Abstract
Background:
Transanal pull-through with laparoscopic assistance is gaining popularity. How much rectal dissection to do laparoscopically and how much transanally is not clear. Laparoscopic rectal mobilization is akin to open pelvic dissection of Swenson's operation — the most physiological procedure. Through this comparative study, we aim to evolve a technique that maximizes the benefits of Swenson's technique and minimizes the problems of a transanal procedure.
Materials and Methods:
Twenty patients (19 boys and one girl, newborn to 6 years) with Hirschsprung's disease (HD) were randomized for laparoscopic-assisted transanal pull-through (LATAPT) either by near complete (Group A) or partial (Group B) laparoscopic mobilization of rectum. Patients were followed up for at least 3 months. Demographic profile; operative details (time taken, blood loss, operative difficulty, and complications); postoperative course (duration of urinary catheter, oral feeding, and hospital stay); and follow-up stooling pattern, consistency, and continence were compared in the two groups.
Results:
The time taken for laparoscopic mobilization was marginally higher in group A, but the time taken for transanal dissection in this group was significantly less than in group B. All other comparisons showed no significant difference in the two groups. Stool frequency and continence improved with time in both groups.
Conclusion:
Extent of laparoscopic mobilization of rectum does not appear to be a factor deciding the outcomes. No recommendations could be made in view of the small number of cases. However, it shows that laparoscopic assistance can be used to maximize the benefits of Swenson type of operation and a transanal pull-through.
KEY WORDS: Endorectal pull-through, Hirschsprung's, laparoscopy, transanal
INTRODUCTION
Ever since the description of Swenson's operation in 1948,[1] its principle of completely resecting the aganglionic rectum and anastomosis of the ganglionic bowel to the anal canal still remains valid as the most physiological approach to Hirschsprung's disease (HD). The essence of this operation lies in a precise pelvic dissection staying on the muscular wall of the rectum, thereby preventing damage to the pelvic nerves behind the bladder. Development of laparoscopic assisted transanal pull-through (LATAPT) by Georgeson et al., in 1995,[2] heralded the era of laparoscopy in the management of HD. The operation was performed in two parts: Laparoscopic and transanal. Laparoscopic part consisted of frozen section biopsy, mobilization of proximal ganglionic bowel, and partial mobilization of the rectum below the peritoneal reflection. The transanal part was similar, but much less extensive, to the transanal pull-through as described by De La Torre-Mondragon in 1998.[3] Both ways of rectal dissection aim to make it safe for the pelvic nerves. Several papers have been published on laparoscopic assisted pull-through, but there has been no consensus on the extent of rectal dissection performed laparoscopically, which perhaps is the most controversial and most varied aspect of the operation. It has varied from complete mobilization (laparoscopic Swenson)[4] to no mobilization (meaning all mobilization done transanally).[5] Some have recommended partial mobilization.[6,7,8] To address this issue we carried out a prospective study on LATAPT with a view to see if the extent of laparoscopic dissection makes any impact on the operation and its outcomes.
MATERIALS AND METHODS
Twenty patients of HD, newborn to six years of age, underwent LATAPT. They were randomized in two groups with 10 patients in each. All other aspects of their operation were same except for the following:
Group A: Near complete laparoscopic mobilization of the rectum was performed, that is, the rectum was dissected laparoscopically all the way down up to the level 2 cm above the anal verge. Below this level the rectum was dissected submucosally through the transanal route [Figure 1a].
Figure 1.
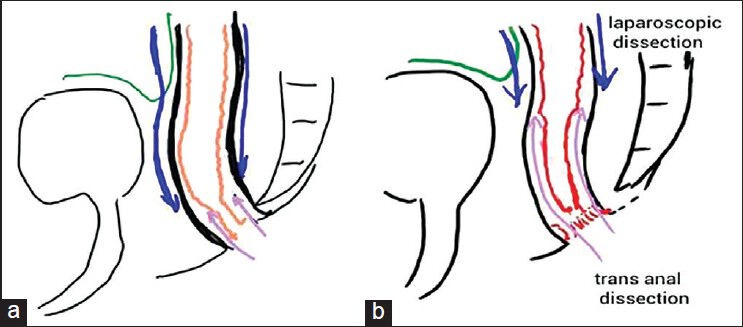
Extent of laparoscopic and transanal rectal mobilization in groups A and B. (a) Blue arrow directed downwards shows major lap dissection. The arrow directed upwards shows minimal transanal dissection. (b) Arrow directed downwards shows minimal lap dissection. The arrow directed upwards shows major transanal dissection
Group B: Partial laparoscopic mobilization of rectum was performed, that is, the rectum was dissected only up to a point 1 cm below the peritoneal reflection. Further rectal dissection was done by transanal route in submucosal plain [Figure 1b].
Patients with aganglionosis beyond splenic flexure, previous pull-through, nonavailability of consent, and contraindication for laparoscopy were excluded from study.
The diagnosis was confirmed in all by rectal biopsy. Contrast enema was done to know the approximate extent of the disease. When the diagnosis was made in the newborn period, the patients were kept on rectal washouts for a minimum 6 weeks before the operation. All information was recorded in a pro forma indicating demography, extent of disease, stoma or no stoma, operating time, blood loss, operative difficulty, duration of urinary catheter, oral feeding, hospital stay, and any other complication. During follow-up stool patterns, stool consistency and continence were recorded. Immediate and follow-up outcomes were compared in the two groups.
Follow-up was arranged at 1 and 3 months in all cases.
Surgical procedure
Mechanical bowel preparation was done in all cases. Under general endotracheal anesthesia, total body preparation was done for intraoperative change of position from laparoscopic to transanal part. The operation was performed in two parts: Laparoscopic and transanal.
Laparoscopic part
After placement of ports, the transition zone was identified. A seromuscular biopsy was obtained from 5 cm proximal to the transition zone for frozen section histology to decide the level of pull-through. A window was made in the sigmoid mesentery and the sigmoid was mobilized. Proximal ganglionic bowel was mobilized preserving the marginal arcade. The peritoneal reflection was incised sharply to facilitate dissection and mobilization of the aganglionic rectum. The procedure varied in the two groups after this step:
Group A: The rectum below the peritoneal reflection was dissected circumferentially all the way up to a level 2 cm above the anal verge.
Group B: The rectum below the peritoneal reflection was minimally mobilized (within 1 cm of peritoneal reflection).
After laparoscopic dissection, the ports were left in situ and the position changed for transanal dissection of the remaining rectum.
Transanal part
Lower rectum in both the groups was mobilized by submucosal dissection as described in the original description of transanal pull-through. Only the extent of this dissection varied in the two groups. In group A since most of the dissection had been carried out laparoscopically only the lower 2 cm of rectum needed mobilization transanally [Figure 2a]. In group B it was more extensive since the laparoscopic dissection was minimal [Figure 2b]. The anastomosis was performed about 5 mm above the dentate line as shown in [Figure 3]. The level of pull-through was at the biopsy proven ganglionic segment. Once the anastomosis was completed, laparoscopy was performed again to check for orientation of the pull-through bowel. Drain was placed and the port sites closed.
Figure 2.
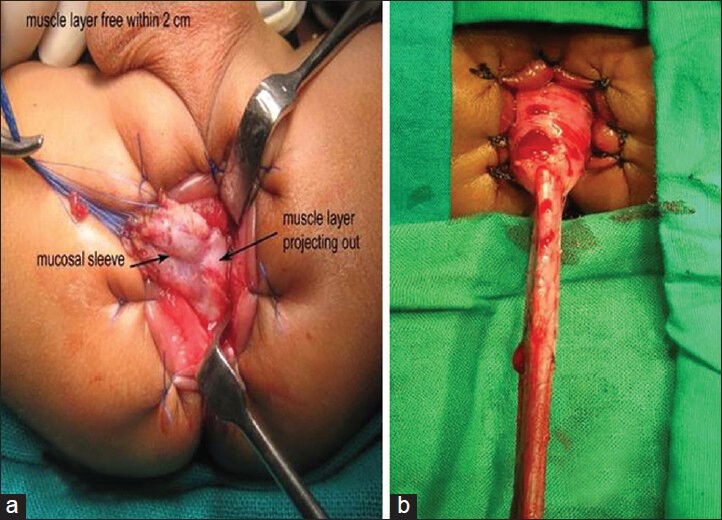
Transanal dissection. (a) Minimal transanal dissection in group A. (b) More transanal dissection is required in group B
Figure 3.
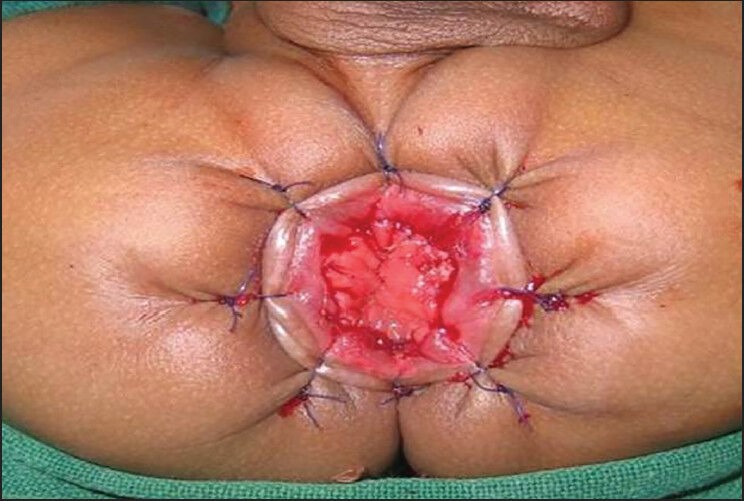
Anastomosis performed about 5 mm above the dentate line
After surgery, patients were kept on intravenous (IV) fluids and nasogastric decompression for 12-24 h. IV antibiotics were given for 48 h and longer if necessary. Oral feeding was introduced when bowel sounds returned. The patient was discharged when tolerating a full oral diet. Rectal examination was performed 3 weeks after surgery. Parents were taught to perform home dilatation with Hegar dilators.
RESULTS
All operations were successfully performed, there being no conversion. Eight patients had a preexisting colostomy (five in Group A and three in Group B) at the time of operation. There were no significant differences between two groups with respect to age at operation and extent of disease [Table 1]. Both the groups were comparable with respect to total duration of surgery, operative difficulties, blood loss, time to oral feeding, and hospital stay.
Table 1.
Demographic profile
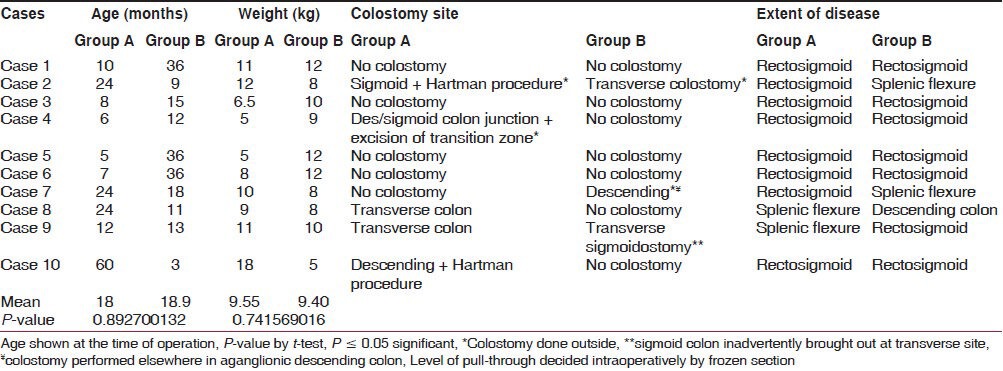
In both groups the duration of surgery was prolonged in the cases that had a preexisting colostomy due to adhesions and variations in port placement. However, its significance could not be estimated because of small number of cases.
Time taken for laparoscopic dissection was higher in group A as compared to group B (P-value 0.027). Time taken for transanal dissection was higher in group B as compared to Group A. However, the total duration of surgery was comparable in the two groups [Table 2].
Table 2.
Time taken for surgery

Urinary catheter was required for 24-48 h in all except one, who had a preexisting bladder dysfunction. Enteral feeding could be established in most by 24 h and in all by 36 h, there being no difference in the two groups.
Tables 3 and 4 show the follow-up regarding stool consistency and soiling, respectively. Although in the early follow-up, the soiling was marginally higher in group B, by the end of 3 months no patient had soiling and stool consistency had improved in all, though one case in group A required a colostomy at 6 weeks after surgery.
Table 3.
Comparison of stool consistency in the two groups
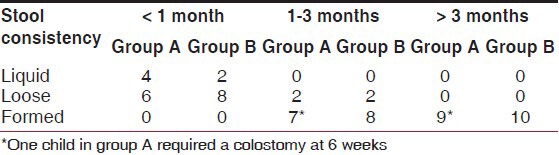
Table 4.
Comparison of soiling in the two groups

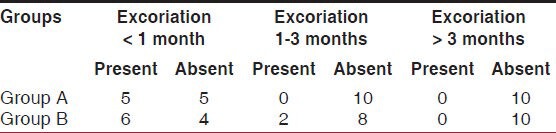
Excoriation was seen in early postoperative period in over 50% cases. However, it was not a problem after 3 months. There was no difference in the two groups as shown in Table 5.
Table 5.
Comparison of perianal skin excoriation in the two groups
All patients in both the groups were kept on home dilatations using Hegar dilators for 3 months. In two cases in group A the compliance was not good resulting in mild narrowing at the anastomosis. It responded to regular dilatation through improved compliance.
DISCUSSION
HD is four times more common in males,[9] though in our study there is only one female. High male predominance in our study may be related to exclusion of total colonic aganglionosis and long segment disease which are more common in females. The other possible reason could be small number of patients. Both the groups were comparable for age, weight, and extent of diseases. Similar criteria of comparison have been used in other reported series.[5,10]
Laparoscopic assistance in the management of HD has seen giant's rides since its introduction by Georgeson et al., in 1995.[2] A major advantage is a frozen biopsy taken very early during surgery, minimal peritoneal trauma, and precise mobilization of the ganglionic bowel preserving the marginal vessels. In addition pelvic dissection of the rectum when performed laparoscopically is perhaps more accurate and precise because of magnification and clear vision in the deep pelvic cavity. We followed the same operative techniques as described by Georgeson et al.,[2] including the use of energy device — monopolar cautery hook for smaller vessels on the rectal muscle and harmonic scalpel for larger mesorectum. This is in variance with Kumar et al.,[4] who preferred only monopolar cautery. Since the ultrasonic scalpel device is only 5 mm and that too quite thick at the end, we feel it should only be used for larger pedicles and not for precise coagulation of very small vessels entering the rectal muscle. Precision is the essence of this aspect of the operation and a 3 mm hook works the best in our experience.
The Swenson operation has been considered a difficult one considering the precision required in the perirectal dissection that too in the deep pelvis. Laparoscopic assistance has made it much easier by providing magnified view and good retraction in the pelvis. There is a concern of diathermy-related injury to the pelvic nerves, but if the plane of dissection is kept just perirectal the injury is minimal to negligible. We have used 3 or 5 mm cautery hook and low settings of cutting current. We have been precise in staying very close to the rectal wall identifying and hooking small vessels entering the rectal muscle. Swenson advocated this plane for dissection and even though it was done by open means, the complications related to nerve injury were small in expert hands.
The completely transanal pull-through (without laparoscopy or laparotomy) although attractive (as it avoid peritoneal dissection completely), is applicable only to classical rectosigmoid disease and also it involves major stretching of the external sphincter to facilitate long submucosal dissection. In group A in our series, we have combined advantages of both the approaches. Major dissection of the rectum has been done through laparoscopy under magnified vision. The transanal dissection was used only for the distal 2 cm which was easy, took less time, and caused less stretching of the sphincter. On the other hand in group B, less dissection was done through laparoscopy and major rectal dissection was done through the transanal approach which took more time, was comparatively difficult, and apparently involved more stretching of the sphincters.
In the original description of the transanal pull-through procedure, the mucosal dissection was carried to a point above the peritoneal reflection, to ensure that there was no injury to pelvic structures. Using this technique, the rectal cuff is quite long, and most authors advocate dividing or excising a part of the cuff to prevent it from rolling down and forming a constricting ring around the pull-through bowel. Although many surgeons continue to leave a long rectal cuff, others have moved to a much shorter cuff. In the technique used in group A, the rectal cuff is very small and that too can be incised posteriorly to completely offset its constrictive effect. The possible advantage of leaving a short cuff or no cuff is the avoidance of a constricting ring or residual aganglionic bowel, with a lower risk of obstruction and enterocolitis.[11] The disadvantage is that dissection on the rectum deep in the pelvis may increase the risk of injury to pelvic nerves and vessels, and to the prostate, urethra, or vagina. The complication associated because of rectal cuff is well-described in literature.[11,12] We in our study have done posterior incision of this cuff in both groups to prevent cuff stenosis. However in group B, where there was more likelihood of cuff-related problem we have not observed any specific problem on the account of longer cuff. This was probably because we always divide the cuff in midline.
Both our groups were found to be comparable as far as the total operative time and blood loss was concerned. Counting the time for laparoscopic dissection and transanal dissection separately it was evident that laparoscopic dissection in group A took significantly more time than in group B. Similarly, transanal dissection took more time in group B. It is logical to think that less transanal dissection should translate into less stretching of the sphincters and hence better continence. In literature also, significant problem have been reported on account of stretching of sphincter.[13] However, stooling pattern has been comparable in the two groups in our study. This may be due to small numbers of cases or maximum number of cases in early infancy. That the effect of stretching on continence is transient is well known.[14] Hence, at the best the ill effect of extensive transanal dissection are short-lived and have no long-term sequel.
The urinary function has not been affected by surgery in either group. In the child who had a neurogenic bladder to begin with, prolonged catheterization was necessary because of the preexisting condition rather than because of the surgery.
A major difference in long-term complication was more incidence of anastomosis narrowing in group A. Sapin et al.,[10] had similar complication and had advocated anal dilation in such patients. Ekema et al.,[15] have recommended routine anal dilation in follow-up in all patients. We have also followed routine home dilatations. Both our patients with anastomotic narrowing were not regular in dilating, and in both the narrowing subsided on resumption of dilatations. It is important to stress the need for dilatations for few weeks till the stool consistency becomes semisolid so that the fecal bolus itself can act as a dilator.
In the literature, concerns have been raised about possibility of incontinence in account of over stretching of sphincters in transanal pull-through, but in most papers including our study it is only transient effect with stooling pattern becoming normal with passage of time. The increase in stool frequency, skin excoriation in Group B on short-term is a reflection of possible transient nature of this effect.
Limitation of our study is its small sample size and limited follow-up data. Number of stools, consistency, and soiling depend on several factors including the level of pull-through. It is difficult to attribute variations on this aspect to the operative procedure alone. Therefore, these parameters may not reflect truly on the operative procedure in the two groups.
CONCLUSIONS
Lap-assisted transanal pull-through is a satisfying operation for HD. The extent of laparoscopic and transanal mobilization of rectum makes no difference in the outcomes. The effect of stretching of external sphincter during transanal dissection appears to be short-lived.
We feel that it should be left to surgeon's discretion who can decide how much to do through laparoscopy and how much to do transanally depending on his training, comfort, expertise, and temperament. If done correctly and precisely, both the approaches to rectal dissection are equally good. Complete mobilization takes marginally higher laparoscopic time than mobilization till just below peritoneal reflection. No recommendations could be made in favor or against any technique based on this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Swenson O, Bill AH., Jr Resection of rectum and rectosigmoid with preservation of the sphincter for benign spastic lesions producing megacolon: An experimental study. Surgery. 1948;24:212–20. [PubMed] [Google Scholar]
- 2.Georgeson KE, Fuenfer MM, Hardin WD. Primary laparoscopic pull-through for Hirschsprung's disease in infants and children. J Pediatr Surg. 1995;30:1017–21. doi: 10.1016/0022-3468(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 3.De la Torre-Mondragón L, Ortega-Salgado JA. Trans-anal endorectal pull-through for Hirschsprung's disease. J Pediatr Surg. 1998;33:1283–6. doi: 10.1016/s0022-3468(98)90169-5. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Macakay A, Borzi P. Laparoscopic swenson procedure-an optimal approach for both primary and secondary pull-through for Hirschsprung's disease. J Pediatr Surg. 2003;38:1440–3. doi: 10.1016/s0022-3468(03)00493-7. [DOI] [PubMed] [Google Scholar]
- 5.Craigie RJ, Conway SJ, Cooper L, Turnock RR, Lamont GL, Baillie CT, et al. Primary pull-through for Hirschsprung's disease: comparison of open and laparoscopic-assisted procedures. J Laparoendosc Adv Surg Tech. 2007;17:809–12. doi: 10.1089/lap.2007.0081. [DOI] [PubMed] [Google Scholar]
- 6.Antao B, Roberts J. Laparoscopic assisted trans anal endorectal colo anal anastomosis for Hirschsprung's disease. J Laparoendos Adv Surg Tech. 2005;15:75–9a. doi: 10.1089/lap.2005.15.75. [DOI] [PubMed] [Google Scholar]
- 7.Georgeson KE, Cohen RD, Hebra A, et al. Primary laparoscopic assisted endorectal colon pull-through for Hirschsprung's disease: a new gold standard. Ann Surg. 1999;229:678–83. doi: 10.1097/00000658-199905000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hau BD, Quynh TA, Anh VH, Liem NT. Early and late outcomes of primary laparoscopic endorectal colon pull-through leaving a short rectal seromuscular sleeve for Hirschsprung's disease. J Laparoendosc Adv Surg Tech. 2011;21:81–3. doi: 10.1089/lap.2009.0482. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsdóttir A, Wester T. Modern Treatment of Hirschsprung's disease. Scandinavian Jour Surg. 2011;100:243–9. doi: 10.1177/145749691110000403. [DOI] [PubMed] [Google Scholar]
- 10.Sapin E, Centonze A, Moog R, Borgnon J, Becmeur F. Trans-anal Coloanal Anastomosis for Hirschsprung's Disease: Comparison between Endorectal and Perirectal Pull-Through Procedures. Eur J Pediatr Surg. 2006;16(5):312–17. doi: 10.1055/s-2006-924523. [DOI] [PubMed] [Google Scholar]
- 11.Nasr A, Langer JC. Evolution of the technique in the trans-anal pull-through for Hirschsprung's disease: effect on outcome. J Pediatr Surg. 2007;42(1):36–40. doi: 10.1016/j.jpedsurg.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 12.De La Torre L, Langer JC. Trans-anal endorectal pull-through for Hirschsprung's disease: technique, controversies, pearls, pitfalls, and an organized approach to the management of postoperative obstructive symptoms. Seminars in pediatric surgery. 2010;19:96–106. doi: 10.1053/j.sempedsurg.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 13.El-Sawaf MI, Drongowski RA, Chamberlain JN, Coran AG, Teitelbaum DH. Are the long-term results of the trans-anal pull-through equal to those of the transabdominal pull-through? A comparison of the 2 approaches for Hirschsprung disease. J Pediatr Surg. 2007;42:41–7. doi: 10.1016/j.jpedsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Elhalaby EA, Hashish A, Elbarbary MM, et al. Trans-anal one stage endorectal pull-though for Hirschsprung's disease: a multicentric study. J Pediatr Surg. 2004;39(3):345–51. doi: 10.1016/j.jpedsurg.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Ekema G, Falchetti D, Torri F, et al. Further evidence on totally trans-anal one-stage pull through procedure for Hirschprung's disease. J pediatr Surg. 2003;38:1434–39. doi: 10.1016/s0022-3468(03)00492-5. [DOI] [PubMed] [Google Scholar]


