This study compared four radiological criteria used to evaluate long-term survival in hepatocellular carcinoma patients treated with sorafenib. The results indicated that Choi criteria, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumors were more appropriate methods than Response Evaluation Criteria in Solid Tumors, version 1.1, for identifying patients with long-term survival.
Keywords: Density, Tumor evaluation, Targeted therapy, Antiangiogenic agents, Computed tomography
Abstract
Introduction.
Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1), may underestimate activity and does not predict survival in patients with hepatocellular carcinoma (HCC) treated with sorafenib. This study assessed the value of alternative radiological criteria to evaluate response in HCC patients treated with sorafenib.
Patients and Methods.
A retrospective blinded central analysis was performed of computed tomography (CT) scans from baseline and the first tumor evaluation in consecutive patients treated with sorafenib over a 2-year period in a single institution. Four different evaluation criteria were used: Choi, European Association for the Study of the Liver (EASL), modified RECIST (mRECIST), and RECIST 1.1.
Results.
Among 82 HCC patients, 64 with Barcelona Clinic Liver Cancer stage B-C were evaluable with a median follow-up of 22 months. Median duration of sorafenib treatment was 5.7 months, and median overall survival was 12.8 months. At the time of the first CT scan, performed after a median of 2.1 months, Choi, EASL, mRECIST, and RECIST 1.1 identified 51%, 28%, 28%, and 3% objective responses, respectively. Responders by all criteria showed consistent overall survival >20 months. Among patients with stable disease according to RECIST 1.1, those identified as responders by Choi had significantly better overall survival than Choi nonresponders (22.4 vs. 10.6 months; hazard ratio: 0.43, 95% confidence interval: 0.15–0.86, p = .0097).
Conclusion.
Choi, EASL, and mRECIST criteria appear more appropriate than RECIST 1.1 to identify responders with long survival among advanced HCC patients benefiting from sorafenib.
Implications for Practice:
In patients with advanced hepatocellular carcinoma, novel radiological criteria such as Choi, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumors (mRECIST) performed within the first 3 months of treatment with sorafenib appear more appropriate than RECIST 1.1 to identify responders with long survival. Alternative response criteria may be useful in routine practice to justify continuing sorafenib among the vast majority of patients with stable disease by RECIST 1.1. As tumor response by RECIST 1.1 does not correlate well with overall survival, those novel criteria, being better correlated with patient outcome, warrant further evaluations in prospective clinical trials exploring novel antiangiogenic agents in patients with advanced hepatocellular carcinoma.
Introduction
Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1), is currently approved as a standard method for the evaluation of tumor response in patients in clinical trials and is considered to be a surrogate endpoint to predict survival outcome in patients with solid tumors [1–3]. Results from one phase II [4] and two large multicenter, double-blind, placebo-controlled randomized phase III trials have led to worldwide approval of sorafenib (Nexavar; Bayer, Leverkusen, Germany, http://www.bayer.com) [5, 6], establishing it as a standard of care prolonging overall survival (OS) in patients with advanced hepatocellular carcinoma (HCC). The OS benefit of sorafenib in HCC is in contrast to the low objective response rate of <5% by RECIST 1.1, raising concerns as to the appropriateness of these criteria as a surrogate endpoint for survival in HCC [4–6]. Similar observations have been reported with sunitinib (Sutent; Pfizer Inc., New York, NY, http://www.pfizer.com), another antiangiogenic multikinase inhibitor, when used to treat patients with advanced HCC. In four studies using sunitinib, three phase II and one phase III, objective response rates ranged from 2% to 3% according to RECIST, which does not reflect the OS benefit seen with this agent [7–10]. Taken together, these reports suggest that antitumor activity of targeted therapies is not adequately captured by RECIST 1.1, which is determined on the sole basis of tumor dimensions [4–6]. Investigation of alternative radiological methods to assess advanced HCC is thus warranted [11].
Other treatment modalities of HCC such as chemoembolization have previously faced similar concerns and led to the development of alternative criteria. These criteria were designed to evaluate the effects of treatment on tumor vascularization based on modifications observed in tumors on contrast-enhanced phase scans. As a result, the European Association for the Study of the Liver (EASL) decided that measuring modifications in the surface of the viable part of the tumor was the optimal method to assess response to chemoembolization [12]. More recently, Lencioni and Llovet developed modified RECIST (mRECIST), restricting measurements to arterially enhanced parts of the tumor only [13]. A recent study suggested that mRECIST might be a potential prognostic factor for survival in advanced HCC patients treated with sorafenib [14]. The use of imatinib, another targeted agent used in gastrointestinal stromal tumors (GIST) prompted Choi et al. to develop composite criteria integrating changes in both tumor size and tumor density, the latter parameter reflecting areas of tumors with reduced vascularization [15, 16]. The Choi criteria appeared to accurately predict imatinib efficacy in GIST and were also shown to be an attractive option to evaluate drug effects in HCC [17].
Although imperfect, RECIST remains the only Food and Drug Administration- and European Medicines Agency (EMA)- validated response criteria to evaluate targeted therapies in advanced HCC. In the current analysis, we aimed to assess alternative criteria including Choi, EASL, and mRECIST, correlating response at the time of first evaluation with OS in advanced HCC patients treated with sorafenib.
Patients and Methods
Patient Selection
This retrospective monocentric cohort analysis included consecutive patients with advanced HCC treated with sorafenib in our institution between 2007 and 2009. The Ethics Committee of the Paris VII University approved this study in accordance with institutional and national guidelines. To be eligible for this study, patients had to have pathologically proven HCC or have been diagnosed according to the European Association for the Study of the Liver/European Organisation for Research and Treatment of Cancer criteria [18], and had to have received a minimal cumulative duration of 4 weeks of sorafenib. Patients had to have undergone baseline thoracic and abdominal-pelvic computed tomography (CT) scans within 6 weeks before sorafenib and had the first tumor evaluation with a second CT scan within 1–3 months after sorafenib initiation. Patients with CT scans that were nonevaluable for technical reasons or with scans performed outside the predefined interval were excluded. Patients with target lesions located in a pretreated area, absence of target lesions, or with missing data due to early death or loss of follow-up were also excluded.
The decision to treat patients with sorafenib was made by an institutional multidisciplinary tumor board composed of hepatologists, oncologists, pathologists, hepatic surgeons, and interventional radiologists, according to EMA Good Practice Recommendations. Sorafenib therapy was selected when no surgical or locoregional approaches were indicated in patients with good performance status (Eastern Cooperative Oncology Group performance status 0-2) and adequate liver function (Child-Pugh score ≤ B7). All patient data were obtained from a prospectively collected clinical and pathological database dedicated to HCC patients.
Treatment and Clinical Follow-Up
Sorafenib was initially given at 800 mg daily (400 mg b.i.d.). After initiating sorafenib, patients were followed on a monthly basis to assess clinical symptoms and tolerance. If needed, doses were adapted according to type and severity of specific toxicities. Dose modifications followed good clinical practice and international recommendations [19]. Sorafenib was maintained until confirmed disease progression or unacceptable toxicity.
CT Scan Procedures
All contrast-enhanced dual-phase CT scans of the chest, abdomen, and pelvis were performed on a 64-section multidetector CT scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, http://www.gehealthcare.com). Technical parameters for CT were as follows: slice thickness and reconstruction interval, 1.25 mm; 120 kVp variable milliamperage determined by x-, y-, and z-axis dose modulation. A nonionic iodinated contrast agent containing 350 mg of iodine mL-1 was administered intravenously (120 mL) through a 16- to 18-gauge catheter via an antecubital vein at 4 mL per second. No oral contrast medium was given. After an unenhanced abdominal scanning, arterial and portal venous phase acquisitions were obtained at 35 and 80 seconds after initiation of contrast medium injection, respectively.
Identification of Target/Nontarget Lesions
At baseline, target lesions had to be at least ≥1 cm for the largest diameter, and nontarget lesions could have a largest diameter of <1 cm. Malignant portal vein thrombosis, malignant ascites or hydrothorax (confirmed by cytologic examination of the fluid), and lymph nodes ≤2 cm were considered as nontarget lesions. According to RECIST 1.1 recommendations, a maximum of two lesions per organ and five lesions in total were selected [2]. For Choi, EASL, and mRECIST, the same number of lesions was selected [12, 13, 15]. Lesions with no intratumoral arterial enhancement were not considered as target lesions. Nonhepatic lesions could be considered as target lesions.
Tumor Response Assessment According to Alternative Criteria
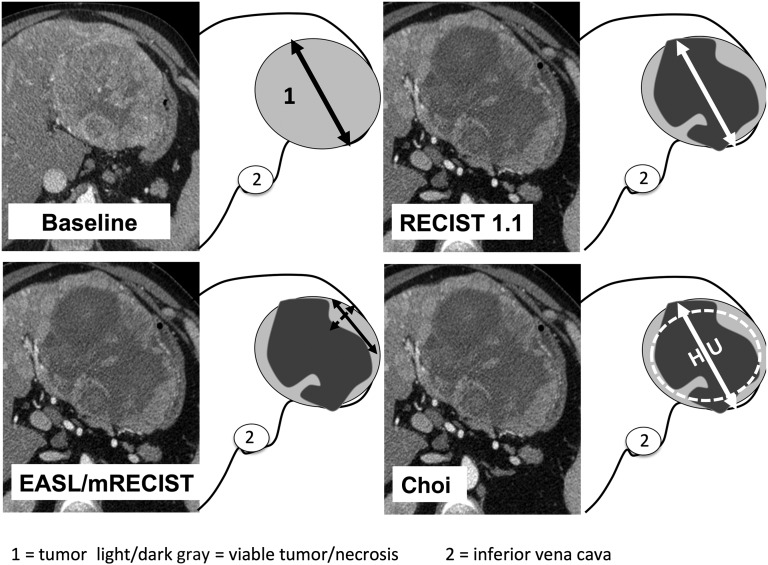
For each target lesion, the maximum diameter, the maximum unidimensional enhanced diameter, the product of the bidimensional enhanced diameters, and tumor density were measured on the arterial phase acquisition. Using these parameters, we evaluated response according to Choi, EASL, mRECIST, and RECIST 1.1 criteria [2, 12, 13, 15]. Figure 1 displays a typical example of tumor evaluation using those criteria. In cases of multinodular enhancement, the largest zone of continuous enhancement was measured. Tumor density was evaluated by selecting a circular region of interest (ROI) on target lesions at baseline and at the time of first evaluation under sorafenib. As no guideline currently exists regarding the use of Choi criteria in HCC, we adapted the original Choi criteria to fit with specific patterns of HCC. First, because HCC are hypervascular tumors, density was measured on the arterial phase instead of the portal phase, as originally described in GIST. Furthermore, because most advanced HCC treated with sorafenib are bulky, heterogeneous, and sometimes multilocular tumor masses often associated with vascular invasion, manual freehand drawing of tumor margins for delineation of ROI, originally described by Choi et al. in GIST, was adapted for HCC. Instead of using a freehand drawing of ROI, a circular ROI was drawn on the axial plane to include the largest surface of the target lesion that presented density modifications in treated tumors compared with the baseline. Then, when the density evaluation was performed on more than one lesion, the global density was calculated as the mean density of the target lesions. We also randomly recollected CT scans at the baseline and following sorafenib treatment in 39 patients to compare freehand-drawn ROI with circular ROI covering the largest possible surface of the tumor.
Figure 1.
Baseline and post-treatment evaluation of response using RECIST 1.1 and alternative radiological criteria. Typical example of response evaluation by RECIST 1.1, mRECIST, EASL criteria, and Choi criteria in a patient with hepatocellular carcinoma treated with sorafenib.
Abbreviations: EASL, European Association for the Study of the Liver; HU, Hounsfield unit; mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECIST 1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
Radiological Evaluation Procedure
A local and central radiological review for imaging interpretation was composed by two radiologists. Readers were experienced abdominal radiologists trained in the HCC field and evaluation of tumor response for 7 and 10 years, respectively. Prior to the study, readers evaluated Choi criteria in a training set of 10 patients who were not included in this study. A debriefing session was used to address difficulties, adopt a common attitude on selecting relevant target lesions, select slice levels, and apply Choi criteria. Then, each radiologist separately performed an independent reading of all CT scans and was blinded to the patients’ clinical data and outcome. Following the independent reading by the two radiologists, any discrepancies between their evaluations were adjudicated by a common third reading of the two readers to reach a final consensus on best response.
For each radiologist, target lesions were selected independently by each radiologist on the baseline CT scan on the basis of size and suitability for accurate measurement and could thus differ between radiologists. Intrahepatic lesions were also classified as nodular or infiltrative [13] for accurate repetitive measurement, and presence of tumor thrombosis was reported.
Overall Survival and Statistical Analysis
OS was measured from the date of initiation of sorafenib to the date of death, regardless of the cause, or censored at the time of the last follow-up visit. Survival curves were prepared using the Kaplan-Meier method and were compared using the log-rank Mantel-Cox test in accordance with the final response outcomes. Cox proportional hazards models were carried out to compare survival according to radiological responses using Choi, EASL, mRECIST, and RECIST 1.1. All statistical tests were two-tailed. A p value of .05 was considered significant and 95% confidence intervals (CI) were calculated. All analyses were performed using the SPSS software (version 17.0; IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/). OS was correlated to response determined at the first tumor evaluation for all four evaluation criteria.
A Fisher’s exact test was used for comparison of frequency. Inter-reader agreements were analyzed using weighted κ statistics. Coefficients between 0.00 and 0.20 indicated slight agreement; 0.21 and 0.40, fair agreement; 0.41 and 0.60, moderate agreement; 0.61 and 0.80, substantial agreement; and 0.81 and 1.00, almost perfect agreement [20, 21].
Results
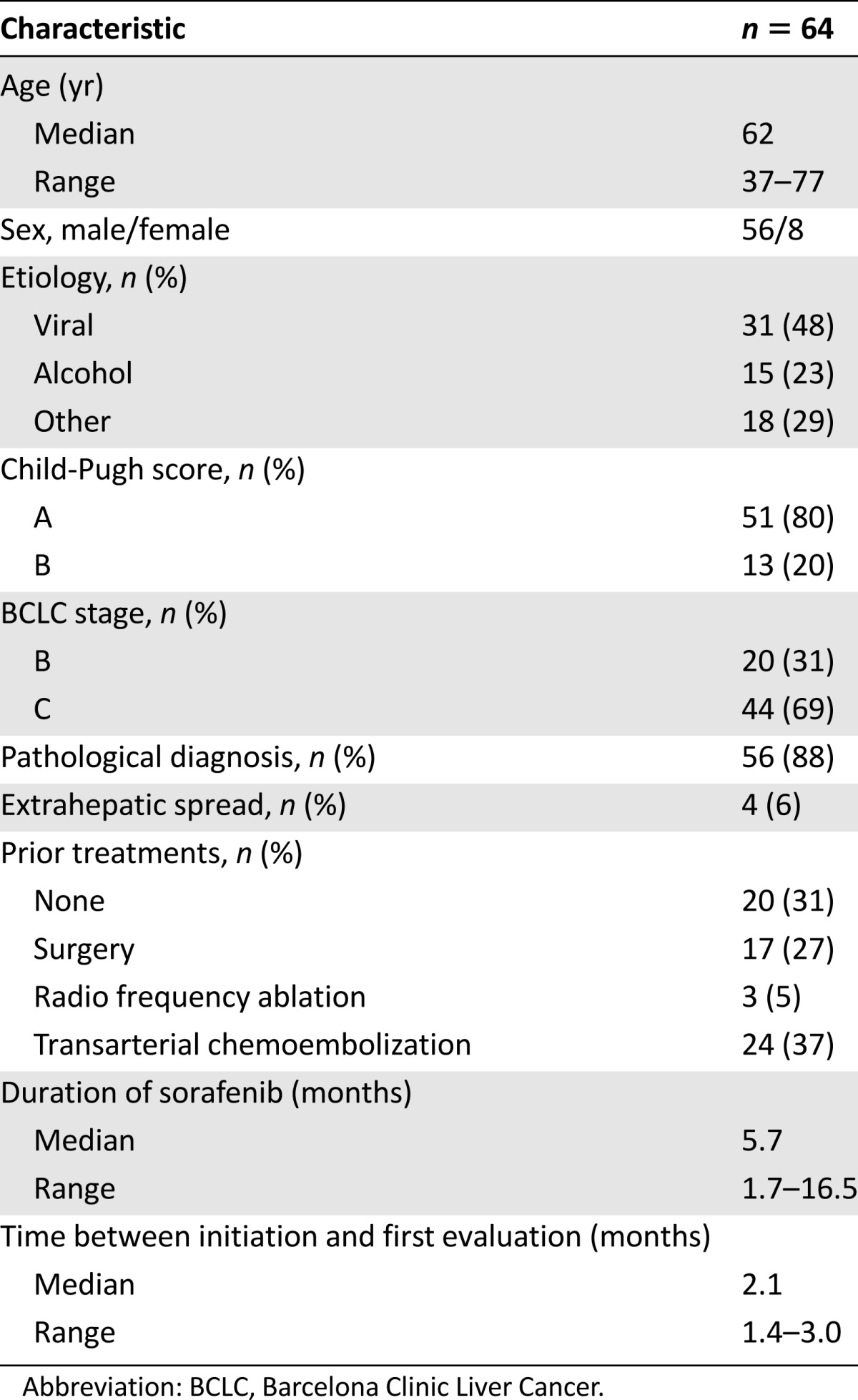
During the period of this analysis, 82 patients were treated with sorafenib for advanced HCC in our institution. A total of 64 patients (56 men, 8 women) met the eligibility criteria and were included in the study. The remaining patients were excluded from the analysis because of nonevaluable CT scans for technical reasons or because evaluations were performed out of the predefined interval (eight patients), target lesions were located in a pretreated area (four patients), absence of target lesions (two patients), and missing data because of early death or loss of follow-up (four patients). Patient demographics and baseline disease characteristics are presented in Table 1. HCC diagnosis was consistent with the Barcelona Clinic Liver Cancer (BCLC) group and American Association for the Study of the Liver Diseases guidelines [12, 18]. In the majority of patients (88%), the diagnosis of HCC was histologically confirmed. The vast majority of patients presented with liver tumors only (58/64 patients, 91%), and the others had extrahepatic spread (lung: 6/64, 9%; peritoneum: 2/64, 3%). The median duration of treatment with sorafenib was 5.7 months (range 1.7–16.5 months). Five patients (7.8%) required dose reduction to 400 mg per day because of side effects (grade 3 asthenia, grade 2 diarrhea), the treatment being further maintained up to CT scan evaluation. The median time between treatment initiation and the first tumor evaluation was 2.1 months (range 1.4–3.0 months). Median duration of the follow-up was 22.2 months (range 1.7–41.2 months).
Table 1.
Patient and baseline characteristics
Variability of Tumor Evaluation Among Radiologists
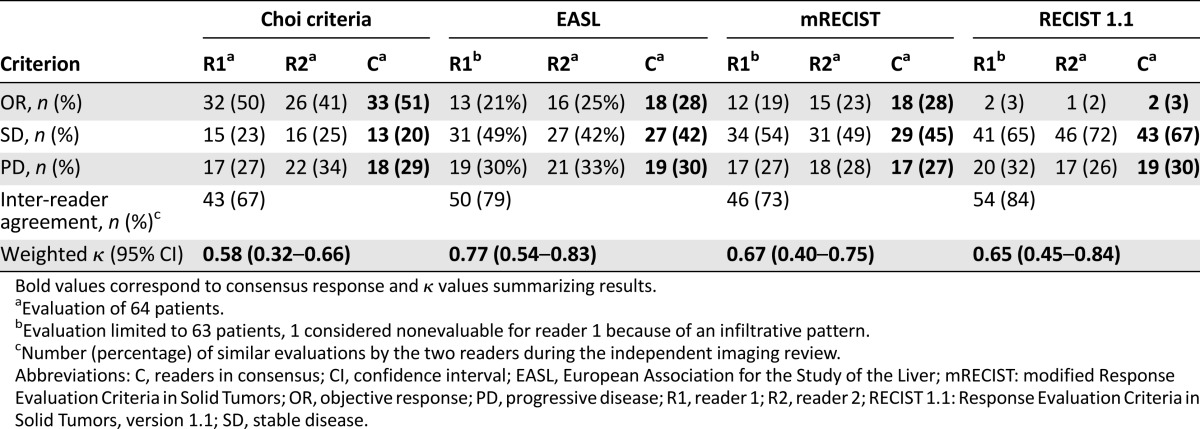
From the blinded readings of all CT scans, differences in response measurements between two radiologists were predominantly observed when evaluating bulky tumors with central necrosis or tumors with infiltrative portions and/or vascular involvement. The inter-reader agreements for Choi, EASL, mRECIST, and RECIST 1.1 were 67%, 79%, 73%, and 84%, respectively. Weighted κ coefficients for Choi, EASL, mRECIST, and RECIST 1.1 criteria were 0.58 (95% CI: 0.32–0.66), 0.77 (95% CI: 0.54–0.83), 0.67 (95% CI: 0.40–0.75), and 0.65 (95% CI: 0.45–0.84), respectively. Inter-reader variability for all criteria is summarized in Table 2.
Table 2.
Response rates according to evaluation criteria for each reader and after consensus with inter-reader agreement (n = 64)
Comparisons of Responses Using Different Methods
According to RECIST 1.1, only 2/64 patients (3%) had an objective response (OR). Using RECIST 1.1, the majority of patients (43/64 patients, 67%) had stable disease (SD). Choi criteria identified 33/64 (51%) patients with OR, and EASL and mRECIST both identified 18/64 (28%) patients with OR. Choi criteria identified significantly more responders than mRECIST and EASL criteria (p = .011). Incidence of patients with progressive disease (PD) was similar in the four groups: 29% for Choi criteria, 30% for EASL, 27% for mRECIST, and 30% for RECIST 1.1. Among patients classified as PD, six developed new lesions, and the others showed an increase in tumor size according to the different criteria. Distributions of consensus responses according to Choi, EASL, mRECIST, and RECIST 1.1 are summarized in Table 2. The two patients with response by RECIST 1.1 were among the 18 patients who responded according to EASL and mRECIST, all of whom were in turn among the 33 patients with OR according to Choi criteria.
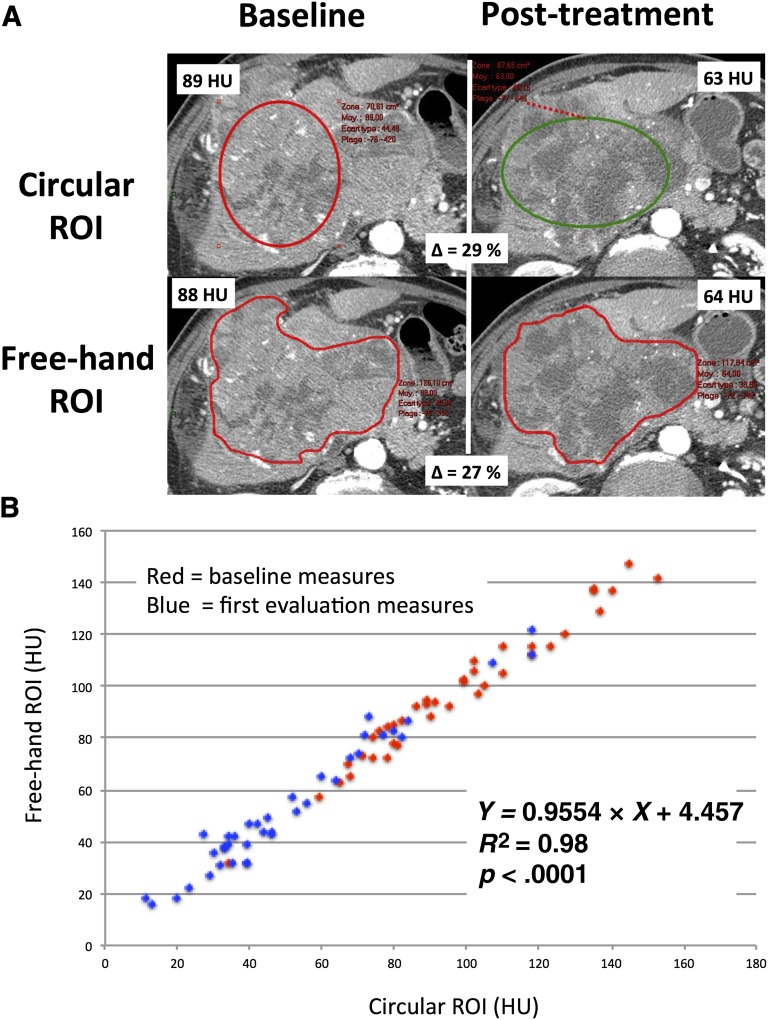
To detect whether the circular ROI used in this study could have impacted the accuracy of density evaluations, we compared freehand ROI delineating the area of the tumor with circular ROI covering the largest possible surface of the tumor in 39 random patients (Fig. 2A). This analysis showed minimal discrepancies between freehand and circular ROI. Our analysis demonstrated that the two methods are highly correlated (Fig. 2B; R2 = 0.98, p < .0001). Similarly, the derived analysis investigating percentage of variations in density induced by sorafenib exposure using “circular ROI” versus “freehand ROI” also showed that the two methods are consistent from one to the other with a very high degree of correlation (R2 = 0.94, p < .0001). From this analysis, we concluded that freehand ROI and circular ROI resulted in very similar evaluations of tumor density.
Figure 2.
Comparison of freehand and circular ROI delineation of the tumor for density evaluation. (A): Representative examples of target lesions at baseline and postsorafenib using a “circular ROI” placed over the tumor and a “freehand ROI” drawing manually delineating the tumor margins before and after sorafenib. (B): Scatterplot comparing “freehand ROI” and “circular ROI” for the evaluation of tumor density in patients with advanced hepatocellular carcinoma before and after treatment with sorafenib.
Abbreviations: HU, Hounsfield unit; ROI, region of interest.
Survival Analysis According to Response Criteria
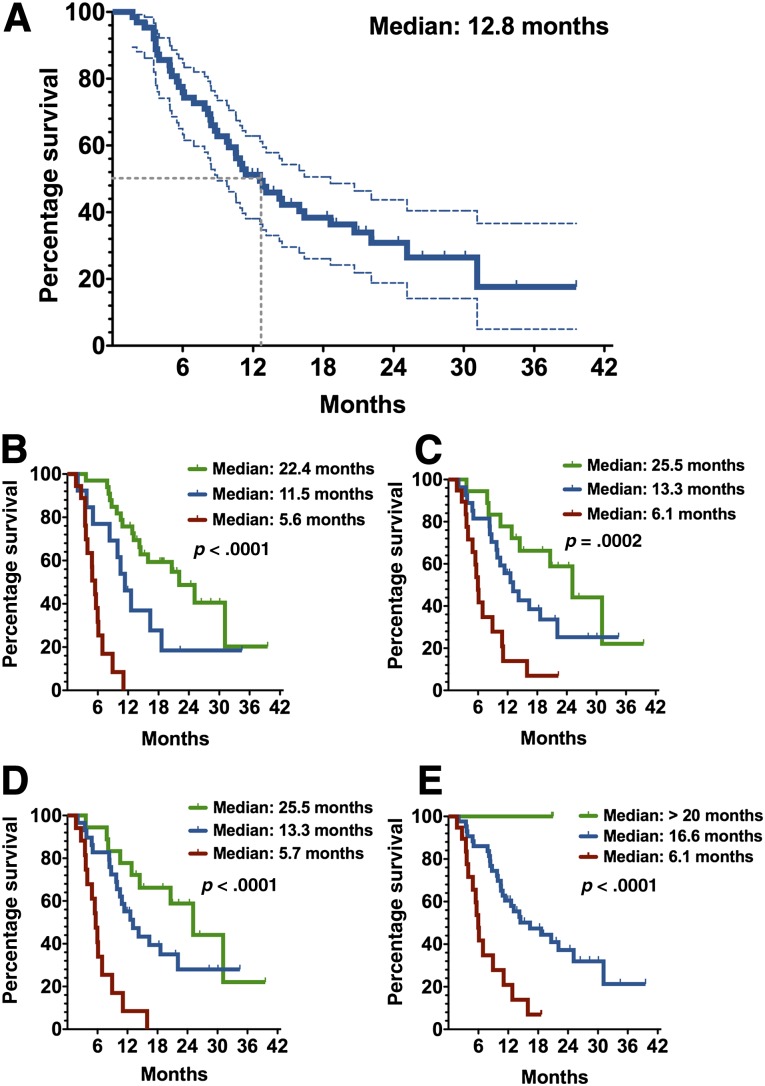
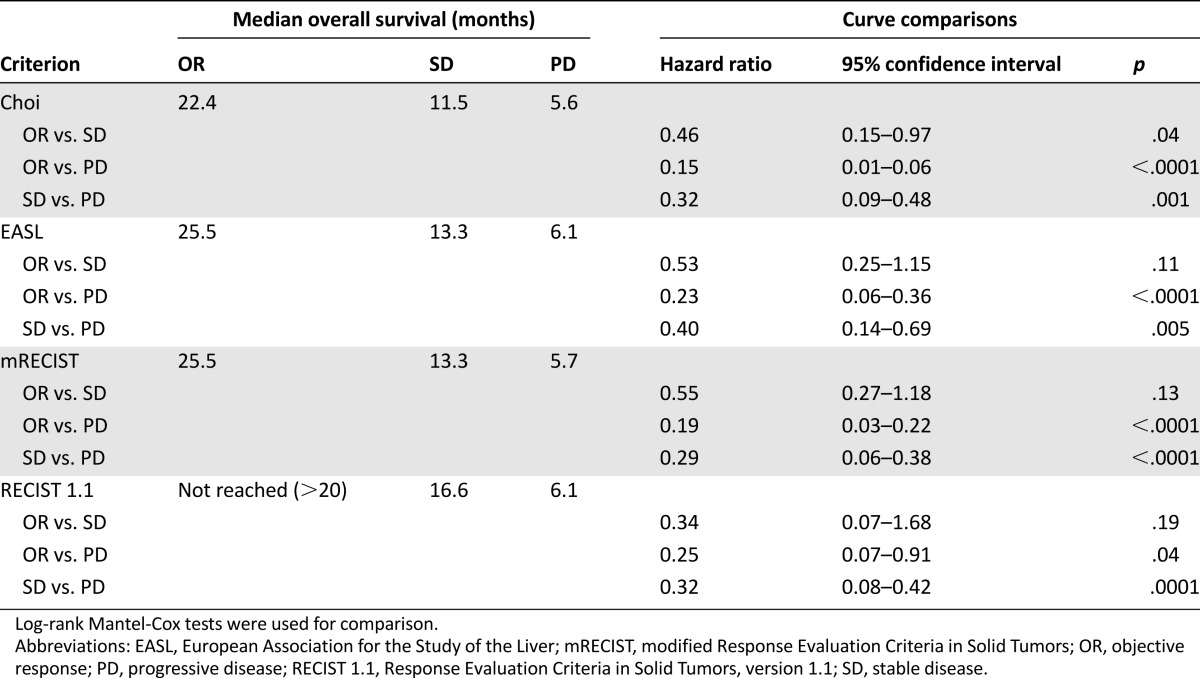
The median OS of patients treated with sorafenib was 12.8 months (range 2.1–40.1) for the overall cohort (Fig. 3A). The OS rates at 6, 12, and 24 months were 75.9%, 51.2%, and 30.9%, respectively. Survival curves according to Choi, EASL, mRECIST, and RECIST 1.1 criteria are shown in Figure 3B–3E. Median OS was ≥20 months in patients with OR and ≤6.1 months in patients with PD according to all four criteria (Table 3). As shown in Table 3, comparisons of hazard ratios (HRs) showed significant differences (ps < .05) between patients with OR versus PD and SD versus PD using Choi, EASL, mRECIST, and RECIST 1.1 criteria. No significant OS differences were detectable between patients with OR and SD using EASL, mRECIST, and RECIST 1.1 criteria, whereas significant differences were detectable between patients with OR and SD using Choi criteria (HR: 0.46, 95% CI: 0.15–0.97, p = .04). Thus, OR using Choi criteria appears to better predict OS as compared with other methods in HCC patients treated with sorafenib.
Figure 3.
Kaplan-Meier overall survival of patients with hepatocellular carcinoma treated with sorafenib. (A): Overall survival (solid blue line) and 95% confidence interval (dotted lines) of patients treated with sorafenib. Overall survival was evaluated according to Choi (B), European Association for the Study of the Liver (C), modified Response Evaluation Criteria in Solid Tumors (D), and Response Evaluation Criteria in Solid Tumors, version 1.1 (E). (B–E): Patients with objective responses (green), stable disease (blue), and progressive disease (red). p values compared the three groups using log-rank tests for trends.
Table 3.
Comparisons of overall survival in patients with hepatocellular carcinoma treated with sorafenib according to their response using each criteria
Choi Criteria in Patients With Stable Disease by RECIST 1.1
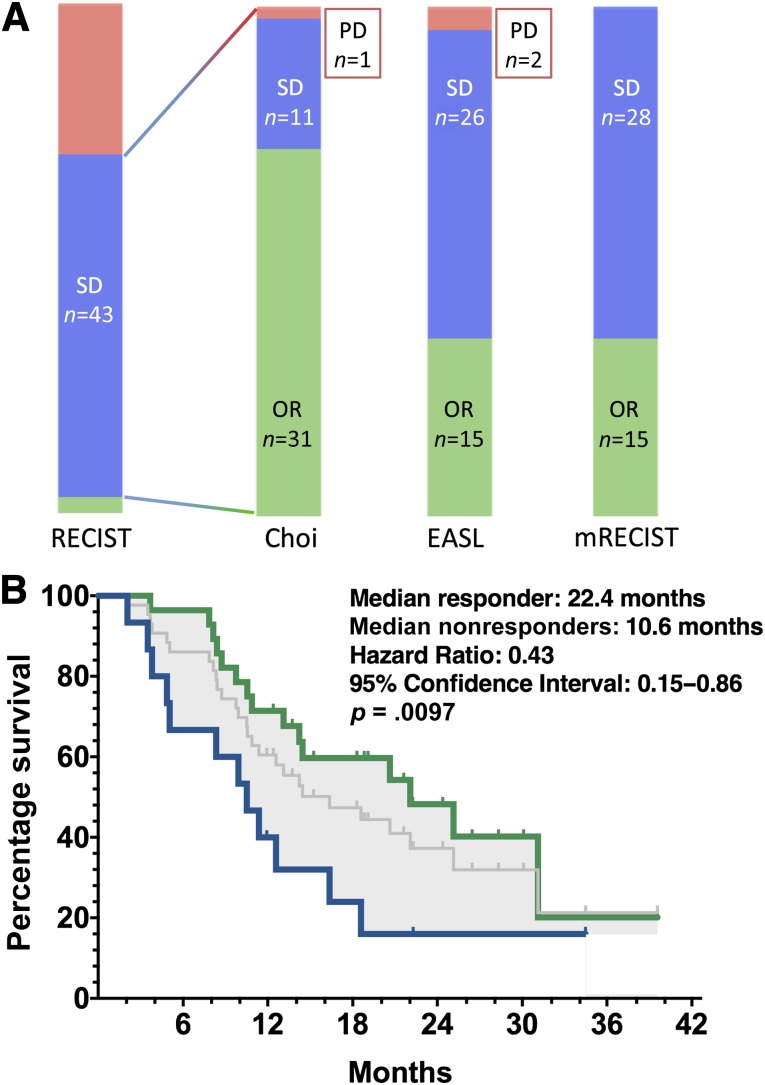
We then performed an analysis in patients with SD by RECIST 1.1, evaluating the proportion of these patients who could be reallocated to OR using other criteria (Fig. 4A). Among 43 patients with SD by RECIST 1.1, 31 patients (72.1%) had OR using Choi criteria and 12 patients (27.9%) were nonresponders (SD or PD). Among patients with SD by RECIST 1.1 (median OS was 16.6 months), those with OR using Choi criteria had a median OS of 22.4 months, which was significantly higher than that of nonresponders (median OS of 10.6 months; HR: 0.43, 95% CI: 015–0.86, p = .0097; Fig. 4B). There was no imbalance for classical prognostic factors (performance status, alpha-fetoprotein level, and portal vein invasion) between responders and nonresponders by Choi criteria. The reallocation of patients with SD by RECIST 1.1 to the groups of patients with OR by Choi criteria appears to be an efficient means of identifying patients with favorable outcome when treated with sorafenib.
Figure 4.
Evaluation of Choi criteria in patients with stable disease by RECIST 1.1. (A): Proportions of patients with objective response, stable disease (SD), and progressive disease using Choi, EASL, and mRECIST among patients with SD according to RECIST 1.1. (B): Kaplan-Meier overall survival of patients with SD by RECIST1.1 is shown in gray. Responders by Choi criteria (green) display significantly higher survival (log-rank test) compared with nonresponders (blue).
Abbreviations: EASL, European Association for the Study of the Liver; mRECIST, modified Response Evaluation Criteria in Solid Tumors; OR, objective response; PD, progressive disease; RECIST 1.1, Response Evaluation Criteria in Solid Tumors, version 1.1; SD, stable disease.
Discussion
More than 50% of patients suffering from HCC present with an advanced disease according to the BCLC staging system [18, 22], some of whom will be offered treatment with sorafenib. In routine practice, the decision to maintain or stop sorafenib is primarily based on continuous reassessment of safety and efficacy. Unfortunately, routine evaluations of response based on RECIST 1.1 are often lacking in precision, as most patients will experience minimal changes in the one-dimensional measurements of target lesions. In clinical trials with sorafenib, the size-based RECIST criteria identify only 2.0%–3.3% of patients with partial response [5, 6], the vast majority of patients with minimal changes in target lesions being considered as having stable disease. Seeking objective criteria that could allow identification of patients with maximal benefit from sorafenib in routine practice and in clinical trials continues to be a major clinical issue. Here, we present a homogenous group of patients with advanced HCC treated with sorafenib and evaluated in a single center with the same contrast-enhanced CT scan technique, comparing three alternative radiological evaluation criteria (Choi, EASL, and mRECIST) with RECIST 1.1. We showed that alternative enhancement-based criteria identified more responders than RECIST 1.1. These results are consistent with previous data published by Edeline et al., who identified 22.6% OR using mRECIST [14]. They are also consistent with previously published studies using transarterial chemoembolization showing no difference between mRECIST and EASL [23, 24, 25]. In our study, it is noteworthy that Choi criteria identified significantly more responders than EASL and mRECIST criteria.
Interobserver agreement with weighted κ values was moderate for Choi (0.58) and substantial for EASL (0.77), mRECIST (0.67), and RECIST 1.1 (0.65). This observation reflects the difficulties involved in performing objective tumor assessment by alternative imaging criteria that appear more likely to be influenced by tumor heterogeneity either at baseline or after sorafenib therapy. Moreover, advanced HCC treated with sorafenib display more bulky tumors than intermediate-stage HCC treated with transarterial chemoembolization or other locoregional therapies, further increasing the likelihood of tumor heterogeneity. In addition, advanced tumors may be more infiltrative, with vascular invasion or extrahepatic disease, ascites, and/or lymph node involvement, making evaluation of multiple target lesions more difficult. After initiation of sorafenib treatment, tumor response may be heterogeneous: few target lesions may respond to treatment, nonvisible small baseline targets may be easier to identify afterward because of hypodensity, tumor thrombosis may increase in size while showing reduced enhancement, or dissociated responses may be observed between liver lesions and extrahepatic metastases. Moreover, Choi criteria could be difficult to apply because of a degree of variation in the interpretation of the initially published criteria in GIST. In our study, Choi criteria were different from those originally described in GIST. Tumor density that is routinely performed during the portal phase in GIST was done on the arterial phase in HCC. Furthermore, instead of manually drawing the tumor margins, circular ROI were used and placed on the largest possible area covering the target lesions in HCC. Applying Choi criteria with those modifications, we did not experience particular difficulty in selecting target lesions for all HCC patients of this study. Comparisons between freehand drawing and circular ROI selection over the largest tumors showed that density evaluations were significantly correlated, providing very similar readouts. These data showed that freehand-drawn ROI was not superior to circular ROI to evaluate tumor density, the latter being sufficiently accurate to predict survival. Moreover, circular ROI was easier and quicker to apply in routine practice. The percentage of agreement between trained radiologists was lower using the alternative contrast-based criteria compared with the simpler criteria used in RECIST 1.1. Several parameters may have contributed to inter-reader variability, such as the choice of CT scan slice level and the selection of target lesions. In our experience, reasons why target selection may have varied between readers appeared mainly related to the complex shape and heterogeneity of advanced HCC, especially in cases of multilocular tumors.
In this study, patients with OR by RECIST 1.1 and mRECIST had better survival outcomes compared with patients with SD and PD, although the magnitude of differences in this small patient population did not allow detection of a significant OS difference between patients with OR and SD. Edeline et al. [14] also showed that the majority of patients with OR according to RECIST 1.1 and mRECIST had improved OS. In our study, OR by Choi criteria observed at the first tumor evaluation was associated with significantly better outcome because a higher OS was reported in comparison with that of patients with SD. We further showed that responders identified by Choi criteria had significantly higher survival compared with nonresponders among patients with SD by RECIST 1.1. Our independent analysis is consistent with published results showing that mRECIST can correlate with OS in a similar population [14]; however, to our knowledge, this is the first demonstration that response by Choi criteria could correlate with OS in HCC patients treated with sorafenib. Because only three patients (5%) received second-line systemic therapy, the overall survival data are likely to reflect almost exclusively the effect of sorafenib.
Conclusion
In summary, Choi, EASL, and mRECIST criteria appear more appropriate than RECIST 1.1 to identify responders with long survival benefiting from sorafenib in advanced HCC. These alternative criteria warrant further evaluation in addition to RECIST 1.1 in prospective trials exploring antiangiogenic drugs in advanced HCC.
Acknowledgments
Maxime Ronot and Mohamed Bouattour contributed equally to this work and should be considered as first authors.
This work was supported by the Foundation Nelia et Amadeo Barletta (http://www.fondationbarletta.org/index.html) and the Association d’Aide à la Recherche et à l’Enseignement en Cancérologie (http://www.aarec-research.org).
We thank Sarah MacKenzie for the final editorial review of the manuscript funded by the Association d’Aide à la Recherche et à l’Enseignement en Cancérologie.
This study was presented in part at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, January 19–21, 2012, San Francisco, California; the International Liver Cancer Association Symposium, September 14–16, 2012, Berlin, Germany; and the American Association for the Study of Liver Diseases Liver Meeting, November 8–13, 2012, Boston, Massachusetts.
Footnotes
For Further Reading: Lorenza Rimassaa, Tiziana Pressiania, Corrado Boni et al. A Phase II Randomized Dose Escalation Trial of Sorafenib in Patients With Advanced Hepatocellular Carcinoma. The Oncologist 2013;18:379–380.
Abstract
Background. Sorafenib has proven survival benefits in patients with advanced hepatocellular carcinoma (HCC). The viability of continuing sorafenib at a higher dosage in patients who experienced radiologic disease progression was investigated.
Methods. Patients who experienced disease progression while on sorafenib 400 mg twice daily were randomized to sorafenib 600 mg twice daily (n = 49) or best supportive care (n = 52). The primary end point was progression-free survival (PFS). Time to progression, overall survival, and safety were also evaluated.
Results. The study did not meet its primary end point. The difference in PFS between the sorafenib arm (3.91 months) and the best supportive care arm (2.69 months) did not reach statistical significance (p = .086). Adverse events were mainly grade 1–2 and similar across both groups. In the sorafenib arm, the most frequent events were diarrhea (80%), weight loss (75%), fatigue (67%), hand-foot-skin reaction (49%), abdominal pain (37%), and stomatitis (26%).
Conclusion. Escalated-dose sorafenib in patients with advanced HCC who progressed while on sorafenib failed to provide any clinical benefit. Second-line treatment still remains an open issue to be explored in appropriate clinical trials.
Author Contributions
Conception/design: Sandrine Faivre, Maxime Ronot, Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, Chantal Dreyer, Valérie Vilgrain, Eric Raymond
Provision of study material or patients: Sandrine Faivre, Maxime Ronot, Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, Chantal Dreyer, Laurent Castera, Valérie Vilgrain, Jacques Belghiti, Eric Raymond
Collection and/or assembly of data: Sandrine Faivre, Maxime Ronot, Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, Chantal Dreyer, Béatrice Larroque, Valérie Vilgrain, Eric Raymond
Data analysis and interpretation: Sandrine Faivre, Maxime Ronot, Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, Chantal Dreyer, Béatrice Larroque, Laurent Castera, Valérie Vilgrain, Jacques Belghiti, Eric Raymond
Manuscript writing: Sandrine Faivre, Maxime Ronot, Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, Chantal Dreyer, Béatrice Larroque, Laurent Castera, Valérie Vilgrain, Jacques Belghiti, Eric Raymond
Final approval of manuscript: Sandrine Faivre, Maxime Ronot, Mohamed Bouattour, Johanna Wassermann, Onorina Bruno, Chantal Dreyer, Béatrice Larroque, Laurent Castera, Valérie Vilgrain, Jacques Belghiti, Eric Raymond
Disclosures
Mohamed Bouattour: Bayer (H); Eric Raymond: Pfizer, Bayer, Novartis, Eli Lilly (C/A, RF). Sandrine Faivre: Pfizer, Bayer, Novartis, Eli Lilly (C/A, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva, Switzerland: World Health Organization; 1979. WHO handbook for reporting results of cancer treatment. [Google Scholar]
- 4.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: An open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koeberle D, Montemurro M, Samaras P, et al. Continuous sunitinib treatment in patients with advanced hepatocellular carcinoma: A Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06) The Oncologist. 2010;15:285–292. doi: 10.1634/theoncologist.2009-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng A, Kang Y, Lin D et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2011;29(suppl):4000a.
- 11.Faivre SJ, Bouattour M, Dreyer C et al. Sunitinib in hepatocellular carcinoma: Redefining appropriate dosing, schedule, and activity end points [letter]. J Clin Oncol 2009;27:e248–e250; author reply e251–e252. [DOI] [PubMed]
- 12.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:052–060. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 15.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 16.Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 17.Faivre S, Zappa M, Vilgrain V, et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504–4512. doi: 10.1158/1078-0432.CCR-10-1708. [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko S, Furuse J, Kudo M, et al. Guideline on the use of new anticancer drugs for the treatment of hepatocellular carcinoma 2010 update. Hepatol Res. 2012;42:523–542. doi: 10.1111/j.1872-034X.2012.00981.x. [DOI] [PubMed] [Google Scholar]
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 21.Svanholm H, Starklint H, Gundersen HJ, et al. Reproducibility of histomorphologic diagnoses with special reference to the kappa statistic. APMIS. 1989;97:689–698. doi: 10.1111/j.1699-0463.1989.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 22.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 23.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–1316. doi: 10.1016/j.jhep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Memon K, Kulik L, Lewandowski RJ et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 2011;141:526–535. [DOI] [PMC free article] [PubMed]
- 25.Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]