Abstract
Treatment resistant latent reservoirs remain a barrier to curing HIV, but the maintenance and properties of these reservoirs are not completely understood. 2-LTR circular HIV DNA has been used to assess ongoing viral replication in HAART treated patients. However, the half-life of this DNA form is still debated with conflicting in vivo and in vitro data. Prior in vitro studies have focused on cell lines or short lived activated cells in cultures of brief duration, while in vivo studies have the added complications of cell migration, division, and death. Therefore, we monitored the stability of 2-LTR circles in primary CD4+T cells in a month long culture and compared it to the stability of integrated HIV DNA and T cell receptor excision circles (TRECs), another circular DNA form that is thought to be stable. We found that 2-LTRs, along with TRECs, were stable, suggesting 2-LTRs do not necessarily indicate ongoing replication.
Keywords: HIV, 2-LTR circles, T cell excision circles, ongoing replication
Introduction
Although highly active antiretroviral therapy (HAART) is effective at controlling HIV replication, it is not a cure as a reservoir of treatment resistant cells persists. These latently infected cells, mainly consisting of resting CD4+T cells, can release infectious virus upon stimulation (Chun et al., 1997; Finzi et al., 1999). How this reservoir is maintained in the face of HAART is still an open question. While several hypotheses exist, one possible explanation is that a low level of ongoing replication occurs, in spite of therapy either in sanctuary sites or due to insufficient drug levels, that replenishes the reservoir over time (Coiras et al., 2009; Lafeuillade and Stevenson, 2011; Palmer et al., 2008). Some evidence for this hypothesis is the existence of circular HIV DNA forms including 2 long terminal repeat circles (2-LTRs) in patients on HAART (Sharkey et al., 2000) and the increase in such circles upon Raltegravir intensification (Buzon et al., 2010). This evidence presupposes a short half-life of these circular forms. However, there is still debate over the longevity of 2-LTR circles (Brussel et al., 2003; Butler et al., 2002; Gillim-Ross et al., 2005; Murray et al., 2012; Pierson et al., 2002; Sharkey et al., 2005; Sharkey et al., 2000; Zhu et al., 2011). While several in vivo studies have suggested 2-LTRs are short-lived (Murray et al., 2012; Sharkey et al., 2005; Zhu et al., 2011), it is difficult to determine if decreases in 2-LTRs reflect degradation of the circles themselves or other complicating factors such as cell half-life, division, and migration. In vitro studies also show conflicting evidence regarding how long 2-LTRs persist. While some studies using cell lines suggest 2-LTRs are short-lived (Sharkey et al., 2000), other studies using cell lines indicate long 2-LTR half-lives, particularly when cell division and viability are controlled (Butler et al., 2002; Pierson et al., 2002). However, these in vitro studies did not use physiologically relevant cells and did not culture the cells for a long period of time (~10 days at most) (Butler et al., 2002; Pierson et al., 2002). Therefore, we chose to examine the stability of 2-LTRs in primary CD4+T cells infected in vitro in a month long culture as some in vivo studies suggest 2-LTR circles have a half-life between 8 and 25 days (Murray et al., 2012; Zhu et al., 2011). We also examined the stability of T cell receptor excision circles (TRECs) as a comparison as TRECs are often assumed to be stable in vivo (Hazenberg et al., 2001; Somech, 2011).
Results and Discussion
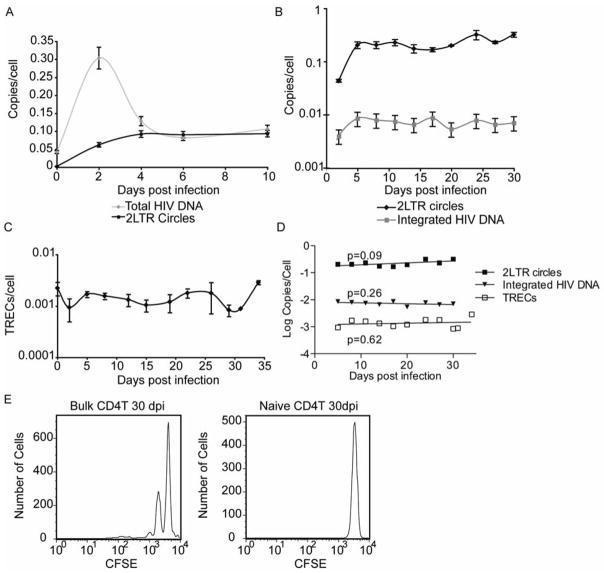
We first examined the dynamics of total HIV DNA and 2-LTRs in infected primary CD4+T cells in a short time-course, similar to previous in vitro studies. We treated the cells with 10ng/mL IL-7 to maintain the cells in culture and with the integrase inhibitor Raltegravir to increase the levels of 2-LTR circles to more easily detectable levels. We found total HIV DNA peaked at 2 days post infection while 2-LTR circles peaked 4 days post infection (Figure 1A), consistent with the literature (Gillim-Ross et al., 2005). Total HIV DNA quickly declined until it reached levels similar to those of 2-LTR circles (~day 4 post infection), suggesting that most of the HIV DNA consisted of 2-LTRs by that time (Figure 1A). This is consistent with the short half-life of linear unintegrated HIV DNA found in vitro (Koelsch et al., 2008). Additionally, we saw no decline in 2-LTRs during the 10 day culture, similar to prior in vitro studies using cell lines (Butler et al., 2002; Pierson et al., 2002).
Figure 1. 2-LTR circles, TRECs and integrated HIV DNA are stable over time in primary CD4+T cells.
In A) CD4+T cells were infected with HIV in the presence of the integrase inhibitor Raltegravir. Cells were cultured in 10nm/mL IL-7. Total HIV DNA and 2-LTR circles were measured between 0 and 10 days post infection. A representative of three experiments is shown. In B) naïve CD4+T cells were infected with HIV and cultured in the presence of Raltegravir, saquinavir, and IL-7. 2-LTR circles and integrated HIV DNA were measured over a period of 30 days. 2-LTRs were not above baseline at t=0. A representative of three experiments is shown. In C) TRECs were measured in the same cells shown in B. Bars represent the standard deviations of the measurements (A–C). A representative of two experiments is shown. In D) the log10 of the values in B and C were graphed over time and a linear regression was performed to calculate half-lives of the different HIV DNA species. In E) infected bulk CD4+T cells or naïve CD4+T cells were labeled with CFSE and cell division measured over time. Graphs shown are for 30 days post infection.
As one of the major criticisms of previous 2-LTR stability studies in vitro was the short duration of the experiments (Sharkey et al., 2005), we wanted to examine the stability of 2-LTRs in primary cells for a longer period of time. In addition, we also wanted to compare the stability of 2-LTRs with integrated HIV DNA and T cell receptor excision circles (TRECs), another type of circular DNA, both of which are thought to persist for the life of the cell. TRECs are generated during TCR recombination and have been used to assess thymic output and the age and division history of T cells (den Braber et al., 2012; Hazenberg et al., 2001; Jamieson et al., 1999; Somech, 2011). While TRECs are assumed to be stable based on in vivo data (Douek et al., 1998; Hazenberg et al., 2001; Livak and Schatz, 1996; Somech, 2011), in vitro data is limited and the precise half-life of TRECs is currently unknown (Hazenberg et al., 2001; Somech, 2011). Therefore, we decided to measure TRECs in addition to 2-LTRs to compare the stability of different circular DNA forms and to add to both the TREC and HIV literature. As TRECs are enriched in naïve CD4+T cells (Douek et al., 1998), we decided to examine both TRECs and 2-LTR circles in naïve cells cultured for a month in vitro.
We therefore infected naïve CD4+T cells with X4 tropic HIV again in the presence of IL-7 and Raltegravir for the reasons stated above. As integrase inhibitors do not completely inhibit integration in our in vitro system (>98% inhibition based on an infected, untreated control, data not shown), we were able to quantify 2-LTR circles, integrated HIV DNA and TRECs. To control for any cell division, we labeled the cells with CFSE and quantified cell division during the culture. We also monitored cell viability to control for cell death.
We found that both 2-LTR circles and integrated HIV DNA were stable in naïve cells during the 30 day culture (Figure 1B). We next examined levels of TRECs over time in vitro and found that TRECs were stable in naïve cells (Figure 1C). To determine the apparent half-lives of the HIV intermediates and TRECs, we used linear regression to generate a best fit line through the plot of log10 quantities per cell over time as previously described (Brussel et al., 2003). We found that none of the slopes of the best fit lines were statistically different than zero, indicating no significant decline in 2-LTRs, integrated HIV DNA or TRECs (Figure 1D).
As cell death or proliferation could affect our results, we examined cell viability and division. We found that the naïve cells did not divide during the month long culture, while bulk CD4+T cells did (Figure 1E), likely due to central memory homeostatic proliferation. This is consistent with prior data indicating that naïve cells only divide in the presence of IL-7 in ~50% of donors (Azevedo et al., 2009). Therefore, cell division did not affect our results. Additionally, we found no significant change in the viability of the cells treated with IL-7 over time, whereas infected cells without additional cytokines died within 7 days (data not shown). Thus, neither cell death nor proliferation altered our findings that 2-LTRs and TRECs are stable in vitro.
Our results indicate that 2-LTRs, like TRECs, are stable in primary CD4+T cells in vitro. While some studies have suggested 2-LTRs are long-lived in vivo (Brussel et al., 2003), recent reports suggest that 2-LTR circles have relatively short half-lives in vivo, between 8 and 28 days (Murray et al., 2012; Zhu et al., 2011). However, these studies admit that the calculations could be due to cellular half-lives or migration of cells containing 2-LTR circles. Our data suggests the short in vivo half-lives of 2-LTRs are likely due to these confounding in vivo factors rather than the stability of the circles themselves. Additionally, the reported short half-life of circular HIV DNA in vivo suggests our month-long culture is sufficient to determine whether the stability of the cells or the 2-LTRs were responsible for 2-LTR declines in patients. While other in vitro studies showed 2-LTR circles were stable in cell lines (Butler et al., 2002; Pierson et al., 2002), their short time courses and use of non-primary cells limited their ability to address both the stability of 2-LTRs in vivo and the ability to measure ongoing replication using this circular HIV intermediate. Our studies show that 2-LTRs are stable in primary over a 30 day culture and that 2-LTR degradation cannot be responsible for their apparent short half-life in vivo. These results have important implications for the HIV field.
Previous studies suggested 2-LTRs could be used as a marker for ongoing replication due to their short half-lives (Sharkey et al., 2005; Sharkey et al., 2000). Our results suggest the presence of 2-LTRs is not sufficient to identify new rounds of replication. Together, our data showing the stability of 2-LTRs in primary cells along with in vivo studies indicating 2-LTRs but not integrated HIV DNA disappear over time suggest that proliferation or migration of cells with 2-LTRs occurs in vivo, confounding interpretations of ongoing replication. However, this is not to say 2-LTRs will not increase during new rounds of replication, merely that 2-LTRs alone may not be sufficient to identify these periods. Overall, our data suggest that 2-LTRs themselves are stable in CD4+T cells but dynamics or migration of infected cells results in the shorter apparent half-life of 2-LTRs in vivo. Thus, the mere presence of 2-LTRs is not sufficient to determine periods of ongoing replication.
Materials and Methods
Cells
Primary CD4+T cells were purified from leukapheresis-enriched PBMC using Rosette Sep (Stemcell Technologies) and were obtained through the University of Pennsylvania’s Center for AIDS Research Human Immunology Core. After infection, cells were cultured in RPMI containing 20% heat inactivated FCS and 10ng/mL IL-7 (R&D Biosystems).
Cell Sorting
CD4+T cells were purified as above and were stained with CD45RO PE-Texas Red (Beckman Coulter) and CCR7 PECy7 (BD Bioscience). Naïve CD4+T cells (CD45RO-, CCR7+) were sorted using a FACSAria II Cell Sorter (BD Bioscience) and were >98% pure.
CFSE staining
An aliquot of infected cells was labeled using CellTrace CFSE Cell Proliferation Kit (Invitrogen) as per the manufacturer’s instructions immediately after infection.
Virus and Infection
NL4-3 viral stocks were prepared by 293T transfections by the University of Pennsylvania’s Center for AIDS Research molecular virology core. Cells were sorted as described above and spinoculated the same day at a concentration of 1×107 cells/mL at 1200×g at 25°C for 2 hours with 280ng of p24 per 1e6 cells. After spinoculation, cells were washed twice in CO2 independent media (Invitrogen) and treated with 50μg/mL Dnase 1 (Roche) and 10mM MgCl2 to remove plasmid DNA. Cells were then cultured in the presence of 1μM Raltegravir (Merck) and 1.25μM saquinavir (Roche).
PCR Measurements
2-LTRs, total HIV DNA (using RU5 primers), and integrated HIV DNA were quantified as previously described (Graf et al., 2011; Pace et al., 2012). Integrated HIV DNA was first measured at 2 days post infection to conserve sample. TRECs were measured as previously described (Douek et al., 1998).
Statistical Analysis
Linear regression analysis was performed using Graphpad Prism 5.
We monitor stability of 2-LTRs, TRECs and integrated HIV in primary CD4+T cells
2-LTRs are stable in primary CD4+T cells for 30 days in culture
The presence of 2-LTR circles is not sufficient to detect ongoing replication
Acknowledgments
Funding
This work was supported by the NIH grant R21 A1087461 as well as additional funding from Merck and AmFAR.
We would like to acknowledge Lindsay Lynch and Sapan Shah for their help in PCR measurements. We would like to thank Daniel Douek for providing the TREC quantification standard. We would also like to acknowledge the University of Pennsylvania CFAR, particularly Farida Shaheen, for the viral stocks. We would like to thank Avinash Bhandoola for productive discussions of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azevedo RI, Soares MV, Barata JT, Tendeiro R, Serra-Caetano A, Victorino RM, Sousa AE. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood. 2009;113:2999–3007. doi: 10.1182/blood-2008-07-166223. [DOI] [PubMed] [Google Scholar]
- Brussel A, Mathez D, Broche-Pierre S, Lancar R, Calvez T, Sonigo P, Leibowitch J. Longitudinal monitoring of 2-long terminal repeat circles in peripheral blood mononuclear cells from patients with chronic HIV-1 infection. AIDS. 2003;17:645–652. doi: 10.1097/00002030-200303280-00001. [DOI] [PubMed] [Google Scholar]
- Butler SL, Johnson EP, Bushman FD. Human Immunodeficiency Virus cDNA Metabolism: Notable Stability of Two-Long Terminal Repeat Circles. Journal of Virology. 2002;76:3739–3747. doi: 10.1128/JVI.76.8.3739-3747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, Palmer S, Stevenson M, Clotet B, Blanco J, Martinez-Picado J. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Coiras M, Lopez-Huertas MR, Perez-Olmeda M, Alcami J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7:798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange SJ, Gallant J, Siliciano RF. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Gillim-Ross L, Cara A, Klotman ME. HIV-1 extrachromosomal 2-LTR circular DNA is long-lived in human macrophages. Viral Immunol. 2005;18:190–196. doi: 10.1089/vim.2005.18.190. [DOI] [PubMed] [Google Scholar]
- Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M, Migueles SA, Connors M, O’Doherty U. Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART. PLoS Pathog. 2011;7:e1001300. doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med (Berl) 2001;79:631–640. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Gunthard HF, Ignacio CC, Campos-Soto P, Little SJ, Shafer R, Robbins GK, D’Aquila RT, Kawano Y, Young K, Dao P, Spina CA, Richman DD, Wong JK. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis. 2008;197:411–419. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- Lafeuillade A, Stevenson M. The search for a cure for persistent HIV reservoirs. AIDS Rev. 2011;13:63–66. [PubMed] [Google Scholar]
- Livak F, Schatz DG. T-cell receptor alpha locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol Cell Biol. 1996;16:609–618. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, McBride K, Boesecke C, Bailey M, Amin J, Suzuki K, Baker D, Zaunders JJ, Emery S, Cooper DA, Koelsch KK, Kelleher AD. Integrated HIV DNA accumulates prior to treatment while episomal HIV DNA records ongoing transmission afterwards. AIDS. 2012;26:543–550. doi: 10.1097/QAD.0b013e328350fb3c. [DOI] [PubMed] [Google Scholar]
- Pace MJ, Graf EH, Agosto LM, Mexas AM, Male F, Brady T, Bushman FD, O’Doherty U. Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 2012;8:e1002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF. Intrinsic stability of episomal circles formed during Human Immunodeficiency Virus Type-1 replication. Journal of Virology. 2002;76:4138–4144. doi: 10.1128/JVI.76.8.4138-4144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey M, Triques K, Kuritzkes DR, Stevenson M. In vivo evidence for instability of episomal human immunodeficiency virus type 1 cDNA. J Virol. 2005;79:5203–5210. doi: 10.1128/JVI.79.8.5203-5210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey ME, Teo I, Greenough TC, Sharova N, Luzuriaga K, Sullivan JL, Bucy RP, Kostrikis LG, Haase AT, Veryard C, Davaro RE, Cheeseman SH, Daly JS, Bova C, Ellison RT, Mady B, Lai KK, Moyle G, Nelson M, Gazzard BG, Shaunak S, Stevenson M. Persistence of episomal HIV-1 intermediates in patients on highly active anti-retroviral therapy. Nat Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somech R. T-cell receptor excision circles in primary immunodeficiencies and other T-cell immune disorders. Curr Opin Allergy Clin Immunol. 2011;11:517–524. doi: 10.1097/ACI.0b013e32834c233a. [DOI] [PubMed] [Google Scholar]
- Zhu W, Jiao Y, Lei R, Hua W, Wang R, Ji Y, Liu Z, Wei F, Zhang T, Shi X, Wu H, Zhang L. Rapid turnover of 2-LTR HIV-1 DNA during early stage of highly active antiretroviral therapy. PLoS One. 2011;6:e21081. doi: 10.1371/journal.pone.0021081. [DOI] [PMC free article] [PubMed] [Google Scholar]