Abstract
Social relationships are essential for many fundamental aspects of life while bond disruption can be detrimental to mental and physical health. Male prairie voles form enduring social bonds with their female partners, allowing the evaluation of partner loss on behavior, physiology, and neurochemistry. Males were evaluated for partner preference formation induced by 24 h of mating, and half were separated from their partner for 4 wk. In Experiment 1, partner loss significantly increased anxiety-like behaviors in the elevated plus maze and light-dark box tests and marginally increased depressive-like behaviors in the forced swim test. In addition, while intruder-directed aggression is common in pair bonded prairie voles, separated males were affiliative and lacked aggression toward an unfamiliar female and an intruding male conspecific. Partner loss increased the density of oxytocin-immunoreactivity (-ir), vasopressin-ir, and corticotrophin-releasing hormone-ir cells in the paraventricular nucleus of the hypothalamus and oxytocin-ir cells in the supraoptic nucleus. Tyrosine hydroxylase-ir was not affected. In Experiment 2, partner preference was observed after 2 wk of partner loss but eliminated after 4 wk partner loss. Body weight gain and plasma corticosterone concentrations were elevated throughout the 4 wk. No effects were observed for plasma oxytocin or vasopressin. Together, partner loss elicits anxiety-like and depression-like behaviors, disrupts bond-related behaviors, and alters neuropeptide systems that regulate such behaviors. Thus, partner loss in male prairie voles may provide a model to better understand the behavior, pathology, and neurobiology underlying partner loss and grief.
Keywords: Vasopressin, Oxytocin, Corticotrophin releasing hormone, Tyrosine hydroxylase, Bond loss, Social stress
1. Introduction
Social living is beneficial for many species, resulting in increased individual survival and fitness. One factor that contributes to such benefits is the anxiolytic effects of social contact with a bonded partner, referred to as social buffering [1–6]. While healthy, committed social relationships provide salubrious effects, social separation or loss of bonded partners is a major risk factor to mental and physical health. With over 800,000 new widows and widowers in the United States annually, spousal bereavement is a significant cause of psychiatric and medical morbidity, and includes psychiatric sequelae such as depression, anxiety, substance abuse, and complicated grief1 [9–11]. For example, after the death of a spouse, there is a greater prevalence of major depressive disorder, post-traumatic stress, panic disorders, and general anxiety disorder [12–16], the odds of a new or worsened physical illness can increase by 1:40 times [17, 18], and mortality rates double for the surviving spouse in the first year [19–21], with bereavement effects strongest immediately after social loss [21, 22]. Even after counseling, this population is at a greater risk for mental disorders [23, 24]. These negative effects may be due, in part, to the stress of losing a loved one, challenges of adapting to widowhood, loss of psychological, social, and economic resources, and social isolation and loneliness that can follow spousal loss. Thus, understanding the behavioral pathologies and neuroendocrine mechanisms that underlie the challenges to mental health and normal behavioral routines associated with social loss are important to improve treatment of subsequent mental disorders.
The socially monogamous prairie vole (Microtus ochrogaster) is a highly social rodent species that has been used to study the neurobiological mechanisms that govern social behaviors [25–28] and consequences of social isolation [29]. Pair bonding in prairie voles reinforce social behavior through activation of the brain reward centers and stress buffering effects of close social contact [6, 25, 30–32]. These driving forces employ a number of neurochemical systems including oxytocin (Oxt), vasopressin (AVP), dopamine (DA), and corticotrophin-releasing hormone (CRH). In addition, the absence of social contact in prairie voles can promote a disruption to normal behavioral routines and biological functions that mimic symptomatology of depression and anxiety disorders in humans [33, 34], though there are gender differences. Female prairie voles isolated from social contact from a same-sex sibling display behaviors relevant to depression and anxiety, observable after 4 days and up to 6 weeks of isolation [33–39]. Interestingly, male prairie voles do not display robust behavioral abnormalities in result to isolation from a familiar same-sex conspecific[37, 40]; however, separation from a female bonded partner can be rather distressing, affecting normal behavioral routines and biological function [40, 41]. Thus, bond loss in male prairie voles provide a model to characterize the impact that bereavement has on normal behavioral routines and function of neuronal systems.
The present study used male prairie voles to evaluate potential effects of partner loss on behavior, physiology, and neurochemistry. Previous studies have shown that 24 hr mating reliably induces partner preference formation in most male prairie voles [42–45]. In the current study, we used this paradigm to pre-screen our male subjects. Subsequently, these pair-bonded males were randomly assigned into one of two experimental groups that were either separated from or continuously housed with their partner for 4 wk to evaluate the impact that this form of social loss on anxiety-like, depression-like, and social behaviors. In addition, we investigated the changes that manifest in body weight, circulating hormones (Oxt, AVP, and corticosterone), and neurochemical systems sensitive to disruptions to the social environment (i.e., Oxt, AVP, DA, and CRH) in brain regions that synthesize these neurochemicals, including the paraventricular and supraoptic nucleus of the hypothalamus (PVN and SON, respectively), ventral tegmental area (VTA), and rostral zona incerta (ZIR). As 4 wks of partner separation led to disruptions in behaviors associated with pair-bonding, we established a second cohort of males that were paired or separated from their female partner for 2 or 4 wk2 to examine the effect of separation durations on partner preference expression. We predict that partner loss will induce a state of distress in male prairie voles that should manifest in increased anxiety-like and depression-like behaviors and physiology without impacting bond-related behaviors.
2. Materials and methods
2.1 Subjects
Male prairie voles (M. ochrogaster) were descended from populations in southern Illinois and captive-bred at Florida State University. Voles were weaned on postnatal day 21 and housed with a same-sex conspecific in plexiglass cages (29 L × 18 W × 13 H cm) containing cedar chip bedding with food and water ad libitum. Colony rooms were maintained on a 14L:10D photoperiod (lights on at 0700 h) and at a temperature range of 21±1 °C. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Florida State University.
2.2 Experimental procedure
2.2.1 Experiment 1: Behavior and neurochemistry after 4 wk partner loss
Sexually naïve males were weighted and paired with an ovariectomized, estrogen-primed female for 24 h then tested in a 3-h partner preference test (PPT), see details below. Males that displayed a partner preference were randomly divided into paired (n = 6) or separated (n = 7) group. Separation involved removing the male from the home cage and female partner and housing it in a new cage, identical to the size of the home cage, alone for 4 wk. In contrast, paired males were moved along with their female partner to a novel cage, similar to the separated males, and housed with their partner for the duration of the experiment. After 4 wk, an array of behavioral testing was conducted on males from both housing conditions to evaluate the impact of partner loss on anxiety-like, depression-like, and social behaviors (see below). Subjects received two behavioral tests each day with the morning test starting at 0900 h and the afternoon at 1500 h. Tested were conducted in the following order: Day 1 - open field (OF), light-dark box (LDB); Day 2 - affiliation test (AFF), resident-intruder test (RIT); Day 3 - elevated plus maze (EPM), and forced swim (FS). All tests were performed in an isolated behavior room maintained under similar temperature- and light-controlled environmental conditions as the colony rooms. To adapt to the environment, animals were brought to the testing room an hour before testing. Behaviors were videotaped and scored later by a single trained observer blind to condition using a computer-assisted data acquisition system (J-watcher, http://www.jwatcher.ucla.edu). For all tests, the light in the room measured ~ 300 l×, except LDB test during which the light in the room measured 850 l×. Subjects were returned to their home cages immediately after each behavioral test. The day after the last behavioral test (i.e. FS), subjects were perfused, and brains were harvested and stored at −80°C until processed.
2.2.2 Experiment 2: Partner preference and physiology during partner loss
A second cohort of males were established using the same paradigm as Experiment 1 in which males cohabitated with a female for 24 h then were tested in a 3-h PPT. Thereafter, they were randomly divided into 2 wk paired (n = 6), 2 wk separated (n = 6), 4 wk paired (n = 6), or 4 wk separated (n = 5) groups. Blood was collected and body weights were taken after 24 h, 2 wk, and 4 wk of separation, or time-matched for paired males (see details below). Males were tested for partner preference behavior using the PPT at the end of 2 wk or 4 wk of separation or pairing.
2.3 OF
Using an established method [47], the 10-min OF test was conducted to evaluate exploratory and anxiety-like behaviors. Briefly, the plastic apparatus (56 L × 56 W × 20 H cm) was divided into 16 squares each measuring 14 cm2 with a visual line grid. Each subject was placed into the center of the OF, and its behaviors were videotaped. Subsequently, anxiety-like behaviors (frequency of center entries and duration spent in the center or corners) and an index for locomotion (frequency of line crosses) were recorded.
2.4 LDB
The 15-min LDB test was conducted to evaluate anxiety-like behaviors. The LDB apparatus consisted of two plastic cages (29 L × 18 W × 13 H cm) that were visually distinct (white vs. black) and connected to one another by a hollow tube (16 L × 7.5 radius cm). Subjects were placed in the dark box facing away from the opening. The amount of time spent in each cage and frequency of cage crosses were quantified.
2.5 AFF
The 60-min AFF test was conducted to evaluate social affiliative behaviors toward an unfamiliar opposite-sex conspecifics [47]. Briefly, the testing apparatus consisted of two plastic cages (29 L × 18 W × 13 H cm) connected by a hollow tube (16 L × 7.5 radius cm). One cage remained empty, and the other cage contained a loosely tethered stimulus animal (a unilateral ovariectomized/contralateral tubal ligated, estrogen-primed adult female at approximately 120 days of age). Subjects were placed in the empty cage facing away from the opening. A series of light beams across the tube and a customized computer program automatically recorded the duration spent in each cage. Affiliative (duration of side-by-side contact) and offensive aggressive (attacks, bites, and chases) behaviors were quantified by a trained observer blind to treatment.
2.6 RIT
The 10-min RIT was conducted to assess intruder-directed aggression toward an unfamiliar same-sex conspecific [43, 48–50]. Twenty-four h before the RIT (immediately following the LDB on Day 2), subjects, along with their partner if in the paired condition, were housed in a large clean cage (45 L × 25 W × 20 H cm) to allow for more movement during the RIT. On the test day, males were allowed to acclimate for 10 min in their home cage after their partner was removed, then the intruder was placed in the cage. Behaviors were recorded and scored for the duration and frequency of offensive aggression (attacks, bites, and chases), social investigation (nose and anogenital sniffing), and affiliative physical contact (side-by-side contact and allogrooming).
2.7 EPM
The 5-min EPM test was conducted to assess anxiety-like behaviors [32, 51]. The testing apparatus was elevated 45 cm off the ground and consisted of two open arms (35 L × 6.5 W cm) and two closed arms (35 L × 6.5 W × 15 H cm) that crossed in the middle. Subjects were placed in the center, facing a closed arm, and its behaviors were videotaped. Several behaviors were quantified by a trained observer, blind to the treatment, for anxiety-like responses (latency to enter the open arm, time spent in open and closed arms, and percentage of open arm entries vs. total arm entries, and frequency of dipping head from open arms) and locomotor activity (total arm entries).
2.8 FS
The 5-min FS test was conducted to assess depression-like behavior [33]. A clear tank (45 L × 25 W × 20 H cm) was filled with tap water (23 ± 1 °C) to a depth of 13 cm. Passive-stress coping/depression-like behaviors (latency to first immobility, frequency of immobility bouts, and the total immobility duration) were quantified by a trained observer blind to treatment.
2.9 PPT
The 3-h PPT was an established behavioral test for pair bonding [50, 52–55]. Briefly, the three-chamber apparatus consisted of a neutral cage (29 L × 18 W × 13 H cm) joined by plastic tubes (16 L × 7.5 radius cm) to two identical cages, each housing a stimulus vole. Male subjects were free to move throughout the apparatus; the stimulus voles were loosely tethered within separate cages to prevent direct contact with each other. During the 24 h, 2 wk, and 4 wk PPT, the familiar partner (the female that had previously been housed with the subject) and an opposite-sex conspecific stranger (a female that had not previously encountered the subject) were used as stimulus voles. Stimulus females were unilateral ovariectomized/contralateral tubal ligated, estrogen-primed. During the PPT, the amount of time the male spent side-by-side contact with the partner and the stranger was quantified.
2.10 Tissue preparation
Subjects were deeply anesthetized with sodium pentobarbital (0.1 mg/10 g body weight) and then transcardially perfused through the ascending aorta with 100 ml 0.9% saline, followed by 100 ml 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Brains were harvested, postfixed for 2 hours in 4% paraformaldehyde, and then stored in 30% sucrose in PBS at 4°C. Brains were cut into 40 µm coronal sections using a sliding microtome.
2.11 CRH, Oxt, AVP, and TH immunohistochemistry
Four sets of floating sections at 240 µm intervals were processed for CRH, Oxt, AVP, and tyrosine hydroxylase (TH) immunostaining using previously established protocols for light microscopy [49, 56]. Briefly, sections were rinsed in 0.1 M PB (PB) for 15 min3; incubated in 1% NaHBO4 in PB for 10 min; rinsed in PB for 15 min; incubated in 3% H2O2 in PB in order to block endogenous peroxidase activity; rinsed in PB for 15 min; and blocked in 10% normal goat serum (NGS) in 0.5% triton-PB (TPB). Each set of sections was incubated with either CRH polyclonal goat IgG antibody (1:5k, Thermo Scientific, Inc., Rockford, IL), Oxt polyclonal rabbit IgG antibody (1:5k, gift from Dr. M. Morris, Wright State University), AVP polyclonal rabbit IgG antibody (1:8k, Millipore Corporation, Temecula, CA), or TH polyclonal rabbit IgG antibody (1:5k, Millipore Corporation, Temecula, CA) in 2% NGS in TPB overnight at 4 °C. Thereafter, sections were rinsed in TPB for 15 min and incubated in a solution containing biotinylated goat anti-rabbit secondary antibody (for Oxt, AVP, CRH, or TH) (1:300 Vector Laboratories, Inc., Burlingame, CA) for 2 h and then in ABC complex (Vector Laboratories, Inc.) for 90 min. Lastly, sections were stained with Nickel-DAB (Vector Laboratories, Inc.) and mounted on slides and cover-slipped. In order to control for variability, all sections for each staining were processed simultaneously. All microscope slides were coded to disguise the treatment condition until data quantification was completed. CRH, AVP, and Oxt densities were measured in the PVN and SON, and TH density was measured in the ZIR and VTA. Density for all stained cells were quantified across three sections and averaged for each vole.
2.12 Blood and body weight collection
Prairie voles were weighed then anesthetized using isoflurane via a precession vaporizer at 1–4% isoflurane with 0.5–3 liters/min oxygen, delivered via inhalation. Before the vole was placed in the isoflurane induction chamber, 1 drop of proparacaine hydrochloride ophthalmic solution (0.5%) was placed into the eye to be bled for post-procedural analgesia. Blood (animal’s body weight [gm] × 0.01 = maximum volume [ml]) was collected from the retro-orbital (RO) sinus with a heparinized end-to-end capillary within a minute by a skilled individual between 0900–1000 h. Blood was immediately transferred into a chilled microcentrifuge vial containing EDTA (4 µl per 100 µl blood) and stored on ice. Blood was then centrifuged at 6000 rpm for 15min at 4°C, plasma will be aspirated, and then centrifuged at 6000 rpm for 10min at 4°C. The second centrifugation is important to reduce damage during incubation and from preventing particular elements that during thawing may be disrupted releasing enzymes that could damage hormones in the sample [57]. Plasma was separated from sediment and transferred to a microcentrifuge vial and stored at −80°C until assayed.
2.13 Hormonal assays
Plasma Oxt (1:8), AVP (1:4), and corticosterone (1:1000) were each measured (in duplicates) using commercial kits previously used and validated in prairie voles [32, 40, 51, 58, 59]. The detecting limits of the kits were 11.7pg/mL for Oxt (ADI-900-153, Enzo Life Sciences, Farmingdale, NY), 3.39 pg/mL for AVP (ADI-900-017, Enzo Life Sciences, Farmingdale, NY), and 7.7 ng/mL for corticosterone (07120102, MP Biomedicals, Orangeburg, NY). The intra-assay and inter-assay coefficient of variation (CV) were 2.55% and 5.01% for Oxt and 3.98% and 3.82% for AVP, respectively. The intra-assay for corticosterone was 2.54%, and no inter-assay CV was calculated for corticosterone samples as all samples were measured in a single assay.
2.14 Data analysis
Data were analyzed using IBM SPSS Statistics 19 (SPSS, Inc., an IBM Company) and were expressed as mean ± SEM. Behaviors displayed during the PPT were analyzed with a mixed-model ANOVA with social condition (2 wk paired, 2 wk separated, 4 wk paired, vs 4wk separated) and female stimuli (partner vs stranger) as the two factors. In addition to the raw data, several variables were calculated for body weight and plasma CORT, Oxt, and AVP concentrations to determine the change in these measures over time during the partner separationor pairing period (measured at 24 h, 2 wk, and 4 wk). These variables were calculated using standard equations previously described [60, 61] and include: Area under the curve with respect to ground (AUCG or area under the curve with respect to zero), Area under the curve with respect to increase (AUCI or area under the curve with respect to the value at 24 h separation), and reactivity (peak value minus the the value at 24 h separation)4. Significant group differences (p < 0.05) were further assessed with a Student–Newman–Keuls post-hoc test. All other behaviors, hormonal, body weight, and neurochemical measures were analyzed using Independent Sample’s T-test (4 wk paired vs 4wk separated). All alpha levels were set at p < 0.05.
3. Results
3.1 Experiment 1: Behavior and neurochemistry after 4 wk partner loss
3.1.1 Anxiety-like behaviors
Anxiety-like behaviors in the EPM and LDB tests were elevated in the 4 wk separated males as compared to the 4 wk paired males (Fig 1). Specifically, males separated from their female partner for 4 wk delayed their entry into the open arm (t11 = 2.42, p < 0.05, Fig 1A), had less percentage of open arm entries (t11 = 2.42, p < 0.05, Fig 1B), and had more percentage of time spent in the closed arms (t11 = 2.53, p < 0.05, Fig 1C). In addition, separated males spent significantly more time in the dark box (t10 = 3.13, p < 0.05) and less time in the light box (t10 = 3.223, p < 0.01) during the LDB test compared to paired male (Fig 1E). No behavioral differences between groups were observed during the OF test (center entry: paired 16.17 ± 3.55, separated 17.00 ± 5.49; center duration: paired 114.17 ± 25.44 s, separated 82.92 ± 16.28 s). No differences were observed in locomotor activities during the EPM (Fig 1D), LDB (Fig. 1F), or OF tests (line crosses: paired 262.50 ± 30.08, separated 249.17 ± 52.86).
Figure 1.
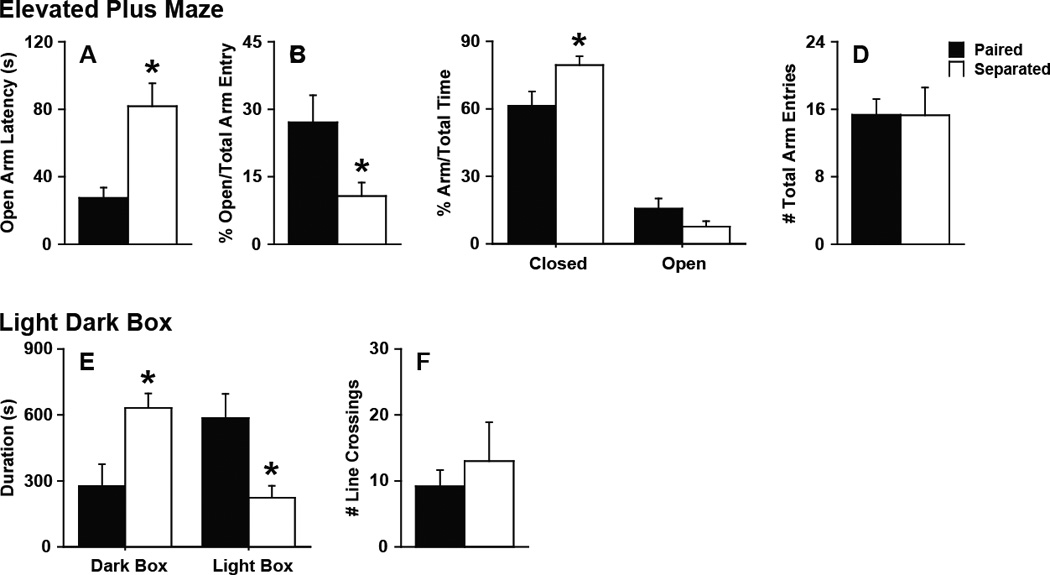
Anxiety-like behaviors. (A–D) Elevated plus maze (EPM) test. Partner loss led to a (A) delay in the latency of males to enter the open arm, (B) decrease in the percentage of open arm entries vs total arm entries by the males, and (C) increase in the percentage of time the males spent in the closed arm in the EPM test. D, No effects on total arm entries were observed. (E–F) Light-dark box (LDB) test. E, Partner loss led to an increase in time spent in the dark box and a decrease in time spent in the light box in the LDB test. F, No effects on line crossings were observed. Bars labeled with asterisks indicate a significant difference between the separated males (Separated) and paired males (Paired) for a specific measure as determined by Independent Sample’s T-test (p < 0.05). A–F, Data are expressed as mean ± SEM.
3.1.2 Depression-like behaviors
Four weeks of separation from the partner also affected depression-like behavior. Separated males became immobile more quickly than paired males during the FS test (t11 = 2.85, p < 0.05, paired 115.08 ± 20.46 s, separated 40.80 ± 16.59 s), though other measures of immobility such as frequency (paired 9.33 ± 5.14, separated 12.57 ± 3.34) and duration (paired 18.17 ± 8.31 s, separated 24.50 ± 14.88 s) were similar among all males.
3.1.3 Social behaviors
The behavioral phenotype for a pair-bonded male prairie vole includes a selective preference and affiliation toward their female partner and robust aggression toward intruding male and female stranger conspecifics. Yet, males separated from their female partner for 4 wk did not display these discriminating social behaviors. While males separated for 4 wk interacted with the unfamiliar female conspecific for a similar duration as the paired males during the AFF test (Fig 2A), the separated males were significantly less aggressive (t11 = 3.46, p < 0.05) and displayed more non-agonistic body contact (t11 = 4.31, p < 0.005) with the stranger female than paired males (Fig 2B). A similar behavioral pattern emerged during the RIT, separated males were more affiliative (frequency: t11 = 5.01, p < 0.001; duration: t11 = 3.42, p < 0.05) and less aggressive (frequency: t11 = 3.45, p < 0.005; duration: t11 = 6.09, p < 0.005) than paired males (Fig 2C–D). In addition, separated males displayed more nose-to-nose (frequency: t11 = 4.01, p < 0.005; duration: t11 = 2.73, p < 0.05) and nose-to-anogenital (frequency: t11 = 3.22, p < 0.01) olfactory investigation than paired males (Fig 2E–F).
Figure 2.
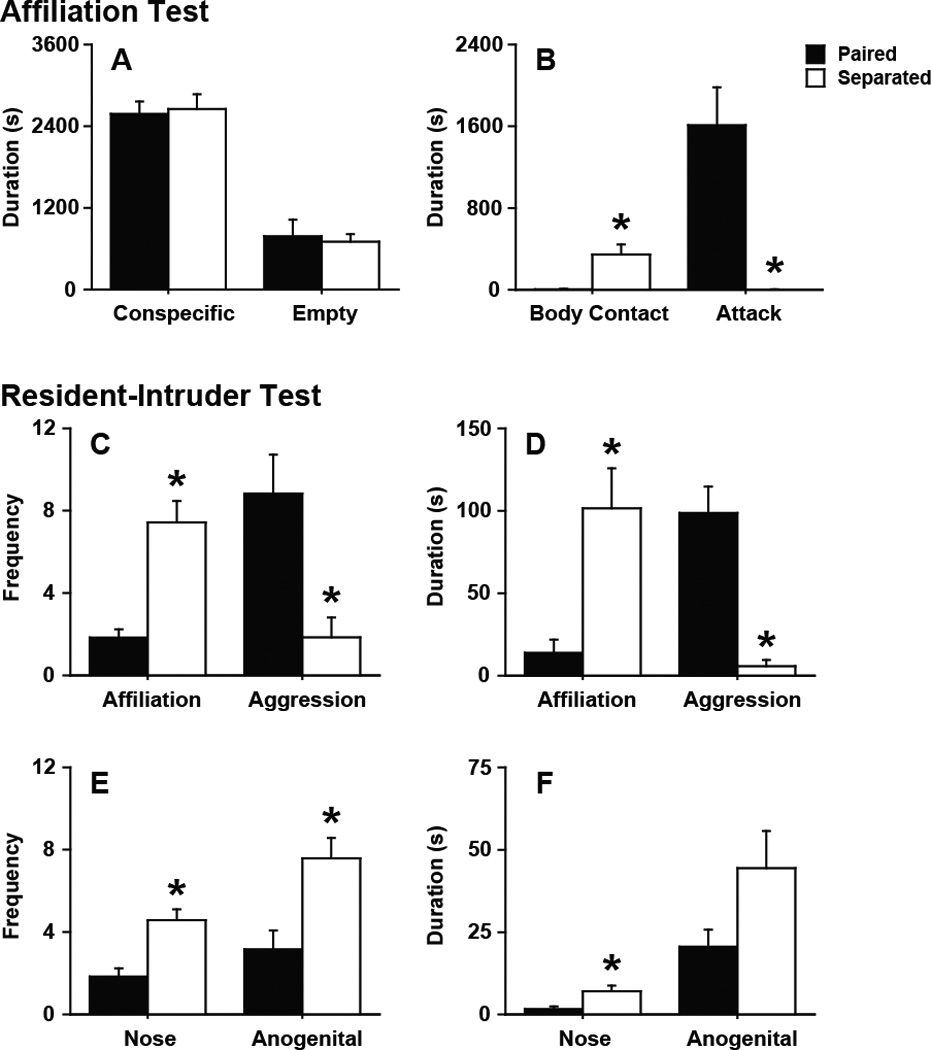
Social affiliation and aggression behaviors. (A–B) Affiliation (AFF) test. Partner loss (A) did not affect the amount of time males spent in either the conspecific or empty cage, (B) but it did led to an increase in non-agonistic body contact and a decrease in attack behavior. (C–F) Resident-intruder test (RIT). Partner loss (C–D) increased total affiliation and decreased aggression in both frequency and duration and (E–F) increased nose-to-nose (Nose) olfactory investigation frequency and duration and nose-to-anogenital (Anogenital) olfactory investigation frequency. Bars labeled with asterisks indicate a significant difference between the separated males (Separated) and paired males (Paired) for a specific measure as determined by Independent Sample’s T-test (p < 0.05). A–F, Data are expressed as mean ± SEM.
3.1.4 Neurochemistry
Partner loss affected the cell density of select neuropeptides. Figure 3 shows an example of CRH-immunoreactivity (-ir), Oxt-ir, AVP-ir, and TH-ir density in brain regions that synthesize these neurochemicals. In separated males, CRH-ir density was significantly more in the PVN (t11 = 2.23, p < 0.05) but not to the SON compared to paired males (Fig 4A). There were more Oxt-ir cells in the PVN (t11 = 2.97, p < 0.05) and SON (t11 = 3.40, p < 0.01) in separated males compared to paired males (Fig 4B). In addition, separated males has more AVP-ir cells in the PVN (t11 = 2.41, p < 0.05) but not the SON compared to paired males (Fig 4C). No group difference for TH-ir, a rate limiting enzyme for DA synthesis, density was observed (Fig 4D).
Figure 3.
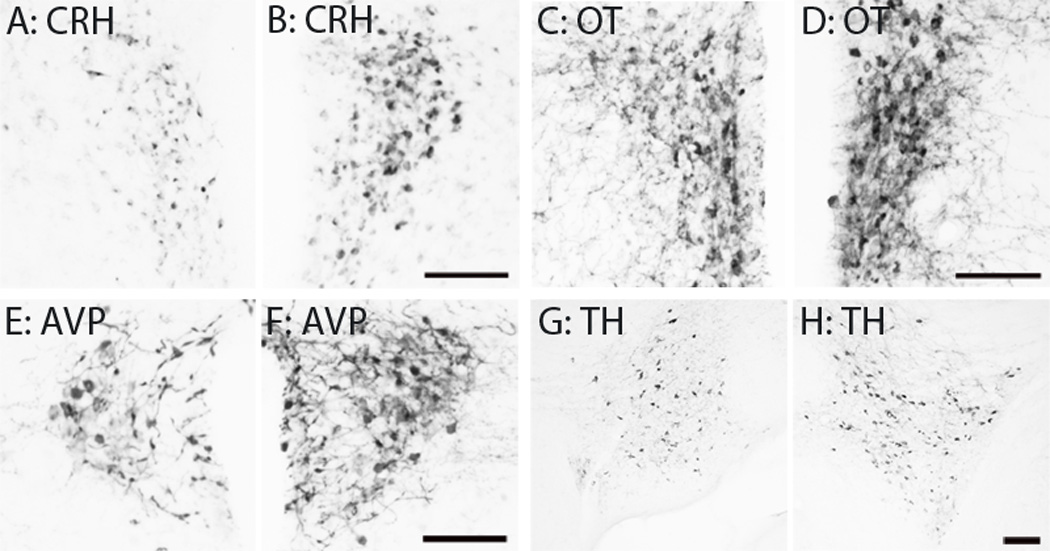
Immunohistochemistry Representative photo images illustrating immunoreactive staining of CRH-ir (A–B), OT-ir (C–D) and AVP-ir (E–F) cells in the PVN as well as TH-ir cells in the VTA (G–H) from male voles that were either paired with (A, C, E & G) or separated from (B, D, F & H) their female partner. Scale bar = 100µm.
Figure 4.
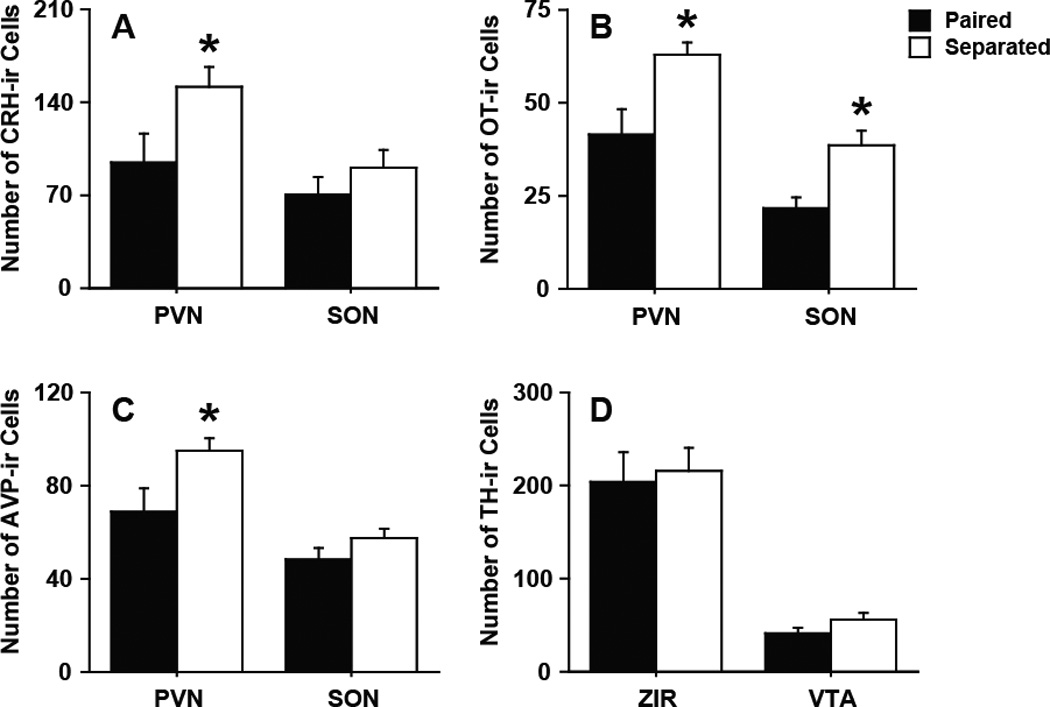
Immunohistochemistry. Partner loss (A) decreased CRH-ir density in the PVN but not the SON, (B) decreased Oxt-ir density in the PVN and SON, (C) decreased AVP-ir density in the PVN but not the SON, and (D) had no effect on TH-ir density in the ZIR or VTA. Bars labeled with asterisks indicate a significant difference between the separated males (Separated) and paired males (Paired) for a specific measure as determined by Independent Sample’s T-test (p < 0.05). A–D, Data are expressed as mean ± SEM.
3.2 Experiment 2: Partner preference and physiology during partner loss
3.2.1 Partner preference
Male partner preference behavior during the PPT was dependent on the duration of their separation from their female partner (F3,19 = 3.66, p < 0.05, Fig 5). Males separated for 4 wk did not display a partner preference during the PPT such that these males spent the same amount of time in side-by-side contact with the stranger female as their original female partner. By comparison, all other males (i.e., 2 wk paired, 2 wk separated, and 4 wk paired males) spent significantly more time during the PPT in side- by-side contact with their female partner compared to the stranger female.
Figure 5.
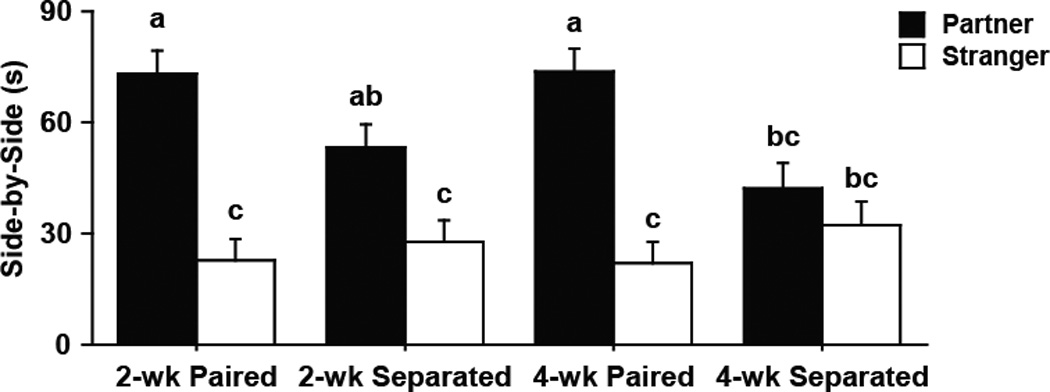
Partner preference test (PPT). Paired males displayed a preference for their familiar partner (black bars) over stranger females (white bars) after both 2 wk or 4 wk pairing. However, males separated from their female only displayed a partner preference after 2 wk separation, and showed no preference after 4 wk separation. Bars labeled with different letters differ significantly by SNK's post-hoc test in which a significant interaction was detected in the mixed-model ANOVA (p < 0.05). Data are expressed as mean ± SEM.
3.5 Body weight and hormonal concentrations
Body weight and the concentrations of circulating hormones were altered by partner loss (Table 1). Specifically, separated males gained more weight over the 4 wk period compared to the paired males, as indicated by the significant difference in body weight reactivity (t9 = 4.71, p < 0.001) and AUCi (t9 = 4.00, p < 0.005). Still, no difference in raw body weight between separated and paired males were observed after 24 h, 2 wk, or 4 wk of housing. In addition, separated males had elevated plasma corticosterone concentrations starting after 24 h separation (t9 = 2.41, p < 0.05) and persisting through 2 wk (t9 = 2.84, p < 0.05) and 4 wk (t9 = 3.86, p < 0.005) separation. This was further highlighted with separated males having a significantly greater AUCG value, indicating a greater total hormonal output, compared to paired males (t9 = 4.66, p < 0.001). No group differences were observed for any of the values measured for plasma Oxt or AVP concentrations throughout the 4 wk testing period.
Table 1.
Weight and hormonal measures after 4 weeks pairing or separation
| Measurement | Paired males | Separated males | |
|---|---|---|---|
| Body Weight (g) | 24 h housing | 36.83 ± 1.75 | 33.86 ± 0.97 |
| 2 wk housing | 36.67 ± 1.57 | 35.48 ± 1.03 | |
| 4 wk housing | 36.35 ± 1.82 | 36.5 ± 1.11 | |
| Reactivity | −0.45 ± 0.44 | 2.64 ± 0.49* | |
| AUCG | 146.52 ± 6.68 | 141.32 ±4.05 | |
| AUCI | −0.82 ± 1.08 | 5.88 ± 1.30* | |
| CORT (ng/mL) | 24 h housing | 516.60 ± 53.54 | 689.07 ± 44.44* |
| 2 wk housing | 615.75 ± 89.22 | 909.16 ± 33.56* | |
| 4 wk housing | 294.12 ± 41.05 | 718.77 ± 110.87* | |
| Reactivity | −149.96 ± 128.27 | 184.74 ± 101.13 | |
| AUCG | 2042.22 ± 196.29 | 3226.14 ± 146.45* | |
| AUCI | −24.18 ± 136.05 | 469.88 ± 234.27 | |
| Oxt (pg/mL) | 24 h housing | 525.45 ± 59.03 | 476.77 ± 63.38 |
| 2 wk housing | 469.59 ± 72.85 | 370.14 ± 24.51 | |
| 4 wk housing | 470.35 ± 90.21 | 386.11 ± 41.29 | |
| Reactivity | −74.98 ± 98.91 | −129.99 ± 84.53 | |
| AUCG | 1934.97 ± 239.75 | 1603.16 ± 89.21 | |
| AUCI | −166.81 ± 199.23 | −303.90 ± 210.07 | |
| AVP (pg/mL) | 24 h housing | 347.44 ± 105.72 | 134.36 ± 43.96 |
| 2 wk housing | 313.59 ± 158.16 | 308.24 ± 127.73 | |
| 4 wk housing | 396.40 ± 132.12 | 123.10 ± 17.23 | |
| Reactivity | 93.12 ± 237.62 | 200.75 ± 141.37 | |
| AUCG | 1313.12 ± 403.39 | 847.05 ± 263.46 | |
| AUCI | 154.98 ± 630.99 | 417.11 ± 308.36 | |
Note.
Indicates a significant group difference, p < 0.05. Body weight and hormonal concentrations were measured in male prairies after either four weeks of pairing (n=6) or separation (n=5) from female partner. Hormones include corticosterone (CORT), oxytocin (Oxt), and vasopressin (AVP). Reactivity = greatest deviation from the 24 h housing condition (value measured during 2 or 4 wk housing condition) minus value at 24 h housing condition. Area Under Curve-Ground (AUCG) = area under the curve with respect to zero, and AUC-Increase (AUCI) = area under curve with respect to the value at 24 h separation.
4. Discussion
Bond loss in humans is accompanied by negative emotional states and can catalyze much of the depression and anxiety symptomology that follows such social disruption [63]. Recent studies indicate male prairie voles express many similar depressive symptomology to humans undergoing social loss [40, 41]. We extend this research to evaluate the impact of bond loss in male prairie voles on behaviors and neurochemical systems that are necessary to maintain a social bond, and in doing so, define the prairie vole as a valuable model for studying the characteristics and neurobiology of bond loss. Consistent with our hypothesis, male prairie voles separated from their female partner over 4 wk displayed anxiety-like and mild depressive-like symptomology that has been associated with social isolation in many gregarious species [33, 64–68] but has not been observed in male prairie voles separated from same-sex conspecifics, with the exception of depressed sucrose intake [37, 40]. Rather unexpectedly, certain pair bonding behaviors were absent after 4 wk of separation, though remained after 2 wk of separation. These changes to behavior were coupled with altered expression of CRH, Oxt, and AVP—neurochemicals involved with various aspects of social bonding and distress in prairie voles.
One limitation of the current study is that we did not evaluate the impact of social isolation separately from bond loss. However, previous literature has systematically weighed the sex-specific impact that social isolation has on prairie vole behavior, physiology, and neurochemistry. Both adolescent and adult female prairie voles isolated from a same-sex sibling or conspecific display an increase in behaviors relevant to depression and anxiety, observable after 4 days and up to 6 weeks of isolation [33–39]. In contrast, male prairie voles response to social isolation in an age-dependent manner, with the exception of depressed sucrose intake [37, 40]. Males chronically isolated during adolescence (6 wk post-weaning) displayed more anxiety-related behaviors and had enhanced gene expression for AVP, Oxt, CRF, and TH in the PVN [47]. In contrast, adult male prairie voles separated from same-sex conspecifics (for 5 days to 4 wk) do not display robust behavioral defects or changes to body weight, peripheral hormones (i.e., plasma Oxt, AVP, ACTH, testosterone, and corticosterone), or neurochemical expression (i.e., Oxt-ir and CHR-ir density in the PVN) [37, 40, 58, 69]. An ecological explanation could be chronic social isolation is an aberrant experience for juvenile male prairie voles, who remain in the parental nest surrounded by extended family members [70, 71], but a normal aspect of adult life for male prairie voles, as 45% of adult males are not a part of a resident group during the breeding season which is approximately 50–75% of the year [72–74].
Despite a relative resilience to social isolation, after female partner loss, adult male prairie voles develop symptoms resembling bond loss in humans. Bosch and colleagues [40] initially observed these effects when evaluating the impact that separation from a same- versus opposite-sex conspecific has on passive coping behavior and CRH expression in male prairie voles [75–77]. Our data expand upon these findings to demonstrate that male prairie voles experiencing partner loss also display increased anxiety-like behaviors in the EPM and LDB tests, greater body weight gains, augmented cell density for CRH-ir, Oxt-ir, and AVP-ir in the PVN, and increased plasma corticosterone concentrations with no change to circulating Oxt or AVP levels. Of course, these data do not account for any impact that previous test history had on the outcome of subsequent tests, an issue to be addressed in future research as several of the behavioral tests were conducted with an inter-test interval that was less than 24 h (e.g., OF and EPM; LDB and FS; AFF and RIT). Still, it is intriguing to note that people grieving the loss of a significant other are reported to be at risk for suffering from major depressive disorder, post-traumatic stress, panic disorders, and general anxiety disorder [12–16], and clinical evidence has associated these various mental health disorders with dysregulated CRH, Oxt, and AVP systems [78–82]. Thus, bond loss in male prairie voles provides a model to characterize the neuroendocrine mechanisms governing the impact of bereavement on normal behavioral routines and emotional states.
Prairie vole pair bonding behaviors have been extensively characterized in the laboratory. Following extended cohabitation, male prairie voles display a selective social preference for their familiar partner [52, 70, 72, 73, 83–85] and an increased aggression toward intruding conspecifics [43, 44, 48–50, 56, 86]. In our study, males displayed a partner preference after 2 wk of separation from their female partner, but not after 4 wk of separation. Previous research has determined that bonded males will maintain their partner preference after 5 days of separation from a female partner [40]. This coincides with field observations that indicate after pair bonding, over 75% of prairie voles will maintain that bond throughout life [70, 87], and even after the death of or abandonment by the female partner, roughly 80% of males never acquire new mates [73]. Based on our data, pair status after partner loss may not be affected by long-term maintenance of pair bonding behavior, given partner preference and intruder aggression were extinguished after 4 wk of partner loss. Thus, our data are the first evidence of such time-dependent dissolving of partner preference behavior in prairie voles.
Pair bonding that induces partner preference also induces selective intruder-directed aggression [43, 86, 88], which serves as a territorial and mating-guarding response [89]. Males separated from their female partner for 4 wk displayed low aggression and high affiliation toward unfamiliar female conspecifics in a neutral environment and unfamiliar male conspecifics in the home cage, a behavioral pattern resembling the non-discriminating sociality of sexually naïve, non-bonded male prairie voles [90]. Isolation-induced aggression has been observed in several species [91–94], including in female prairie voles [37]. However, adult male prairie voles isolated from a same-sex conspecific for 4 wk do not display altered aggressive behavior during a RIT compared to males housed with a same-sex conspecific [37]. Therefore, the decrease in aggressive behavior during the RIT observed in the current study is unlikely a result of social isolation but rather due to a dissolving of the pair-bond.
Pair bonding in prairie voles reinforces social behavior through employment of a number of neurochemical systems including CRH, Oxt, AVP, and DA. For example, male vole bond formation is promoted via stress-induced hypothalamic-pituitary-adrenal (HPA) axis activity, and more specifically, increased CRH neural activity [95–97]. CRH treatments can facilitate partner preference formation in male prairie voles [98]. However, blocking CRH receptors pharmacologically does not prevent the display of partner preference after it has formed, even in male prairie voles separated from their female partner [40]. Thus, the increase in CRH-ir in the PVN associated with bond loss in this study may not affect the lack of partner preference behavior. Still, separation from same-sex conspecifics does not affect CRH-ir or CRH mRNA in the PVN in male prairie voles [37, 40]. For that reason, the increase in CRH-ir cells in the PVN in males may be facilitated by the loss of the partner, rather than simply the social isolation. Individuals with depression experience increased cortisol in circulation [99, 100], CRH-expressing neurons in the PVN in depressed individuals [78], and synergetic interaction between CRH and AVP [78–80, 101]. Therefore, increased circulating corticosterone and increased CRH-ir and AVP-ir density in the PVN could be ascribed to chronic stress and hyperactivity of the HPA axis in male prairie voles experiencing partner loss.
In addition, social separation from a same-sex conspecific does not affect Oxt-ir or AVP-ir density in the PVN in male prairie voles [35, 37], but our data indicate partner loss increases Oxt-ir and AVP-ir density in the PVN, without effecting DA expression, as measured by TH-ir cell density, in the VTA or ZIR. AVP and Oxt in the PVN are released into limbic areas, either via afferent projections or diffusion through extracellular fluid, where their effects on male prairie vole pair bonding behavior has been reported [28], including partner preference [102, 103] and intruder-directed aggression [43, 48–50]. Still, Oxt-ir and AVP-ir density do not reflect species differences in social or mating systems in the Microtus genus, rather it is changes to receptor distribution and activity [56]. An increase in the number of cells that are immunoreactive for these neuropeptides could translate to a decrease in release and limited receptor activity, rather than an increase in production. If this is the case, a change in Oxt-ir and AVP-ir density in the PVN, spurned by partner loss, could reflect a neurochemical mechanism of pair bond disruption. Furthermore, an increase in Oxt-ir and AVP-ir in the PVN and Oxt-ir in the SON could reflect a significant surge of the peripheral release of these neuropeptides as a portion of each is released from these brain regions into systemic circulation through the posterior pituitary [104, 105]. However, plasma Oxt and AVP concentrations throughout the 4 wk of partner loss were not altered, similar to chronic social isolation [37, 58]. Thus, any effects of the Oxt and AVP systems on male social behaviors would have arisen from a central mechanism.
Ultimately, it may be more adaptive, than maladaptive, to relinquish versus maintain a bond after social loss, or permanent social separation [106, 107]. This is highlighted by the consequences of complicated grief and other mental disorders that may arise after lingering intense attachments after social loss [12–16]. It remains unclear which underlying processes are related to adaptive or maladaptive bereavement outcomes. Prairie voles have already been used as a model to evaluate the effects of social isolation. While this form of social stress is adequate to induce a distressing state for female prairie voles, males display more resilience. Two recent studies demonstrated that male prairie voles are negatively affected by partner loss, displaying similar depressive-like symptoms [40, 41]. Our study attempts to expand among these findings to evaluate a broader array of behaviors, physiology, and neurochemistry that may be susceptible to effects of partner loss. As a result, we have observed male prairie voles do in fact display maladaptive behaviors, reflective of depression or anxiety symptomology, as well as experience a time-dependent dissolving of their social bonds, all concomitantly occurring with neurochemical changes. Together, our data provide an evaluation of the behavioral consequencesand neurochemical correlations associated with loss of social partner, and further research using this model should render a better understanding of the neurobiology underlying partner loss and grief.
Highlights.
Male prairie voles express bond loss via increased stress behavior and physiology
Partner loss disrupt bond-related behavior in a time-dependent manner in male voles
Partner loss alter neuropeptide systems involved in vole pair bonding
We review the distinct effects of social isolation and bond loss in voles
Acknowledgement
This work was supported by the Natural Science Foundation of China NSFC 30870370 to PS, National Science Foundation Graduate Research Fellowship and National Institutes of Health grant NIMHF31-095464 to AS, and the NIH grants NIMHR01-058616 and NIMHR01-89852 to ZW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
It is considered normal for individuals who lost a loved one to experience intrusive thoughts of the deceased, sadness, and yearning for reunion, all common symptoms of grief [7] Bonanno GA, Wortman CB, Lehman DR, Tweed RG, Haring M, Sonnega J, et al. Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. J Pers Soc Psychol. 2002;83:1150-64.. However, individuals who fail to dampen symptoms within 18 months may be diagnosed with complicated grief [8] Zisook S, Simon NM, Reynolds CF, 3rd, Pies R, Lebowitz B, Young IT, et al. Bereavement, complicated grief, and DSM, part 2: Complicated grief. J Clin Psychiatry. 2010;71:1097-8.
Previous literature has demonstrated that after 24 h cohabitation, female prairie voles maintain a partner preference over an opposite-sex stranger for at least 2 wk, even in the absence of further exposure to the male mate [46] Insel TR, Hulihan TJ. A gender specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behavioral Neuroscience. 1995;109:782-9.. Bosch and colleagues [38] demonstrated that 5-day paired males display a partner preference even after 5 days of separation from their female partner. Our pilot experiments confirmed that male prairie voles sustain a selective partner preference for 2 wk following the initial 24h cohabitation, either with or without further exposure to their female mate (data not shown). Therefore, we utilized this 2 wk cohabitation/separation paradigm as a temporal control in the current study.
The rinses consisted of three 5 min washes, totaling 15 min. The buffer was transferred after every rinse cycle.
AUC and reactivity are common computations in endocrinological and neuroscience research to dissect information about changes in a biological marker that can be gleaned from repeated measurements over a specific period [60] Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916-31, [61] Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651-9, [62] French JA, Smith AS, Birnie AK. Maternal gestational androgen levels in female marmosets (Callithrix geoffroyi) vary across trimesters but do not vary with the sex ratio of litters. Gen Comp Endocr. 2010;165:309-14.. AUCG reflects the total body weight or amount of hormonal output of a vole during the 4 wk period. AUCI designates the sensitivity of voles to separation/pairing by emphasizing the significant change in the body weight or hormonal concentrations over the 4 wk period as it relates to the initial value after 24 h separation/pairing. Reactivity reflects the peak change in body weight or hormonal concentrations during the 4 wk separation/pairing period from the value measured after 24 h separation/pairing.
References
- 1.Flannery RB, Wieman D. Social support, life stress, and psychological distress: An empirical assessment. J Clin Psychol. 1989;45:867–872. doi: 10.1002/1097-4679(198911)45:6<867::aid-jclp2270450606>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 3.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 4.Karelina K, DeVries AC. Modeling social influences on human health. Psychosom Med. 2011;73:67–74. doi: 10.1097/PSY.0b013e3182002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maulik PK, Eaton WW, Bradshaw CP. The effect of social networks and social support on common mental disorders following specific life events. Acta Psychiat Scand. 2010;122:118–128. doi: 10.1111/j.1600-0447.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonanno GA, Wortman CB, Lehman DR, Tweed RG, Haring M, Sonnega J, et al. Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. J Pers Soc Psychol. 2002;83:1150–1164. doi: 10.1037//0022-3514.83.5.1150. [DOI] [PubMed] [Google Scholar]
- 8.Zisook S, Simon NM, Reynolds CF, 3rd, Pies R, Lebowitz B, Young IT, et al. Bereavement, complicated grief, and DSM, part 2: Complicated grief. J Clin Psychiatry. 2010;71:1097–1098. doi: 10.4088/JCP.10ac06391blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwert F, Christakis NA. Wives and ex-wives: A new test for homogamy bias in the widowhood effect. Demography. 2008;45:851–873. doi: 10.1353/dem.0.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elwert F, Christakis NA. The effect of widowhood on mortality by the causes of death of both spouses. Am J Public Health. 2008;98:2092–2098. doi: 10.2105/AJPH.2007.114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozenzweig A, Prigerson H, Miller MD, Reynolds CF., 3rd Bereavement and late-life depression: Grief and its complications in the elderly. Annu Rev Med. 1997;48:421–428. doi: 10.1146/annurev.med.48.1.421. [DOI] [PubMed] [Google Scholar]
- 12.Byrne GJ, Raphael B. The psychological symptoms of conjugal bereavement in elderly men over the first 13 months. Int J Geriatr Psychiatry. 1997;12:241–251. doi: 10.1002/(sici)1099-1166(199702)12:2<241::aid-gps590>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Byrne GJ, Raphael B. Depressive symptoms and depressive episodes in recently widowed older men. Int Psychogeriatr. 1999;11:67–74. doi: 10.1017/s1041610299005591. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen P, Weisaeth L, Heir T. Bereavement and mental health after sudden and violent losses: A review. Psychiatry. 2012;75:76–97. doi: 10.1521/psyc.2012.75.1.76. [DOI] [PubMed] [Google Scholar]
- 15.Onrust SA, Cuijpers P. Mood and anxiety disorders in widowhood: A systematic review. Aging Ment Health. 2006;10:327–334. doi: 10.1080/13607860600638529. [DOI] [PubMed] [Google Scholar]
- 16.Zivin K, Christakis NA. The emotional toll of spousal morbidity and mortality. Am J Geriatr Psychiatry. 2007;15:772–779. doi: 10.1097/JGP.0b013e318050c9ae. [DOI] [PubMed] [Google Scholar]
- 17.Chen JH, Bierhals AJ, Prigerson HG, Kasl SV, Mazure CM, Jacobs S. Gender differences in the effects of bereavement-related psychological distress in health outcomes. Psychol Med. 1999;29:367–380. doi: 10.1017/s0033291798008137. [DOI] [PubMed] [Google Scholar]
- 18.Thompson LW, Breckenridge JN, Gallagher D, Peterson J. Effects of bereavement on self-perceptions of physical health in elderly widows and widowers. J Gerontol. 1984;39:309–314. doi: 10.1093/geronj/39.3.309. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PJ, Feng Z, Raab GM. Does widowhood increase mortality risk?: Testing for selection effects by comparing causes of spousal death. Epidemiology. 2011;22:1–5. doi: 10.1097/EDE.0b013e3181fdcc0b. [DOI] [PubMed] [Google Scholar]
- 20.Kaprio J, Koskenvuo M, Rita H. Mortality after bereavement: A prospective study of 95,647 widowed persons. Am J Public Health. 1987;77:283–287. doi: 10.2105/ajph.77.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer C, Quesenberry CP, Jr, Wi S. Mortality following conjugal bereavement and the effects of a shared environment. Am J Epidemiol. 1995;141:1142–1152. doi: 10.1093/oxfordjournals.aje.a117387. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein P, Gatz M, Berg S. A twin study of mortality after spousal bereavement. Psychol Med. 1998;28:635–643. doi: 10.1017/s0033291798006692. [DOI] [PubMed] [Google Scholar]
- 23.Kato PM, Mann T. A synthesis of psychological interventions for the bereaved. Clinical psychology review. 1999;19:275–296. doi: 10.1016/s0272-7358(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 24.Jordan JR, Neimeyer RA. Does grief counseling work? Death studies. 2003;27:765–786. doi: 10.1080/713842360. [DOI] [PubMed] [Google Scholar]
- 25.Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): An animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Carter CS, DeVries AC, Getz LL. Physiological substrates of monogamy: The prairie vole model. Neurosci Biobehav R. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 27.Young LJ, Wang Z. The neurobiology of pair-bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 28.Smith AS, Lei K, Zuoxin W. Neurobiology of social attachment. In: Charney D, Buxbaum J, Sklar P, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2013. [Google Scholar]
- 29.Grippo AJ, Trahanas DM, Zimmerman IiRR, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammack SE, Cooper MA, Lezak KR. Overlapping neurobiology of learned helplessness and conditioned defeat: Implications for PTSD and mood disorders. Neuropharmacology. 2012;62:565–575. doi: 10.1016/j.neuropharm.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann ID. The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Frontiers in Neuroendocrinology. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. doi: 10.1016/j.biopsych.2013.09.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grippo AJ, Pournajafi-Nazarloo H, Sanzenbacher L, Trahanas DM, McNeal N, Clarke DA, et al. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress: The International Journal on the Biology of Stress. 2012;15:149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuroendocrinology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, et al. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci. 2013 doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gubernick DJ. Biparental care and male-female relations in mammals. In: Parmigiani S, Saal FSv, editors. Infanticide and Parental Care, Chur. Switzerland: Harwood Academic Publishers; 1994. pp. 427–463. [Google Scholar]
- 43.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 44.Insel TR, Preston S, Winslow JT. Mating in the monogamous male: Behavioral consequences. Physiology & Behavior. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- 45.Insel TR, Young L. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 46.Insel TR, Hulihan TJ. A gender specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behavioral Neuroscience. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 47.Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. P Natl Acad Sci USA. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. Journal of Comparative Neurology. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- 50.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 51.Smith AS, Lieberwirth C, Wang Z. Behavioral and physiological responses of female prairie voles to various stressful conditions. Stress: The International Journal on the Biology of Stress. 2013;16:531–539. doi: 10.3109/10253890.2013.794449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Wang Z. Nucleus accumbens dopamine and oxytocin interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 54.Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. The Journal of Neuroscience. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H, Wang J, Cacioppo JT, Glaser R, Kiecolt-Glaser JK, Malarkey WB. Chronic stress associated with spousal caregiving of patients with Alzheimer's dementia is associated with downregulation of B-lymphocyte GH mRNA. J Gerontol A Biol Sci Med Sci. 1999;54:M212–M215. doi: 10.1093/gerona/54.4.m212. [DOI] [PubMed] [Google Scholar]
- 56.Wang ZX, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. Journal of Comparative Neurology. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 57.Orth DN. Adrenocorticotropic hormone (ACTH) In: Jaffe BM, Behrman HR, editors. Methods of Hormone Radioimmunoassay. New York: Academic Press; 1979. pp. 245–278. [Google Scholar]
- 58.Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, et al. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides. 2013;43:20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- 60.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 61.Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- 62.French JA, Smith AS, Birnie AK. Maternal gestational androgen levels in female marmosets (Callithrix geoffroyi) vary across trimesters but do not vary with the sex ratio of litters. Gen Comp Endocr. 2010;165:309–314. doi: 10.1016/j.ygcen.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shear K, Shair H. Attachment, loss, and complicated grief. Developmental Psychobiology. 2005;47:253–267. doi: 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- 64.Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 65.French JA, Fite JE, Jensen HA, Oparowski KM, Rukstalis M, Fix H, et al. Treatment with CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii) Am J Primatol. 2007;69:877–889. doi: 10.1002/ajp.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus jacchus) Biol Psychiatry. 1996;40:317–337. doi: 10.1016/0006-3223(95)00397-5. [DOI] [PubMed] [Google Scholar]
- 67.Shepherd RE, French JA. Comparative analysis of sociality in lion tamarins (Leontopithecus rosalia) and marmosets (Callithrix kuhli): Responses to separation from long-term pairmates. J Comp Psychol. 1999;113:24–32. [Google Scholar]
- 68.Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, DeVries AC. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: A potential role for oxytocin. Psychosom Med. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 69.Klein SL, Hairston JE, Devries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Horm Behav. 1997;32:30–39. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- 70.Getz LL, Carter CS. Prairie-vole partnerships. American Scientist. 1996;84:56–62. [Google Scholar]
- 71.McGuire B, Getz LL, Hofmann J, Pizzuto T, Frase B. Natal dispersal and philopatry in prairie voles (Microtus ochrogaster) in relation to population density, season, and natal social environment. Behav Ecol Sociobiol. 1993;32:293–302. [Google Scholar]
- 72.Getz LL, Carter CS, Gavish L. The mating system of prairie voles, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology. 1981;8:189–194. [Google Scholar]
- 73.Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J Mammal. 1993;74:44–58. [Google Scholar]
- 74.Solomon NG, Jacquot JJ. Characteristics of resident and wandering prairie voles, Microtus ochrogaster. Can J Zool. 2002;80:951–955. [Google Scholar]
- 75.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatment. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 76.Armario A, Gavaldà A, Martí OA. Forced swimming test in rats: Effect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility and defecation rate. Eur J Pharmacol. 1988;158:207–212. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- 77.Martín J, Armario A. Effects of diazepam and desipramine in the forced swimming test: Influence of previous experience with the situation. Eur J Pharmacol. 1993;236:295–299. doi: 10.1016/0014-2999(93)90601-d. [DOI] [PubMed] [Google Scholar]
- 78.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 79.Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 80.Dinan TG, Lavelle E, Scott LV, Newell-Price J, Medbak S, Grossman AB. Desmopressin normalizes the blunted adrenocorticotropin response to corticotropin-releasing hormone in melancholic depression: Evidence of enhanced vasopressinergic responsivity. J Clin Endocrinol Metab. 1999;84:2238–2240. doi: 10.1210/jcem.84.6.5723. [DOI] [PubMed] [Google Scholar]
- 81.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 82.Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- 83.Dewsbury DA. Diversity and adaptation in rodent copulatory behavior. Science. 1975;190:947–954. doi: 10.1126/science.1188377. [DOI] [PubMed] [Google Scholar]
- 84.Dewsbury DA. The comparative psychology of monogamy. Nebr Symp Motiv. 1987;35:1–50. [PubMed] [Google Scholar]
- 85.Gray GD, Dewsbury DA. A quantitative description of copulatory behavior in prairie voles (Microtus ochrogaster) Brain Behav Evol. 1973;8:426–452. [PubMed] [Google Scholar]
- 86.Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- 87.Getz LL, McGuire B, Carter CS. Social behavior, reproduction and demography of the prairie vole, Microtus ochogaster. Ethol Ecol Evol. 2003;15:105–118. [Google Scholar]
- 88.Gobrogge KL, Wang Z. Genetics of aggression in voles. In: Huber R, Bannasch DL, Brennan P, editors. Aggression. San Diego, CA: Academic Press; 2011. pp. 121–150. [Google Scholar]
- 89.Carter CS, Getz LL. Monogamy and the prairie vole. Scientific American. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 90.Shapiro LE, Dewsbury DA. Differences in affiliative behavior, pair bonding, and vaginal cytology in two species of vole (Microtus ochrogaster and M. montanus) J Comp Psychol. 1990;104:268–274. doi: 10.1037/0735-7036.104.3.268. [DOI] [PubMed] [Google Scholar]
- 91.Hoffman HS, Boskoff KJ, Eiserer LA, Klein SH. Isolation- induced aggression in newly hatched ducklings. J Comp Physiol Psychol. 1975;89:447–456. doi: 10.1037/h0077063. [DOI] [PubMed] [Google Scholar]
- 92.Adams DB. The relation of scent-marking, olfactory investigation, and specific postures in the isolation-induced fighting of rats. Behaviour. 1976;56:286–297. doi: 10.1163/156853976x00064. [DOI] [PubMed] [Google Scholar]
- 93.Uyeno ET. Inhibition of isolation-induced attack behavior of mice by drugs. Proc West Pharmacol Soc. 1966;9:62. [PubMed] [Google Scholar]
- 94.Da Vanzo JP, Daugherty M, Ruckart R, Kang L. Pharmacological and biochemical studies in isolation-induced fighting mice. Psychopharmacologia. 1966;9:210–219. doi: 10.1007/BF02198481. [DOI] [PubMed] [Google Scholar]
- 95.DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. P Natl Acad Sci USA. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeVries AC. Interaction among social environment, the hypothalamic-pituitary-adrenal axis, and behavior. Horm Behav. 2002;41:405–413. doi: 10.1006/hbeh.2002.1780. [DOI] [PubMed] [Google Scholar]
- 97.Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 99.Thase ME. Neurobiological aspects of depression. In: Gotlieb IH, Hammen CL, editors. Handbook of Depression. New York: Guilford Press; 2009. pp. 187–217. [Google Scholar]
- 100.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 101.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: Implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 102.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1080. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 103.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 104.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:630–668. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 105.Nair HP, Young LJ. Vasopressin and pair-bond formation: Genes to brain to behavior. Physiology & Behavior. 2006;21:146–152. doi: 10.1152/physiol.00049.2005. [DOI] [PubMed] [Google Scholar]
- 106.Field NP. Continuing bonds in adaptation to bereavement: Introduction. Death Stud. 2006;30:709–714. doi: 10.1080/07481180600848090. [DOI] [PubMed] [Google Scholar]
- 107.Stroebe M, Schut H, Boerner K. Continuing bonds in adaptation to bereavement: Toward theoretical integration. Clin Psychol Rev. 2009;30:259–268. doi: 10.1016/j.cpr.2009.11.007. [DOI] [PubMed] [Google Scholar]


