorhinolaryngol Relat Spec. 2010;72:84–90. doi: 10.1159/000297576. [DOI] [PubMed] [Google Scholar]
- 71.Abdala-Valencia H, Berdnikovs S, McCary CA, Urick D, Mahadevia R, Marchese ME, et al. Inhibition of allergic inflammation by supplementation with 5-hydroxytryptophan. Am J Physiol Lung Cell Mol Physiol. 2012;303:L642–60. doi: 10.1152/ajplung.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernández S, Wasowski C, Paladini AC, Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol Biochem Behav. 2004;77:399–404. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 73.Miraldi E, Ferri S, Mostaghimi V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran) J Ethnopharmacol. 2001;75:77–87. doi: 10.1016/s0378-8741(00)00381-0. [DOI] [PubMed] [Google Scholar]
- 74.Malva JO, Santos S, Macedo T. Neuroprotective properties of Valeriana officinalis extracts. Neurotox Res. 2004;6:131–40. doi: 10.1007/BF03033215. [DOI] [PubMed] [Google Scholar]
- 75.Müller LG, Salles LA, Stein AC, Betti AH, Sakamoto S, Cassel E, et al. Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:101–9. doi: 10.1016/j.pnpbp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 76.Leathwood PD, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1982;17:65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 77.Müller CE, Schumacher B, Brattström A, Abourashed EA, Koetter U. Interactions of valerian extracts and a fixed valerian-hop extract combination with adenosine receptors. Life Sci. 2002;71:1939–49. doi: 10.1016/s0024-3205(02)01964-1. [DOI] [PubMed] [Google Scholar]
- 78.Schumacher B, Scholle S, Hölzl J, Khudeir N, Hess S, Müller CE. Lignans isolated from valerian: Identification and characterization of a new olivil derivative with partial agonistic activity at A (1) adenosine receptors. J Nat Prod. 2002;65:1479–85. doi: 10.1021/np010464q. [DOI] [PubMed] [Google Scholar]
- 79.Ortiz JG, Rassi N, Maldonado PM, González-Cabrera S, Ramos I. Commercial valerian interactions with [3H] Flunitrazepam and [3H] MK-801 binding to rat synaptic membranes. Phytother Res. 2006;20:794–8. doi: 10.1002/ptr.1960. [DOI] [PubMed] [Google Scholar]
- 80.Tang JY, Zeng YS, Chen QG, Qin YJ, Chen SJ, Zhong ZQ. Effects of Valerian on the level of 5-hydroxytryptamine, cell proliferation and neurons in cerebral hippocampus of rats with depression induced by chronic mild stress. Zhong Xi Yi Jie He Xue Bao. 2008;6:283–8. [PubMed] [Google Scholar]
- 81.Hendriks H, Bos R, Woerdenbag HJ, Koster AS. Central nervous depressant activity of valerenic acid in the mouse. Planta Med. 1985;51:28–31. doi: 10.1055/s-2007-969384. [DOI] [PubMed] [Google Scholar]
- 82.Sudati JH, Fachinetto R, Pereira RP, Boligon AA, Athayde ML, Soares FA, et al. In vitro antioxidant activity of Valeriana officinalis against different neurotoxic agents. Neurochem Res. 2009;34:1372–9. doi: 10.1007/s11064-009-9917-8. [DOI] [PubMed] [Google Scholar]
- 83.Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143:34–8. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Hou C, Zhao H, Li W, Cai S. Hydrogen peroxide induces high mobility group bo×1 release in human bronchial epithelial cells. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1131–4. [PubMed] [Google Scholar]
- 85.Jung JY, Lee KY, Lee MY, Jung D, Cho ES, Son HY. Antioxidant and antiasthmatic effects of saucerneol D in a mouse model of airway inflammation. Int Immunopharmacol. 2011;11:698–705. doi: 10.1016/j.intimp.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 86.Miyasaka LS, Atallah AN, Soares BG. Valerian for anxiety disorders. Cochrane Database Syst Rev. 2006;4:CD004515. doi: 10.1002/14651858.CD004515.pub2. [DOI] [PubMed] [Google Scholar]
- 87.Torssell K, Wahlberg K. Isolation, structure and synthesis of alkaloids from Valeriana officinalis L. Acta Chem Scand. 1967;21:53–62. doi: 10.3891/acta.chem.scand.21-0053. [DOI] [PubMed] [Google Scholar]


