Abstract
Purpose of review
The arrival of large datasets and the on-going refinement of neuroimaging technology have led to a number of recent advances in our understanding of visual pathway disorders. This work can broadly be classified into two areas, both of which are important when considering optimal management strategies. The first looks at delineation of damage, teasing out subtle changes to (specific components of) the visual pathway, which may help evaluate severity and extent of pathology. The second uses neuroimaging to investigate neuroplasticity, via changes in connectivity, cortical thickness, and retinotopic maps within the visual cortex.
Recent findings
Here we give consideration to both acquired and congenital patients with damage to the visual pathway, and how they differ. Congenital disorders of the peripheral visual system can provide insight into the large-scale reorganisation of the visual cortex: these are investigated with reference to disorders of the optic chiasm and anophthalmia (absence of the eyes). In acquired conditions, we consider recent work describing patterns of degeneration, both following single insult and in neurodegenerative conditions. We also discuss developments in functional neuroimaging, with particular reference to work on hemianopia and the controversial suggestion of cortical reorganisation following acquired retinal injury.
Summary
Techniques for comparing neuro-ophthalmological conditions with healthy visual systems provide sensitive metrics to uncover subtle differences in gray and white matter structure of the brain. It is now possible to compare the massive reorganisation present in congenital conditions with the apparent lack of plasticity following acquired damage.
Keywords: hemianopia, optic chiasm, neuroimaging, fMRI, blindness
Introduction
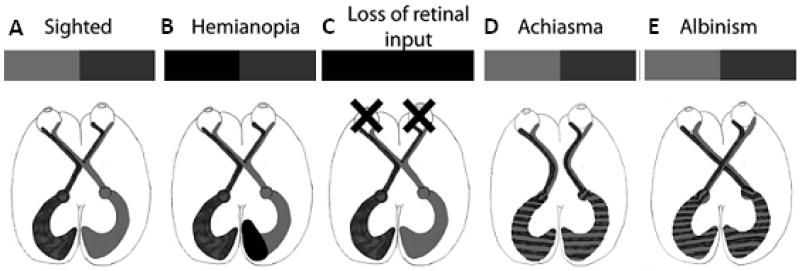
The advances in non-invasive neuroimaging techniques over the past few years have allowed the detailed investigation of a number of neuro-ophthalmological diseases. In particular, the use of magnetic resonance imaging (MRI) has provided insight into differences of both structure and function of the visual pathway, shown in Figure 1A. While the vast majority of research into such disorders is acquired using 3T scanners, the number of 7T scanners is increasing and more studies will benefit from increasing signal-to-noise ratio in the next few years.
Figure 1. Neuroimaging can be applied to investigate neuro-ophthalmological disorders at multiple points in the visual pathway.
A indicates the normal visual pathway where input from the eyes is reorganised at the optic chiasm such that one cortical hemisphere represents one side of visual space. B is the extreme condition where damage to the eyes prevents any visual input reaching the brain. C and D illustrate disorders of the chiasm in which cortical organization is abnormal (C Achiasma; D Albinism). E shows the effects of cortical visual damage resulting in homonymous hemianopia, a loss of vision on one side of visual space.
To understand the altered function of the brain in neuro-ophthalmological disorders, functional MRI (fMRI) is usually the method of choice, as a standard 3T research scanner provides high spatial resolution, up to around 2 × 2 × 2 mm3. Any functional changes or differences can be related to structural measures of both connectivity and volume. A summary of some current methods is provided below.
Diffusion-weighted imaging can be used to determine the integrity of white matter pathways within the brain. It provides a sensitive measure to pick up small differences that are unlikely to be obvious from an inspection of a T1- or T2-weighted structural image. Fractional anisotropy (FA) provides a measure of directionality of water molecule movement indicating the level of white matter integrity in fiber bundles, while increases in mean diffusivity (MD) can indicate a loss of axonal integrity.
Quantification of brain size and shape projected on the cortical surface permits the measurement of cortical thickness and the depth of gyrification. Furthermore, software such as freesurfer [1] can also assign visual areas based on cortical folding patterns, allowing comparison even without retinotopic mapping of these areas.
fMRI can be used to investigate functional reorganisation that occurs in neuro-ophthalmological disorders. Data can be acquired either during a task designed to investigate a specific type of visual processing or changes in correlation between visual areas while subjects are at rest (resting state analysis).
Neuroimaging may permit understanding of pathways to residual vision in hemianopia
In hemianopia, where damage along the post-chiasmatic visual pathway causes loss of vision (Figure 1B), structural imaging has proven a useful adjunct in assessing degeneration of pathways following damage such as stroke or traumatic brain injury. In the last few years, any controversy regarding trans-synaptic retrograde degeneration (TRD) of retinal ganglion cells following acquired postnatal occipital lobe damage appears to have been settled [2]; TRD has been clearly demonstrated following adult acquired occipital lobe lesions by showing retinal nerve fiber layer thinning using optical coherence tomography (OCT) [3] and thinning of the optic tract using MRI [4,5] in moderate sample sizes.
Structural imaging is also useful to relate functional deficits to the extent and localization of brain damage. One interesting example with clinical application [6] relates performance at collision avoidance with lesion location on MRI in a group of 26 hemianopic patients. This well-known technique of function-lesion mapping is gaining resurgence through use of large sample sizes, facilitated by the advent of shared imaging data sets.
Functional MRI in patients with hemianopia is important not only to further our understanding of blindsight, plasticity, and recovery after brain injury, but also to increase our understanding of the visual system. ‘Blindsight’ is the ability for a visual stimulus inside the blind field to influence behavior despite a patient often having no awareness of seeing it [7]. This is most consistent for moving stimuli, illustrated by fMRI activation of motion area V5/MT+ for high contrast motion within the blind field [8-10].
Affective blindsight is the non-conscious recognition of emotional stimuli in the absence of primary visual cortex [11]. DeGelder and colleagues have made considerable progress in this field in well-described patient GY [12], and a patient with cortical blindness due to bilateral occipital cortex damage. Having initially demonstrated selective activation of the amygdala for emotional versus neutral faces [13,14], they have now shown specific activity for directed versus averted gaze [15], and that even whole-body emotional actions can elicit differential activation. Although these are single case studies, the results provide increasing support for the role of subcortical pathways bypassing V1 in blindsight. These studies also suggest that the spatial resolution of residual pathways may be reasonably high since the critical information about pupil direction is contained within a 0.5° window. This clearly warrants further investigation.
The incidence of blindsight, and whether it reflects plasticity or residual pathways remains somewhat contentious [16], and one approach to investigate this question is to compare peri-natal lesions to those acquired later in life. Tinelli and colleagues [17] used psychophysics tests to show that children with congenital hemianopia could perform direction and orientation discrimination (notoriously hard to elicit in blindsight subjects), which those with acquired hemianopia could not. They also performed a type of quadrantic-retinotopic mapping in congenital cases, finding ipsilateral representation of the visual field in early visual cortex in some cases. The equivalent mapping was not, however, performed in the acquired cases so it is not clear whether such reorganization may also be present in those cases or whether this ‘plasticity’ relates to their behavioral results.
One recent study has looked at retinotopy in acquired cases, and is the largest study of its kind to date. It reports that two in 25 patients [18] with adult-onset visual cortex pathology (versus one in two congenital cases) demonstrate unusual ipsilateral representation on retinotopic mapping. This proportion is small, and only one acquired patient had fixation recorded outside the scanner with approximately 80% of gaze within the central 2°. However, the authors present an interesting case for reorganisation via unmasking of ipsilateral retino-geniculate afferents that may be worth exploring further.
Neuroimaging of patients with V1 damage can also be used to inform understanding of ‘normal’ neural processing. Bridge et al. [19] investigated the role of V1 in visual mental imagery was in a patient with bilateral damage. Despite almost complete cortical blindness in subject SBR, visual mental imagery significantly activated specific dorsal and ventral extrastriate regions. This is important because whilst V1 has previously been shown to be insufficient for visual imagery, this is the first suggestion that V1 may also not be necessary.
Posterior cortical atrophy shows a distinct pattern of gray and white matter degeneration
Posterior cortical atrophy (PCA) is a progressive neurodegenerative disease, often described as the visual variant of Alzheimer’s disease. First described by Benson et al. [20], the disorder is characterized by an early presentation of higher order visual processing deficits and only mild memory impairments, although memory deteriorates with progression of the disease. In the past, poor performance on visual field tests would often be solely attributed to cognitive visuospatial deficits. Whilst this is often the case, it is now agreed that true visual loss can also occur in a substantial proportion of patients [21,22], such as homonymous hemianopia due to asymmetrical onset. The condition is then often diagnosed late or not at all, due to a focus on seeking ophthalmological causes underlying symptoms [23].
Neuroimaging has illustrated the pattern of atrophy that distinguishes PCA from other dementias, resulting in the visual deficits indicative of the disorder. Voxel-based morphometry (VBM) has been used in a number of studies to investigate the extent and location of grey matter atrophy in PCA [24-27]. Alves et al. [28] recently conducted a meta-analysis of several of these studies, concluding that the key region showing greater degeneration in PCA than Alzheimer’s was the right occipital gyrus. Diffusion-weighted imaging suggests that the atrophy is not limited to the gray matter, but extends to the white matter in the parietal and occipital lobes [29] and indeed may be present throughout the major white matter pathways involved in visuo-spatial processing [27]. However these studies have been case studies and, given the heterogeneity of the disorder, replication is required in larger patient groups. Nonetheless, it appears that the impairments exhibited by PCA patients result from a combination of gray and white matter damage. There is a lack of fMRI studies to determine changes in visual activation patterns, with only a handful of studies on single cases [30-32]. Only one study [31] quantified visual activation and found decreased activation in areas V1 and V2.
The neurological basis for asymmetrical field loss has yet to be systematically investigated using neuroimaging. Nonetheless, a significant number of studies have noted that atrophy in PCA appears to be greater in the right hemisphere [24,33], particularly in patients with more ‘dorsal’ symptoms [27]. However it has been argued that this asymmetry is largely due to selection biases towards patients with extensive visual dysfunction [34]. Thus while the left-right asymmetry noted may not be characteristic of the PCA population as a whole, it may be a facet of the pattern of atrophy underlying those with the most profound visual loss, as exhibited in those with hemianopia.
Neuroimaging reveals considerable reorganisation of the visual pathway in bilateral congenital anophthalmia
In bilateral anophthalmia the eyes fail to develop early in gestation, or development begins and is arrested at an early stage (Figure 1C). It is a rare condition, only 1.8 per 100 000 (including microphthalmia) [35], and in around 50% cases there are other associated developmental disorders. Given the rarity of the condition, little was known of the changes in function of the ‘visual’ pathway in individuals with this condition until recently. Watkins et al. [36] performed the first functional study of a group of anophthalmic subjects, employing fMRI to look at both language task-related brain activity and changes in resting state correlations throughout the brain. Interestingly, while structural imaging shows only subtle differences in anophthalmic subjects [37], the scale of changes in function are considerable. Using a language task they found that the lateral occipital cortex (LOC) which normally responds to visual objects, was activated. In contrast, the calcarine region, normally V1 responded equally to both the language task and to backwards speech. Interestingly this contrasts with ‘early blind’ subjects who also show a specialisation for language in V1 [38]. Using resting state fMRI data [39], Watkins et al. further determined that LOC in the left hemisphere also became included in a left lateralized ‘language’ network, suggesting a tight coupling of response with the language regions.
These results suggest that the changes in the ‘visual’ pathway that are present in bilateral anophthalmia may differ from those seen when loss of vision occurs post-natally and there has been potential for pathway stimulation, either with light or endogenous retinal waves.
Cortical changes following early visual pathway damage in adulthood may not reflect plasticity
Until recently it was considered that cortical reorganisation might still occur following retinal pathology in adulthood [40], although this view has been contested (e.g. [41], reviewed in [42]). The first study with a relatively large cohort of patients (n=16) with macular degeneration used quantitative retinotopic-mapping to demonstrate an increase in population receptive field size and eccentricity at the occipital pole in patients compared to controls [43]. Intriguingly this difference disappears when a central scotoma is simulated in controls during testing. Whilst they could not rule out small-scale remapping at the edges of ‘lesion-projection zones’, they suggest that instead of plasticity, population receptive fields can be displaced and enlarged just by silencing central visual field stimulation. They postulate that this may simply reflect the underlying properties of neuronal receptive fields, and perhaps modulatory feedback signals from extrastriate visual cortex [44].
Congenital defects in crossing at the optic chiasm have a profound effect on visual organisation
There are two main conditions in which there is congenitally abnormal crossing at the chiasm: achiasma where the optic chiasm is absent and hence there is no crossing (Figure 1D) [45], and albinism where the majority of fibers cross at the chiasm (Figure 1E) [46]. The application of modern non-invasive imaging approaches has begun to unveil how the visual cortex copes with this abnormal input.
Two papers in the last year have applied modern imaging techniques to 3 subjects with achiasma to understand the functional organisation in the cortex [47,48]. In both studies, diffusion-weighted imaging showed that the white matter integrity of the splenium, through which the visual regions pass information between the two hemispheres, was similar to controls. This was also the case for the optic tract and optic radiation where measured.
Since the structural integrity of the visual system appears maintained, and visual fields are complete, the visual system must reorganise functionally to use this abnormal input. In both studies stimulation of an eye resulted in activation of the ipsilateral hemisphere, as predicted by the anatomy. Furthermore, fMRI-based mapping of the visual hemifields demonstrated retinotopic maps of each hemifield, superimposed in the visual cortex. Employing a population receptive field approach, Hoffmann and colleagues showed that V1 voxels were better modeled with a bilateral receptive fields, rather than standard contralateral representations. Both sets of authors conclude that, given the apparently normal visual fields, the likely encoding within the early visual cortex is at a macroscopic level. One method of achieving this would be an interleaved representation of ipsi- and contralateral hemifields, analogous to the ocular dominance columns present in control subjects. In the next few years imaging at sufficiently high resolution (~0.5mm3) may uncover visualisation of the difference in response to the two hemifields.
In human albinism, there is abnormal crossing at the chiasm, such that the majority of temporal retinal ganglion fibers cross (Figure 1E). The abnormal pattern of activation of the occipital cortex, in which each hemisphere contains a full representation of visual space has been shown using fMRI [49]. Like achiasma, each eye projects to one hemisphere, but in this condition it is the hemisphere contralateral to the eye, rather than ipsilateral. Intriguingly, in albinism cortical representation of the two visual hemifields is in close agreement to that described for achiasma, ie. as superimposed retinotopic maps [50]. These superimposed representations appear to support normal visual fields [51] and independent visual perception in both visual hemifields [52]. The visual system in albinism is also affected by the lack of foveal development which reduces the visual acuity of those with the condition, often resulting in considerable visual impairment. A recent study using structural scans from a large number of subjects with albinism employed quantitative measures of cortical morphology to uncover subtle changes in cortical structure [53]. Specifically, one of the differences was an increase in V1 cortical thickness in albinism compared to control subjects. Cortical thickness has previously been shown to be greater in blind subjects [54] and those with anophthalmia[37], hypothesised to be due to a lack of axonal pruning due to the absence of visual input. Intriguingly, the data from the subjects with albinism also showed a significant negative correlation between V1 thickness and visual acuity, suggesting that better vision leads to thinner visual cortex.
Conclusions
The neurological basis of several disorders described here has recently been uncovered by the use of various non-invasive imaging techniques. There is a large discrepancy in the plasticity and reorganisation shown in the case of congenital and acquired neuro-ophthalmological conditions. Congenital disorders generally lead to more reorganisation compared to acquired cases, but this also depends on whether the condition affects the cortex (e.g. hemianopia) or peripheral visual system (i.e. retina or chiasm). It is important not only to focus on situations of reorganisation, but also significant negative findings, and to quantitatively delineate areas in the visual pathway that have been damaged. This may help to target certain therapies within a stable visual system, and to evaluate those situations where rehabilitation may be most beneficial.
Key Points.
Neuroimaging has uncovered both degeneration and reorganisation in a range of neuro-ophthalmological disorders
Cortical reorganisation is greater in congenital, compared to acquired conditions
Functional fMRI can indicate brain regions that are active even in the absence of perception
Disorders of the optic chiasm result in abnormal functional organization, with relatively normal perception
Acknowledgements
This work was funded by a Royal Society University Research Fellowship to HB, a Wellcome Trust Clinical Research Training Fellowship to SA and an MRC studentship to RM. We would like to thank Annabel Hollins for the illustrations in Figure 1.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- 1.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 2.Bridge H, Plant GT. Conclusive evidence for human transneuronal retrograde degeneration in the visual system. Journal of Clinical and Experimental Ophthalmology. 2012;S3 [Google Scholar]
- 3.Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135:534–541. doi: 10.1093/brain/awr324. [DOI] [PubMed] [Google Scholar]
- 4.Bridge H, Jindahra P, Barbur J, Plant GT. Imaging reveals optic tract degeneration in hemianopia. Invest Ophthalmol Vis Sci. 2011;52:382–388. doi: 10.1167/iovs.10-5708. [DOI] [PubMed] [Google Scholar]
- 5.Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain. 2011;134:2149–2157. doi: 10.1093/brain/awr125. [DOI] [PubMed] [Google Scholar]
- 6.Papageorgiou E, Hardiess G, Wietholter H, Ackermann H, Dietz K, Mallot HA, Schiefer U. The neural correlates of impaired collision avoidance in hemianopic patients. Acta Ophthalmol. 2012;90:e198–205. doi: 10.1111/j.1755-3768.2011.02315.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- 8.Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res. 2001;41:1459–1474. doi: 10.1016/s0042-6989(01)00069-4. [DOI] [PubMed] [Google Scholar]
- 9.Nelles G, Pscherer A, de Greiff A, Gerhard H, Forsting M, Esser J, Diener HC. Eye-movement training-induced changes of visual field representation in patients with post-stroke hemianopia. J Neurol. 2010;257:1832–1840. doi: 10.1007/s00415-010-5617-1. [DOI] [PubMed] [Google Scholar]
- 10.Bridge H, Hicks SL, Xie J, Okell TW, Mannan S, Alexander I, Cowey A, Kennard C. Visual activation of extra-striate cortex in the absence of V1 activation. Neuropsychologia. 2010;48:4148–4154. doi: 10.1016/j.neuropsychologia.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Gelder B, Vroomen J, Pourtois G, Weiskrantz L. Non-conscious recognition of affect in the absence of striate cortex. Neuroreport. 1999;10:3759–3763. doi: 10.1097/00001756-199912160-00007. [DOI] [PubMed] [Google Scholar]
- 12.Van den Stock J, Tamietto M, Sorger B, Pichon S, Grezes J, de Gelder B. Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1) Proc Natl Acad Sci U S A. 2011;108:16188–16193. doi: 10.1073/pnas.1107214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124:1241–1252. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- 14.Pegna AJ, Khateb A, Lazeyras F, Seghier ML. Discriminating emotional faces without primary visual cortices involves the right amygdala. Nat Neurosci. 2005;8:24–25. doi: 10.1038/nn1364. [DOI] [PubMed] [Google Scholar]
- 15.Burra N, Hervais-Adelman A, Kerzel D, Tamietto M, de Gelder B, Pegna AJ. Amygdala Activation for Eye Contact Despite Complete Cortical Blindness. J Neurosci. 2013;33:10483–10489. doi: 10.1523/JNEUROSCI.3994-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridge H, Thomas O, Jbabdi S, Cowey A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain. 2008;131:1433–1444. doi: 10.1093/brain/awn063. [DOI] [PubMed] [Google Scholar]
- 17.Tinelli F, Cicchini GM, Arrighi R, Tosetti M, Cioni G, Morrone MC. Blindsight in children with congenital and acquired cerebral lesions. Cortex. 2013;49:1636–1647. doi: 10.1016/j.cortex.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Reitsma DC, Mathis J, Ulmer JL, Mueller W, Maciejewski MJ, Deyoe EA. Atypical retinotopic organization of visual cortex in patients with central brain damage: congenital and adult onset. J Neurosci. 2013;33:13010–13024. doi: 10.1523/JNEUROSCI.0240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridge H, Harrold S, Holmes EA, Stokes M, Kennard C. Vivid visual mental imagery in the absence of the primary visual cortex. J Neurol. 2012;259:1062–1070. doi: 10.1007/s00415-011-6299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- 21.Pelak VS, Smyth SF, Boyer PJ, Filley CM. Computerized visual field defects in posterior cortical atrophy. Neurology. 2011;77:2119–2122. doi: 10.1212/WNL.0b013e31823e9f2a. [DOI] [PubMed] [Google Scholar]
- 22.Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 23.Ruis C, van den Berg E, van Zandvoort MJ, Boshuisen K, Frijns CJ. Ophthalmic impairment or higher-order visual deficit? Posterior cortical atrophy: a case report. Appl Neuropsychol Adult. 2012;19:153–157. doi: 10.1080/09084282.2012.670165. [DOI] [PubMed] [Google Scholar]
- 24.Migliaccio R, Agosta F, Toba MN, Samri D, Corlier F, de Souza LC, Chupin M, Sharman M, Gorno-Tempini ML, Dubois B, et al. Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex. 2012;48:1298–1309. doi: 10.1016/j.cortex.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann M, Barnes J, Ridgway GR, Ryan NS, Warrington EK, Crutch SJ, Fox NC. Global gray matter changes in posterior cortical atrophy: a serial imaging study. Alzheimers Dement. 2012;8:502–512. doi: 10.1016/j.jalz.2011.09.225. [*A well conducted longitudinal study measuring the progression of gray matter loss in a sizeable group of patients with posterior cortical atrophy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitwell JL, Jack CR, Jr., Kantarci K, Weigand SD, Boeve BF, Knopman DS, Drubach DA, Tang-Wai DF, Petersen RC, Josephs KA. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28:1051–1061. doi: 10.1016/j.neurobiolaging.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migliaccio R, Agosta F, Scola E, Magnani G, Cappa SF, Pagani E, Canu E, Comi G, Falini A, Gorno-Tempini ML, et al. Ventral and dorsal visual streams in posterior cortical atrophy: a DT MRI study. Neurobiol Aging. 2012;33:2572–2584. doi: 10.1016/j.neurobiolaging.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves J, Soares JM, Sampaio A, Goncalves OF. Posterior cortical atrophy and Alzheimer’s disease: a meta-analytic review of neuropsychological and brain morphometry studies. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9236-1. [DOI] [PubMed] [Google Scholar]
- 29.Duning T, Warnecke T, Mohammadi S, Lohmann H, Schiffbauer H, Kugel H, Knecht S, Ringelstein EB, Deppe M. Pattern and progression of white-matter changes in a case of posterior cortical atrophy using diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2009;80:432–436. doi: 10.1136/jnnp.2008.153148. [DOI] [PubMed] [Google Scholar]
- 30.Barbarulo AM, Pappata S, Puoti G, Prinster A, Grossi D, Cotrufo R, Salvatore M, Trojano L. Rehabilitation of gesture imitation: a case study with fMRI. Neurocase. 2008;14:293–306. doi: 10.1080/13554790802363688. [DOI] [PubMed] [Google Scholar]
- 31.Feldmann A, Trauninger A, Toth L, Kotek G, Kosztolanyi P, Illes E, Pfund Z, Komoly S, Nagy F, Illes Z. Atrophy and decreased activation of fronto-parietal attention areas contribute to higher visual dysfunction in posterior cortical atrophy. Psychiatry Res. 2008;164:178–184. doi: 10.1016/j.pscychresns.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Delazer M, Karner E, Zamarian L, Donnemiller E, Benke T. Number processing in posterior cortical atrophy--a neuropsycholgical case study. Neuropsychologia. 2006;44:36–51. doi: 10.1016/j.neuropsychologia.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Migliaccio R, Agosta F, Possin KL, Rabinovici GD, Miller BL, Gorno-Tempini ML. White matter atrophy in Alzheimer’s disease variants. Alzheimers Dement. 2012;8:S78–87. e71–72. doi: 10.1016/j.jalz.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11:170–178. doi: 10.1016/S1474-4422(11)70289-7. [*A comprehensive review of posterior cortical atrophy, from description of the clinical features to an overview of the current state of understanding of the disorder through neuroimaging and genetic studies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw GM, Carmichael SL, Yang W, Harris JA, Finnell RH, Lammer EJ. Epidemiologic characteristics of anophthalmia and bilateral microphthalmia among 2.5 million births in California, 1989-1997. Am J Med Genet A. 2005;137:36–40. doi: 10.1002/ajmg.a.30840. [DOI] [PubMed] [Google Scholar]
- 36.Watkins KE, Cowey A, Alexander I, Filippini N, Kennedy JM, Smith SM, Ragge N, Bridge H. Language networks in anophthalmia: maintained hierarchy of processing in ‘visual’ cortex. Brain. 2012;135:1566–1577. doi: 10.1093/brain/aws067. [DOI] [PubMed] [Google Scholar]
- 37.Bridge H, Cowey A, Ragge N, Watkins K. Imaging studies in congenital anophthalmia reveal preservation of brain architecture in ‘visual’ cortex. Brain. 2009;132:3467–3480. doi: 10.1093/brain/awp279. [DOI] [PubMed] [Google Scholar]
- 38.Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc Natl Acad Sci U S A. 2011;108:4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25:614–618. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuda Y, Dumoulin SO, Nakadomari S, Wandell BA. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb Cortex. 2008;18:2483–2493. doi: 10.1093/cercor/bhm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci. 2009;10:873–884. doi: 10.1038/nrn2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baseler HA, Gouws A, Haak KV, Racey C, Crossland MD, Tufail A, Rubin GS, Cornelissen FW, Morland AB. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat Neurosci. 2011;14:649–655. doi: 10.1038/nn.2793. [DOI] [PubMed] [Google Scholar]
- 44.Haak KV, Cornelissen FW, Morland AB. Population receptive field dynamics in human visual cortex. PLoS One. 2012;7:e37686. doi: 10.1371/journal.pone.0037686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apkarian P, Bour L, Barth PG. A unique achiasmatic anomaly detected in non-albinos with misrouted retinal-fugal projections. Eur J Neurosci. 1994;6:501–507. doi: 10.1111/j.1460-9568.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 46.Guillery RW. Neural abnormalities of albinos. Trends in Neurosciences. 1985;9:364–367. [Google Scholar]
- 47.Davies-Thompson J, Scheel M, Jane Lanyon L, Sinclair Barton JJ. Functional organisation of visual pathways in a patient with no optic chiasm. Neuropsychologia. 2013;51:1260–1272. doi: 10.1016/j.neuropsychologia.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann MB, Kaule FR, Levin N, Masuda Y, Kumar A, Gottlob I, Horiguchi H, Dougherty RF, Stadler J, Wolynski B, et al. Plasticity and stability of the visual system in human achiasma. Neuron. 2012;75:393–401. doi: 10.1016/j.neuron.2012.05.026. [**A careful investigation of the structure and function of the visual pathway in two achiasmatic subjects. The use of fMRI to look at visual repesentation, and the normal visual function allowed the authors to conclude that there is likely a columnar organisation for ipsi- and contralateral visual fields allowing separate processing of this information at a macro-scopic level. This illustrates the ability of the brain to rewire considerably in this congenital condition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morland AB, Hoffmann MB, Neveu M, Holder GE. Abnormal visual projection in a human albino studied with functional magnetic resonance imaging and visual evoked potentials. J Neurol Neurosurg Psychiatry. 2002;72:523–526. doi: 10.1136/jnnp.72.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann MB, Tolhurst DJ, Moore AT, Morland AB. Organization of the visual cortex in human albinism. J Neurosci. 2003;23:8921–8930. doi: 10.1523/JNEUROSCI.23-26-08921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann MB, Seufert PS, Schmidtborn LC. Perceptual relevance of abnormal visual field representations: static visual field perimetry in human albinism. Br J Ophthalmol. 2007;91:509–513. doi: 10.1136/bjo.2006.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klemen J, Hoffmann MB, Chambers CD. Cortical plasticity in the face of congenitally altered input into V1. Cortex. 2012;48:1362–1365. doi: 10.1016/j.cortex.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Bridge H, von dem Hagen EA, Davies G, Chambers C, Gouws A, Hoffmann M, Morland AB. Changes in brain morphology in albinism reflect reduced visual acuity. Cortex. 2012 doi: 10.1016/j.cortex.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Jiang J, Zhu W, Shi F, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Thick visual cortex in the early blind. J Neurosci. 2009;29:2205–2211. doi: 10.1523/JNEUROSCI.5451-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]