This review article provides evidence of how older patients may differ from younger patients, an approach to assessing older patients, and clinical recommendations to guide treatment recommendations and management of older patients with localized esophageal cancer.
Keywords: Esophageal cancer, Elderly, Chemoradiotherapy, Geriatric oncology
Abstract
Most patients with gastroesophageal cancers are older than 65 years of age. The management of older patients poses challenges because they have multiple comorbidities and physiological changes associated with aging. Furthermore, data are limited on tolerance of cancer therapy and the use of combined-modality treatments in this patient population to guide their treatment. In this article, we focus on the management of older patients with localized esophageal cancer, highlighting the role of comprehensive geriatric assessment to identify and better tailor treatment approaches in this patient population. We review the literature and discuss the role of surgical resection and potential complications specific to an older patient. We review the rationale of combined-modality treatment and the potential benefits of a chemoradiotherapy-based approach in this patient population.
Implications for Practice:
Esophageal cancer is an aggressive malignancy. Tailoring treatment for older patients with esophageal cancer is challenging. This review article provides physicians with evidence of how older patients may differ from younger patients and an approach to assessing older patients. In addition, we offer clinical recommendations to guide oncologists on treatment recommendations and management of older patients with localized esophageal cancer.
Introduction
In the U.S., people older than 65 years of age are expected to compose 20% of the population, or 72 million people, by 2030 [1]. Cancer is a disease associated with aging, and oncologists need to be prepared for this demographic shift. The median age of patients with esophageal and gastroesophageal junction is 68 years, and more than 30% of patients are more than 75 years old at diagnosis [2]. The incidence of esophageal adenocarcinoma is rising, with the highest percentage change in the 65- to 74-year-old age group, heralding an increase in the number of older patients with esophageal cancer.
There is often reluctance to have elderly patients undergo recommended treatment modalities because they may have increased comorbidities, polypharmacy, and physiological changes associated with older age. Surgical resection has been the mainstay of curative treatment. Recent trials in neoadjuvant therapy have clearly shown decreases in recurrence and improvements in overall survival; however, combined-modality treatments with chemotherapy and radiation are often felt to be too toxic for most elderly patients with localized esophageal cancer. Older patients with esophageal cancer are less likely to be referred to a cancer specialist and, if referred, are less likely to receive surgery or chemotherapy, regardless of tumor stage or comorbidities [3]. This finding is not surprising, given the underrepresentation of elderly patients in clinical trials and a paucity of data in this age group.
The scope of this review is to discuss and clarify the management strategies for localized esophageal cancer in the older patient, with a specific focus on evaluation of functional age rather than chronological age as the determinant for decision making.
Assessment of the Older Patient With Esophageal Cancer
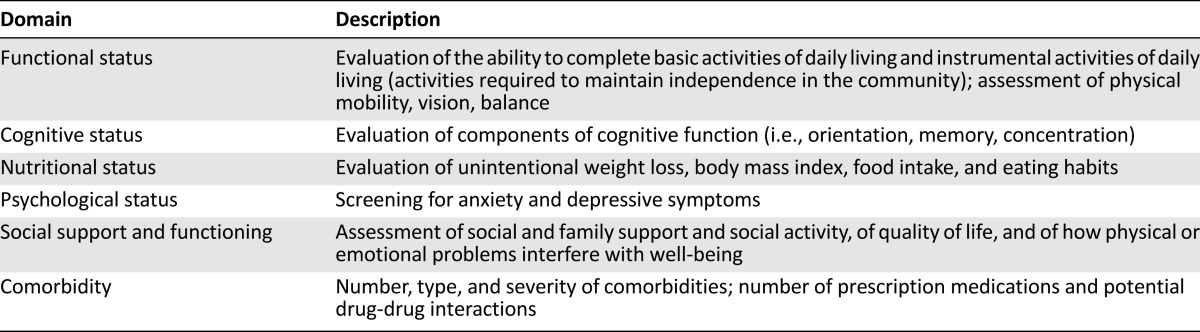
Older patients with esophageal cancer have unique issues that require careful consideration including age and life expectancy, functional status, risk of treatment-related morbidity, competing comorbidities, and desire to receive therapy. The elderly population is characterized by significant variability in the rate of aging; therefore, chronological age does not reflect a patient’s ability to tolerate chemotherapy, radiation, or surgery. Patients of the same age may be robust, with good physical activity levels, or frail, with decreased functional capacity and limited ability to carry out activities of daily living (ADLs). The comprehensive geriatric assessment aims to better evaluate the overall health status and address vulnerabilities in older patients. Geriatricians perform a multidisciplinary assessment that measures independent clinical predictors of morbidity and mortality in older adults [4] (Table 1). This assessment typically has not been used in the oncology setting.
Table 1.
Domains of a comprehensive geriatric assessment
Hurria et al. developed a cancer-specific comprehensive geriatric assessment tool that is mainly self-administered by the patient and is feasible in the setting of an outpatient oncology clinic [5]. The Cancer and Aging Research Group used this tool in a multicenter prospective study to develop a predictive model for chemotherapy toxicity in patients aged ≥65 years [6]. The model identified age of ≥72 years, tumor type (gastrointestinal or genitourinary cancers), polychemotherapy, anemia, creatinine clearance, and geriatric assessment variables (hearing, number of falls, and functional status) as risk factors for toxicity. Although tumor-specific validation of this risk model is needed, these data begin to fill the critical gaps in the knowledge of predictors for chemotherapy toxicity in older adults. These variables are common to patients diagnosed with gastroesophageal cancers, and consideration of toxicity and potential therapy tolerance must be paramount in developing a treatment plan.
We believe that these factors should be carefully considered in older esophageal cancer patients prior to initiating a treatment course, but we also acknowledge that a full geriatric assessment is not feasible to perform in a busy oncology clinic. Some elements of the geriatric assessment, such as inquiring about falls, assessing ability to perform ADLs, and performing a basic cognitive screen with a Mini-Mental Status Exam or a clock drawing test, can be readily incorporated into a history and physical to help identify frail patients, address modifiable risk factors, and make shared and informed decisions. Geriatricians and primary care providers are in optimal positions to help assess the patient’s medical, social, and functional ability to pursue treatment and should be used in the decision-making process. A multidisciplinary network of specialists including nurses, pharmacists, nutritionists, and social workers should be available to support patients through their care.
Esophagectomy in the Older Patient
Surgery has been the mainstay of treatment for localized, resectable esophageal cancer. Endoscopic mucosal resection (EMR) can be therapeutic in patients with high-grade dysplasia and T1 disease limited to the muscularis mucosae who are adequately staged by endoscopic ultrasound [7, 8]; however, most patients present with more advanced disease and are not candidates for EMR. Esophagectomy is a high-risk surgery with serious postoperative complications and reported in-hospital mortality rates ranging from 1% to 23% [9]. Large-population database reviews have demonstrated a consistent adverse effect of increasing age on mortality following esophagectomy [10, 11]. Finlayson et al. used the Nationwide Inpatient Sample and Surveillance, Epidemiology, and End Results-Medicare data to review esophagectomy outcomes of 27,957 patients aged 65 years or older and showed that operative mortality significantly increased with age from 8.8% in the group aged 65–69 years to 13.4% in the group aged 70–79 years and to 19.9% in patients older than 80 years [12].
This increased risk of postsurgical morbidity in older patients has been debated in the literature and appears to be closely related to hospital volume, surgical expertise, and patient selection [13, 14]. Although some high-volume centers report equivalent mortality rates despite patient age, most of the data suggest that older patients are at increased risk for cardiovascular and pulmonary complications and perioperative mortality [15–21].
In weighing the optimal treatment approach in a geriatric patient population, the relative benefit of surgery as part of the management of local disease needs to be considered in the context of the high proportion of patients developing metastatic disease within the first 2 years of diagnosis, even with initial presentation of localized disease. Although surgery as primary treatment is the mainstay of early stage I disease, the risk of metastatic disease development escalates to 60% or higher in patients with T2 or higher T-stage disease, and node positivity at surgery portends a risk in excess of 70%–80% of developing metastatic disease [22, 23].
Preoperative Geriatric Assessment
The role of esophagectomy in an elderly patient needs to be considered carefully in a selected group of robust individuals with high performance status. The functional reserve of a patient, related to aging, has been shown to be a more robust predictor of surgical outcomes than medical comorbidities such as cardiac or pulmonary disease [24].
Preoperative geriatric assessment can enhance the surgical decision-making process for elderly patients with cancer. The inability to independently perform ADLs and impaired cognition were independent predictors of postoperative complications in patients undergoing thoracic surgery [25]. Robinson et al. showed that the preoperative presence of four or more geriatric variables (dependency on ADLs, falls, poor nutrition, anemia, cognitive impairment, and medical comorbidities) has high sensitivity of 81% and specificity of 86% for 6-month postoperative mortality [26]. Audisio et al. studied the combination of traditional surgical risk-assessment tools combined with the comprehensive geriatric assessment to develop a validated instrument in an older patient with cancer, called the Preoperative Assessment of Cancer in the Elderly, or PACE, tool [27]. Preoperative geriatric assessments have not yet been studied specifically in esophagus cancer.
Optimizing postoperative care is important in older patients who are surgical candidates for esophagectomy. This includes using measures of early mobilization, avoiding polypharmacy, and recognizing postoperative delirium early, all of which have been shown to reduce complications and optimize treatment outcomes [28]. A model of comanagement by geriatricians and orthopedic surgeons has been shown to improve outcomes in patients with hip fractures [29]. They reported decreased length of stay, fewer postoperative infections, and decreased number of complications including delirium and cardiopulmonary events. Older patients undergoing esophagectomy may have similar benefits from being followed postoperatively by a geriatrically focused service during hospitalization when possible.
Chemoradiotherapy for Locally Advanced Disease
Despite surgical resection, most patients with locally advanced esophageal cancer (T3 or higher or node-positive disease) will develop recurrent disease. The risk of early systemic spread has led to the evaluation of preoperative therapies to improve outcomes. Preoperative chemoradiotherapy (CRT) has become an accepted standard approach in the U.S. based on the findings of the CROSS trial [30]. In this study, 368 patients with endoscopic ultrasound staged T1N1 or T2–3N0–1 esophageal or gastroesophageal junction cancers were randomized to surgery alone or weekly carboplatin and paclitaxel for 5 weeks with concurrent radiotherapy followed by surgery. The CRT arm showed nearly 2-year improvement in median survival (49 months vs. 26 months) and 13% improvement in 5-year survival rates over surgery alone. Treatment was generally well tolerated, with 23% of patients in the CRT arm reporting grade 3/4 toxicity. Pathologic complete response (pCR) was achieved in 23% of patients with adenocarcinoma and 49% with squamous cancer.
Despite being well-tolerated, the median age in the CROSS study was 60 years (range: 36–79 years). No subset analysis for age was provided in the CROSS study, making it difficult to generalize the findings to an older geriatric patient population. What is the evidence to guide clinicians for chemoradiotherapy in the geriatric population?
Is Chemoradiotherapy Tolerated by the Older Patient?
Data are limited regarding the use of chemoradiotherapy in localized esophageal cancer in older adults. Tougeron et al. reviewed 109 patients aged >70 years (mean age: 74 years; range: 70–88 years) who received radiation with cisplatin-based chemotherapy [31]. Clinical complete response (CCR) was seen in 58% of patients, with 2-year survival of 36%. Grade 3 or greater toxicity was seen in 24% patients, and one death from febrile neutropenia was reported. Similar rates of efficacy and acceptable toxicity were reported by Anderson et al. in 25 patients older than 65 years treated with CRT using 5-fluorouracil (5-FU) and mitomycin [32]. In this small study, the CCR rate was 68%, with 2-year survival of 64% and 36% experiencing significant toxicity. Contrary findings were reported by Takeuchi et al. [33]. In this study, older patients (aged 71 years) had worse survival and higher toxicity compared with younger patients treated with cisplatin and 5-FU (CF regimen) and radiation. Despite their poorer outcomes, the median survival of the older population was still 14.7 months (compared with 35.1 months in younger patients), with 3-year survival of 29% (compared with 49% for younger patients), which is consistent with rates seen in the other studies.
One small prospective study was conducted in 22 elderly patients aged ≥75 years with the primary endpoint of feasibility and efficacy of CRT (5,000 centigrays [cGy] with cisplatin) [34]. The mean age was 79 years, 68% had squamous carcinoma, and the mean Charlson index score was 1. CCR was seen in 64%, with 1-year survival of 62%, and 18% had long-term durable complete remission at a follow-up of 26 months to 5.5 years. Eastern Cooperative Oncology Group performance status was initially stable but then worsened slightly during the last 2 weeks of treatment. Moreover, 22% had grade 2 vomiting, but no nephrotoxicity or toxicity-related death was seen.
The available literature supports the use of chemoradiation in elderly patients with locally advanced esophageal cancers, demonstrating that older patients can tolerate and benefit from combined-modality treatment. However, it is important to note the limitations regarding these data and their applicability to the “average” elderly patient with esophageal cancer. The studies are small and predominantly retrospective in nature. The fact that these older patients were selected by their physicians to undergo chemoradiotherapy suggests that they were more robust at baseline than those who likely received more palliative treatments.
Management of Surgical Candidates: Chemoradiotherapy Plus Surgery
In the physiologically “younger,” robust older patient who is clearly a surgical candidate based on thorough assessment of comorbidities and functional status, preoperative chemoradiation followed by esophagectomy is recommended based on the CROSS data.
Very few studies have examined the tolerability and efficacy of preoperative chemoradiation followed by esophagectomy in older patients. In a retrospective review of 260 patients who underwent neoadjuvant CRT before esophagectomy, Fogh et al. reported no significant differences in mortality in patients >70 years old (7%) compared with younger patients (5%) but found that older patients were more likely to develop supraventricular arrhythmias and respiratory failure requiring intubation [35]. A similar report by Ruol et al. also showed no difference in mortality rates in patients aged <70 years and >70 years receiving neoadjuvant chemoradiation followed by surgery. The median survival rates were equivalent in older and younger patients (23.1 months vs. 23.7 months), and pCR rates were similar (26% vs. 23%) [36]. There were increased cardiovascular complications, defined as myocardial infarction, severe arrhythmia, pulmonary edema, or pulmonary embolism, in the older patients. A third study, by Camerlo et al., reported very similar results [37].
These studies indicate that a very selected group of fit elderly patients who are surgical candidates appear to tolerate neoadjuvant CRT with no significant increase in mortality or morbidity compared with younger cohorts. Older patients are at increased risk for cardiopulmonary complications, likely related to the surgery and not the CRT; similar increased complications are seen in the surgical literature examining esophagectomy alone in the elderly.
Management of Nonsurgical Candidates: Definitive Chemoradiotherapy
Due to frailty, medical comorbidities, advanced age, or patient preference, many older patients with localized esophageal cancer are not candidates for surgical resection. For elderly patients, definitive chemoradiotherapy is an alternative to surgery that can achieve long-term disease control and that is potentially curative. For squamous cancers, primary chemoradiotherapy is acceptable practice with reservation of surgery as salvage for biopsy-positive persistent local disease [38, 39].
The use of primary or definitive chemoradiation has been established by the Radiation Therapy Oncology Group 85-01 trial in locally advanced esophageal cancer treated without surgery [40]. In this study, patients treated with chemoradiotherapy (CF regimen and 5,000 cGy) demonstrated improved overall survival (38% vs. 10% at 2 years and 26% vs. 0% at 3 years) and median survival (12.5 months vs. 8.9 months) compared with the radiation-only arm (6,400 cGy). Surgery was reserved only for recurrences or complications. Given these results, radiation alone is not recommended as a primary strategy for patients with localized disease. Subsequent randomized studies have confirmed these findings of definitive CRT with a CCR rate of approximately 50%–65%, median overall survival of 12–26 months, and a 2-year survival rate of 30%–40%, with higher responses seen in squamous cell histology [38, 40–42].
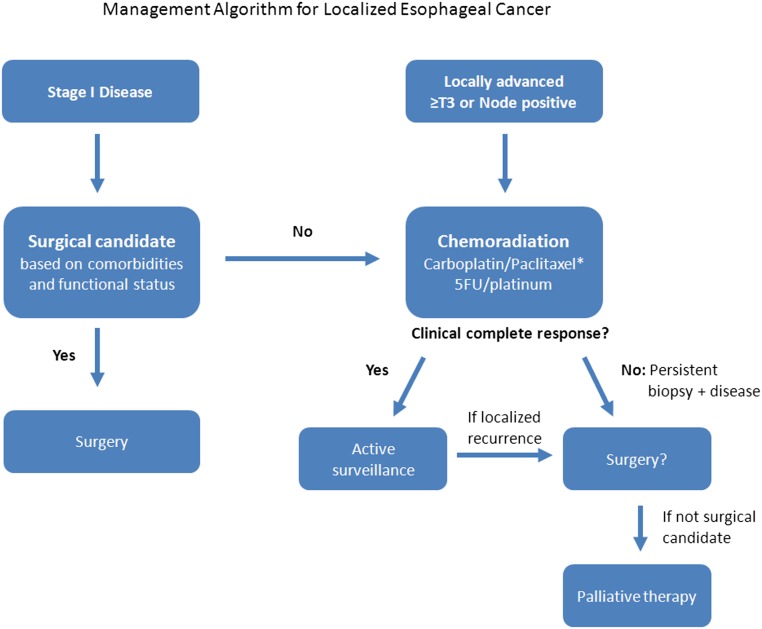
With the exception of the fittest, physiologically intact patients, in elderly patients with locally advanced resectable disease (T3–4, any regional node-positive tumors), our recommendation would be to treat with primary chemoradiotherapy and elective consideration for subsequent surgery dependent on response to therapy and on medical comorbidities rather than treatment with primary, upfront surgery. In patients with early stage disease (T1–2N0) who are not surgical candidates, we recommend chemoradiation as an alternative to surgery (Fig. 1).
Figure 1.
Management algorithm for localized esophageal cancer. *, Preferred regimen.
Abbreviation: 5-FU, 5-fluorouracil.
We favor this approach to chemoradiotherapy over surgical resection alone based on the potential to avoid or defer esophagectomy for some elderly patients and the evidence demonstrating improved outcomes when chemoradiotherapy precedes surgery. In addition, individuals with more aggressive biology, who will develop distant metastatic disease early in their treatment course, would be spared surgical resection if there were to be early development of distant disease.
Although pre- or perioperative chemotherapy have been shown to improve outcomes [43–45], this approach would be less preferred in a geriatric population, given the very low rates of clinical or pathologic complete responses and the necessary requirement for surgery. If there is consideration, particularly in a geriatric population, to avoid surgery as part of disease management, combined chemoradiotherapy with the ability to achieve pathological complete response makes this treatment approach more optimal for this population.
The available literature supports the use of chemoradiation in elderly patients with locally advanced esophageal cancers as an alternative to mandatory surgical resection. It is effective, with a 40%–60% clinical complete response rate (higher for squamous histology) and a fair percentage of patients achieving 2-year survival endpoints. Toxicity of CRT when patients are closely monitored is acceptable. The relatively low rate of toxicity for the CROSS regimen—a modern regimen that substitutes the far better tolerated carboplatin for cisplatin and that uses weekly paclitaxel compared with the more cumbersome infusional 5-FU—argues for consideration of use of this regimen, even in the definitive CRT setting. Rates of pathologic complete response compare with those of CF-based preoperative CRT trials, and the 49% rate of pCR in squamous cancers on the CROSS trial is one of the highest reported [30]. We believe this approach of definitive chemoradiotherapy offers a potentially curative and tolerable treatment for the majority of elderly patients for whom surgical resection would not be indicated.
The available literature supports the use of chemoradiation in elderly patients with locally advanced esophageal cancers as an alternative to mandatory surgical resection. It is effective, with a 40%–60% clinical complete response rate (higher for squamous histology) and a fair percentage of patients achieving 2-year survival endpoints.
Post-Treatment Surveillance After Chemoradiotherapy
As per the 2013 National Comprehensive Cancer Network guidelines for the care of esophageal and gastroesophageal junction cancers, all patients should undergo assessment with endoscopy and biopsy 5–6 weeks after completion of therapy [46]. Repeat computed tomography (CT) imaging is recommended at this time to rule out interval development of metastatic disease. Patients who achieve a clinical complete response, as indicated by a negative endoscopy and biopsy and imaging, should continue to be monitored carefully. There are no clear guidelines for ongoing endoscopic surveillance after chemoradiation. Our practice for surveillance is for clinical visits every 3–4 months for the first 1–2 years, with endoscopy and CT imaging repeated at 6 months and 1 year after completion of treatment or earlier if clinically indicated. For patients who continue to maintain a response, we recommend annual endoscopy surveillance, with CT imaging and clinical visits twice yearly for 3–5 years and followed thereafter with an annual endoscopy and clinical visit.
Treatment Options for Persistent Disease After Chemoradiotherapy
Salvage Surgery
Chemoradiotherapy can be followed by surgical resection in the subset of robust elderly patients who want aggressive treatment and would tolerate a subsequent esophagectomy and should be considered in patients with biopsy-positive residual disease after completion of CRT (Fig. 1). For patients who initially achieved a clinical complete response and then developed biopsy-proven local-regional recurrence, delayed salvage esophagectomy after failed CRT may be a potential option for a selected group of patients.
Salvage esophagectomy has been associated with high rates of anastomotic leak and conduit loss, complications that carry major morbidity and mortality [47]. One recent study suggests that salvage esophagectomy may result in survival outcomes and postoperative morbidity and mortality similar to those of patients undergoing immediate surgery. Marks et al. reported the M.D. Anderson Cancer Center experience of 65 patients with esophageal adenocarcinoma who underwent salvage esophagectomy after failed CRT compared with age-matched patients who received preoperative CRT followed by planned esophagectomy [48]. The median age was 63 years, and median time to surgery from completion of CRT for the salvage group was 216 days. The major postoperative events (major pulmonary event, conduit leak, readmission to the intensive care unit) were not increased in the salvage group, occurring in 35% of patients compared with 31% in the age-matched planned resection group (p = .719). Respective median and 3-year overall survival rates were not statistically different between the two groups (salvage group: 32 months, 48%; planned group: 48 months, 57%; p = .222). Salvage esophagectomy may be a viable treatment option for a subset of robust older patients who fail definitive chemoradiotherapy.
Endoscopic Mucosal Resection
Patients with locally persistent or recurrent disease, if correctly identified, are potential candidates for local therapy, especially those who are not candidates for salvage surgery. Endoscopic mucosal resection can treat T1a disease, carcinoma limited to the lamina propria or muscularis mucosae, in the absence of lymph node metastasis, lymphovascular differentiation, or poor differentiation grade. Studies from Japan have reported the outcomes of EMR after CRT in esophageal squamous cell carcinoma and showed 3-year survival rates of 56%–64% [49, 50]. In a pooled analysis of patients undergoing EMR, the most common complications included bleeding and stenosis (occurring in 10% and 6% of patients, respectively) [51].
Brachytherapy
Intraluminal brachytherapy has been used as a palliative treatment for advanced esophageal cancer. High-dose-rate brachytherapy can deliver a higher dose to the superficial lesions of the esophagus while delivering much lower doses to surrounding tissues. This modality has been explored in patients with recurrent disease following a definitive course of external beam radiotherapy. Folkert et al. reported their institutional experience of 14 patients (median age: 76 years) treated with high-dose-rate brachytherapy given as three weekly fractions [52]. Of the 10 patients with recurrent disease who received prior external beam radiotherapy, the overall freedom from failure was 11.1% and overall survival was 55.6% at 18 months. Approximately half of the patients received concurrent chemotherapy with capecitabine (1,000-mg flat dose twice daily for 5 days per week) given throughout the duration of their treatment. Esophageal stricture grade 2 was seen in 7%, and one patient developed a grade 3 stricture and, subsequently, a tracheoesophageal fistula.
Sharma et al. treated 21 patients with post-treatment recurrent tumors [53]. Dysphagia was improved in 6 of 21 (28%) for a median of 6 months, with median overall survival of 7 months. The most common serious complications were esophageal strictures in 5 of 21 patients (24%) and esophageal ulceration in 3 of 21 (14%). This modality may be a modest palliative option for older patients with recurrent localized disease who are not candidates for salvage surgery but may be appropriate for patients able to receive further radiation therapy.
Systemic Chemotherapy and Best Supportive Care
For patients with localized disease who are not candidates for combined-modality chemoradiation, who have recurrent disease, or who develop distant metastasis after initial therapy, improving and maintaining quality of life and symptom relief are paramount goals. Best supportive care is an appropriate treatment option for patients with declining functional status. Chemotherapy should be individualized based on a patient’s functional status, comorbidities, and goals of care.
Dysphagia is the most common symptom in patients with esophageal cancer. Assessment of swallowing impairment and initiation of appropriate interventions is essential. Older patients with esophageal cancer are likely to be at the highest risk of malnutrition because of increased medical comorbidities compounded by normal physiological changes of decreased muscle mass and geriatric issues of altered cognition, mobility, mood, and social support and access to nutrition [54]. Poor nutrition has been shown to be a predictive risk factor for death in elderly patients undergoing chemotherapy for cancer [55].
Older patients with esophageal cancer are likely to be at the highest risk of malnutrition because of increased medical comorbidities compounded by normal physiological changes of decreased muscle mass and geriatric issues of altered cognition, mobility, mood, and social support and access to nutrition. Poor nutrition has been shown to be a predictive risk factor for death in elderly patients undergoing chemotherapy for cancer.
Chemotherapy can potentially improve or maintain stability of quality of life and relieve dysphagia in 60%–80% of patients [56–58], but with short time to progression, patients often need additional palliation for their dysphagia symptoms. Palliative methods for management of dysphagia include endoscopic dilatation, stent placement, and brachytherapy [59, 60].
A multidisciplinary approach involving palliative care specialists, social work, and primary care physicians is essential to treating all patients with esophageal cancer. Advanced directives and goals of care should be discussed with the patient and caregivers in a sensitive but timely manner. Addressing issues of dysphagia, pain, nausea, dyspnea, and psychological distress is an integral part of oncology care.
Conclusion
As our patient population ages, oncology research and treatment guidelines need to adapt accordingly. Older patients present with complex coexisting conditions and frailty, making it difficult to extrapolate trial data to this population. Age alone should not be the determinant for the selection of patients for treatment in esophageal cancer. Older patients clearly can benefit from surgery, chemotherapy, and radiation.
Several recommendations are proposed for the management of localized esophagus and gastroesophageal junction cancers in the geriatric population. First, stage I patients should undergo primary surgery. Chemoradiotherapy can be used as primary treatment if functional status and comorbidities preclude surgery. Second, for patients with locally advanced tumors (T3 or higher or node positive), definitive chemoradiotherapy is preferred, with consideration of upfront surgery for a selected subset of patients who are surgical candidates based on function, comorbidities, and life expectancy. Higher rates of clinical and pathological complete response are achieved with CRT, and the potential to selectively apply or defer surgery argues in favor of this approach. Preoperative chemotherapy is less preferred because it mandates surgical resection. Third, for squamous cell carcinoma, primary CRT is an accepted standard approach, with selective application of surgery for persistent disease. Fourth, carboplatin and paclitaxel should be considered as a preoperative and definitive CRT regimen, given the low rates of toxicity and high rates of pCR demonstrated in the CROSS data.
Treatment strategies should be based on thorough assessment of the individual’s functional status and potential deterioration of organ function and physiological reserve caused by aging as well as consideration of patient wishes. Fit older patients may derive the same benefit from aggressive treatments as younger patients, and functional age should guide treatment decisions. Age-specific modifications of some treatment paradigms may be appropriate because therapy tolerance and risk of toxicities vary according to patient age and burden of comorbidities. It is important to monitor closely for adverse effects, to make appropriate adjustments, and to offer sufficient care in the follow-up stages of treatment. Prospective trials involving older patients are critically important to develop guidelines for treatment and to understand tolerance of anticancer therapies in this rapidly growing population.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Footnotes
For Further Reading: Patrick M. Ford, Ronan J. Kelly. Genomic Alterations in Advanced Esophageal Cancer May Lead to Subtype-Specific Therapies. The Oncologist 2013;18:823–832.
Implications for Practice: The disease burden of esophageal cancer is increasing in the United States and worldwide, primarily driven by higher rates of adenocarcinoma risk factors, including obesity and Barrett's esophagus. Chemotherapy has moderate efficacy for locally advanced and metastatic esophageal cancer, but new approaches to treatment are urgently needed. This article focuses on potential oncogenic targets in esophageal cancer and comprehensively reviews the current state of the art in targeted therapy for esophageal and gastroesophageal junction tumors. Anti-human epidermal growth factor receptor-2 therapy has provided benefit for a small proportion of patients; however, despite signs of efficacy in early phase clinical trials, results with anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapy have been generally disappointing. Experience to date with targeted agents suggests that collaborative trials of target-specific agents in those subgroups of patients who have potential oncogenic drivers represent the best opportunity for bringing novel agents to the clinic.
Author Contributions
Conception/Design: Elizabeth Won, David H. Ilson
Collection and/or assembly of data: Elizabeth Won
Data analysis and interpretation: Elizabeth Won, David H. Ilson
Manuscript writing: Elizabeth Won, David H. Ilson
Final approval of manuscript: Elizabeth Won, David H. Ilson
Disclosures
David H. Ilson: Lilly/Imclone, AMGEN (C/A); AMGEN, Bayer, Bristol-Myers Squibb (RF); Genentech (H). The other author indicates no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Profile of older Americans. Available at http://www.aoa.gov/AoARoot/Aging_Statistics/Profile/index.aspx Accessed April 23, 2013.
- 2.Previous version: SEER cancer statistics review, 1975-2009 (vintage 2009 populations). Updated August 20, 2012. Available at http://seer.cancer.gov/csr/1975_2009_pops09/ Accessed October 18, 2013.
- 3.Steyerberg EW, Neville B, Weeks JC, et al. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: A population-based analysis of elderly patients. J Clin Oncol. 2007;25:2389–2396. doi: 10.1200/JCO.2006.09.7931. [DOI] [PubMed] [Google Scholar]
- 4.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 5.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: A feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 6.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 8.Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16–23. doi: 10.1016/s0016-5107(05)00319-6. [DOI] [PubMed] [Google Scholar]
- 9.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: A ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–222. doi: 10.1016/s0003-4975(02)04368-0. discussion 222. [DOI] [PubMed] [Google Scholar]
- 11.Ra J, Paulson EC, Kucharczuk J, et al. Postoperative mortality after esophagectomy for cancer: Development of a preoperative risk prediction model. Ann Surg Oncol. 2008;15:1577–1584. doi: 10.1245/s10434-008-9867-4. [DOI] [PubMed] [Google Scholar]
- 12.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J Am Coll Surg. 2007;205:729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 13.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126–1132. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 14.Kuo EY, Chang Y, Wright CD. Impact of hospital volume on clinical and economic outcomes for esophagectomy. Ann Thorac Surg. 2001;72:1118–1124. doi: 10.1016/s0003-4975(01)02962-9. [DOI] [PubMed] [Google Scholar]
- 15.Adam DJ, Craig SR, Sang CTM, et al. Esophagectomy for carcinoma in the octogenarian. Ann Thorac Surg. 1996;61:190–194. doi: 10.1016/0003-4975(95)00932-9. [DOI] [PubMed] [Google Scholar]
- 16.Bonavina L, Incarbone R, Saino G, et al. Clinical outcome and survival after esophagectomy for carcinoma in elderly patients. Dis Esophagus. 2003;16:90–93. doi: 10.1046/j.1442-2050.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 17.Rice DC, Correa AM, Vaporciyan AA, et al. Preoperative chemoradiotherapy prior to esophagectomy in elderly patients is not associated with increased morbidity. Ann Thorac Surg. 2005;79:391–397. doi: 10.1016/j.athoracsur.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 18.Moskovitz AH, Rizk NP, Venkatraman E, et al. Mortality increases for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2006;82:2031–2036. doi: 10.1016/j.athoracsur.2006.06.053. discussion 2036. [DOI] [PubMed] [Google Scholar]
- 19.Morita M, Egashira A, Yoshida R, et al. Esophagectomy in patients 80 years of age and older with carcinoma of the thoracic esophagus. J Gastroenterol. 2008;43:345–351. doi: 10.1007/s00535-008-2171-z. [DOI] [PubMed] [Google Scholar]
- 20.Pultrum BB, Bosch DJ, Nijsten MW, et al. Extended esophagectomy in elderly patients with esophageal cancer: Minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572–1580. doi: 10.1245/s10434-010-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2010;90:900–907. doi: 10.1016/j.athoracsur.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 22.Abate E, DeMeester SR, Zehetner J, et al. Recurrence after esophagectomy for adenocarcinoma: Defining optimal follow-up intervals and testing. J Am Coll Surg. 2010;210:428–435. doi: 10.1016/j.jamcollsurg.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Gu Y, Swisher SG, Ajani JA, et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer. 2006;106:1017–1025. doi: 10.1002/cncr.21693. [DOI] [PubMed] [Google Scholar]
- 24.Zenilman M. Geriatric surgery: Past, present, and future. Arch Surg. 2012;147:10. doi: 10.1001/archsurg.2011.1040. [DOI] [PubMed] [Google Scholar]
- 25.Fukuse T, Satoda N, Hijiya K, et al. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–891. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 27.Audisio RA, Pope D, Ramesh HS, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65:156–163. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 29.Friedman SM, Mendelson DA, Kates SL, et al. Geriatric co-management of proximal femur fractures: Total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56:1349–1356. doi: 10.1111/j.1532-5415.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- 30.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 31.Tougeron D, Di Fiore F, Thureau S, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer. 2008;99:1586–1592. doi: 10.1038/sj.bjc.6604749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson SE, Minsky BD, Bains M, et al. Combined modality chemoradiation in elderly oesophageal cancer patients. Br J Cancer. 2007;96:1823–1827. doi: 10.1038/sj.bjc.6603821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi S, Ohtsu A, Doi T, et al. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol. 2007;30:607–611. doi: 10.1097/COC.0b013e3180ca7c84. [DOI] [PubMed] [Google Scholar]
- 34.Servagi-Vernat S, Bosset M, Crehange G, et al. Feasibility of chemoradiotherapy for oesophageal cancer in elderly patients aged >or=75 years: A prospective, single-arm phase II study. Drugs Aging. 2009;26:255–262. doi: 10.2165/00002512-200926030-00006. [DOI] [PubMed] [Google Scholar]
- 35.Fogh SE, Yu A, Kubicek GJ, et al. Do elderly patients experience increased perioperative or postoperative morbidity or mortality when given neoadjuvant chemoradiation before esophagectomy? Int J Radiat Oncol Biol Phys. 2011;80:1372–1376. doi: 10.1016/j.ijrobp.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 36.Ruol A, Portale G, Castoro C, et al. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2007;14:3243–3250. doi: 10.1245/s10434-007-9455-z. [DOI] [PubMed] [Google Scholar]
- 37.Camerlo A, D’Journo XB, Ouattara M, et al. Adenocarcinoma of the esophagus and esophagogastric junction in patients older than 70 years: Results of neoadjuvant radiochemotherapy followed by transthoracic esophagectomy. J Vis Surg. 2012;149:e203–e210. doi: 10.1016/j.jviscsurg.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 39.Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 40.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 41.Coia LR, Minsky BD, Berkey BA, et al. Outcome of patients receiving radiation for cancer of the esophagus: Results of the 1992-1994 Patterns of Care study. J Clin Oncol. 2000;18:455–462. doi: 10.1200/JCO.2000.18.3.455. [DOI] [PubMed] [Google Scholar]
- 42.Ohtsu A, Boku NO, Muro K, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–2921. doi: 10.1200/JCO.1999.17.9.2915. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 44.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 45.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed October 18, 2013.
- 47.Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg. 2007;94:1059–1066. doi: 10.1002/bjs.5865. [DOI] [PubMed] [Google Scholar]
- 48.Marks JL, Hofstetter W, Correa AM, et al. Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg. 2012;94:1126–1132. doi: 10.1016/j.athoracsur.2012.05.106. discussion 1132–1133. [DOI] [PubMed] [Google Scholar]
- 49.Hattori S, Muto M, Ohtsu A, et al. EMR as salvage treatment for patients with locoregional failure of definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc. 2003;58:65–70. doi: 10.1067/mge.2003.306. [DOI] [PubMed] [Google Scholar]
- 50.Yano T, Muto M, Minashi K, et al. Endoscopic mucosal resection (EMR) and photodynamic therapy (PDT) as curative salvage treatments for local failure after definitive chemoradiotherapy (CRT) for esophageal cancer (EC) [abstract] Gastrointest Endosc. 2007;65:AB143. doi: 10.1016/s0016-5107(05)00545-6. [DOI] [PubMed] [Google Scholar]
- 51.McCann P, Stafinski T, Wong C, et al. The safety and effectiveness of endoscopic and non-endoscopic approaches to the management of early esophageal cancer: A systematic review. Cancer Treat Rev. 2011;37:11–62. doi: 10.1016/j.ctrv.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Folkert MR, Cohen GN, Wu AJ, et al. Endoluminal high-dose-rate brachytherapy for early stage and recurrent esophageal cancer in medically inoperable patients. Brachytherapy. 2013;12:463–470. doi: 10.1016/j.brachy.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Sharma V, Mahantshetty U, Dinshaw KA, et al. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002;52:310–315. doi: 10.1016/s0360-3016(01)01822-3. [DOI] [PubMed] [Google Scholar]
- 54.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 56.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: The V-325 Study Group. J Clin Oncol. 2007;25:3210–3216. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 57.Ribi K, Koeberle D, Schuller JC, et al. Is a change in patient-reported dysphagia after induction chemotherapy in locally advanced esophageal cancer a predictive factor for pathological response to neoadjuvant chemoradiation? Support Care Cancer. 2009;17:1109–1116. doi: 10.1007/s00520-008-0570-6. [DOI] [PubMed] [Google Scholar]
- 58.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 59.Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: Multicentre randomised trial. Lancet. 2004;364:1497–1504. doi: 10.1016/S0140-6736(04)17272-3. [DOI] [PubMed] [Google Scholar]
- 60.Ross WA, Alkassab F, Lynch PM, et al. Evolving role of self-expanding metal stents in the treatment of malignant dysphagia and fistulas. Gastrointest Endosc. 2007;65:70–76. doi: 10.1016/j.gie.2006.04.040. [DOI] [PubMed] [Google Scholar]