Abstract
Mucositis may limit the therapeutic window for mammalian target of rapamycin inhibitor-based combination therapy, necessitating treatment interruptions and/or dose reductions. Optimizing treatment or prophylactic interventions for mucositis will enable patients to continue effective treatment while maintaining good quality of life.
Introduction
Temsirolimus is a novel mammalian target of rapamycin (mTOR) inhibitor, approved by the U.S. Food and Drug Administration for advanced renal cell carcinoma [1, 2]. mTOR inhibitors have demonstrated encouraging efficacy results in patients with a range of advanced malignancies, alone or in combination with other chemotherapy agents or targeted therapies in trials [3–9]. Different molecules have been combined with temsirolimus to overcome resistance to single-agent mTOR inhibitors [10].
Mucositis, one of the most common dose-limiting toxicities, is a common side effect of mTOR inhibitor-based treatment, is dose related, and occurs in earlier cycles [2, 3, 11, 12]. The mucositis incidence related to single-agent temsirolimus treatment was 41.3% (86 of 208 patients) in patients with advanced renal cell carcinoma, with 2.8% (6 of 208) at grade 3 or higher [2]. However, a recent review of all temsirolimus-based treatment demonstrated that the mucositis incidence rate was 60.8% (819 of 1,347 patients), with 5.2% (70 of 1,347) of patients developing grade 3 or 4 lesions [13].
This institutional review board-approved retrospective data review focused on three open label phase I clinical trials of temsirolimus-based combination therapy for which the second agent is not known to cause significant mucositis. These three trials used temsirolimus combined with metformin (ClinicalTrials.gov identifier NCT01529593) or cixutumumab, a fully humanized monoclonal antibody that blocks against insulin-like growth factor-1 receptor (ClinicalTrials.gov identifier NCT00678769), or pimasertib (also known as MSC1936369B), a mitogen-activated kinase (MEK) 1/2 inhibitor (ClinicalTrials.gov identifier NCT01378377). We investigated whether there was an association between the severity of mucositis and tumor response to the temsirolimus-based combination treatment. Temsirolimus was administered as intravenous infusion once weekly over a 21-day or 28-day cycle. The starting dose of temsirolimus was 12.5 mg by intravenous administration (i.v.) weekly when MSC1936369B was used as a combination agent. For the two other trials, a standard dose of 25 mg by i.v. weekly was used in cohort 1.
Mucositis diagnoses were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4 [14]. Patients with stable disease lasting 6 months or longer were considered to have durable stable disease.
Mucositis Treatment and Efficacy Evaluation
Treatment for the management of mucositis was started at its initial presentation. The regimens used were previously described by Naing et al. [7]. Based on physician discretion, some patients received one drug or more from the above regimens for mucositis. Response to mucositis treatment was defined as downgrade of mucositis of at least one level according to the CTCAE [14]. For example, a patient will have achieved a response to mucositis if the patient had grade 2 mucositis that later decreased to grade 1 when treated with one drug or more from the mucositis regimen.
Results
There were 77 patients who received a temsirolimus dose of 25 mg by i.v. weekly. Mucositis occurred in 56 of 87 patients (64.4%; 95% confidence interval: 53%–74%) treated in one of the three combination studies. The mucositis grades at initial presentation for the 56 patients were grade 1 (78.6%, n = 44) and grade 2 (21.4%, n = 12). No grade 3 or 4 mucositis was noted at initial presentation.
Eight patients eventually developed grade 3 mucositis. All eight patients had a dose delay because of grade 3 mucositis, and four patients had dose reductions because of grade 3 mucositis only. Three patients never resumed treatment because of progression of disease.
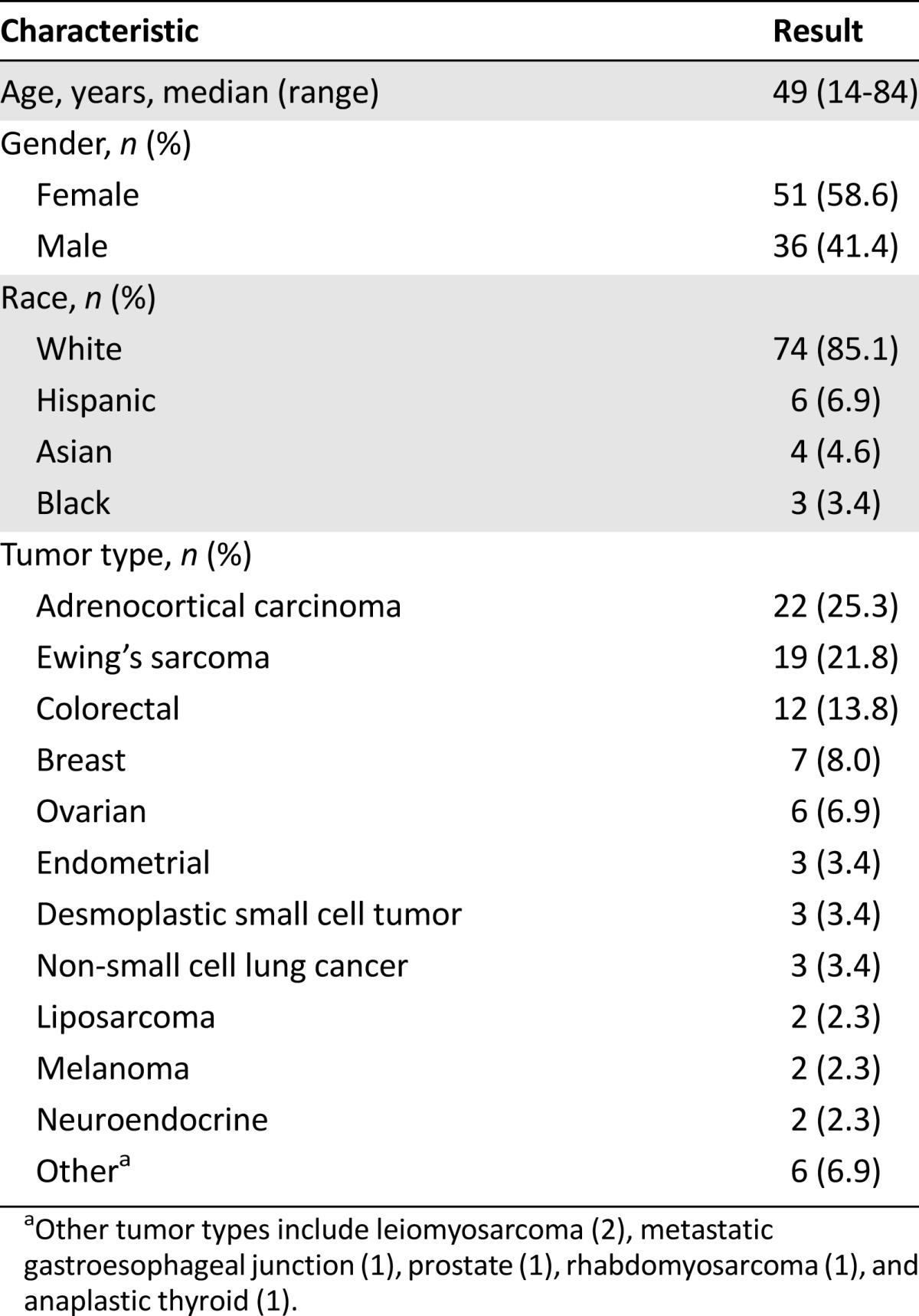
The median onset time (either reported by the patient or observed by the physician) of initial mucositis was 14 days after the start of the treatment. The association between gender and ethnicity to the incidence of mucositis was inconclusive (p > .05) (Table 1).
Table 1.
Demographics of patients (n = 87)
Discussion
The incidence of mucositis in our temsirolimus-based combination trials was significantly greater than that of single-agent temsirolimus treatment (41.3%, p = .0003). Moreover, the incidence rate in the group with mucositis higher than grade 2 was 9.2% higher than the 3% rate in temsirolimus single-agent treatment group [2].
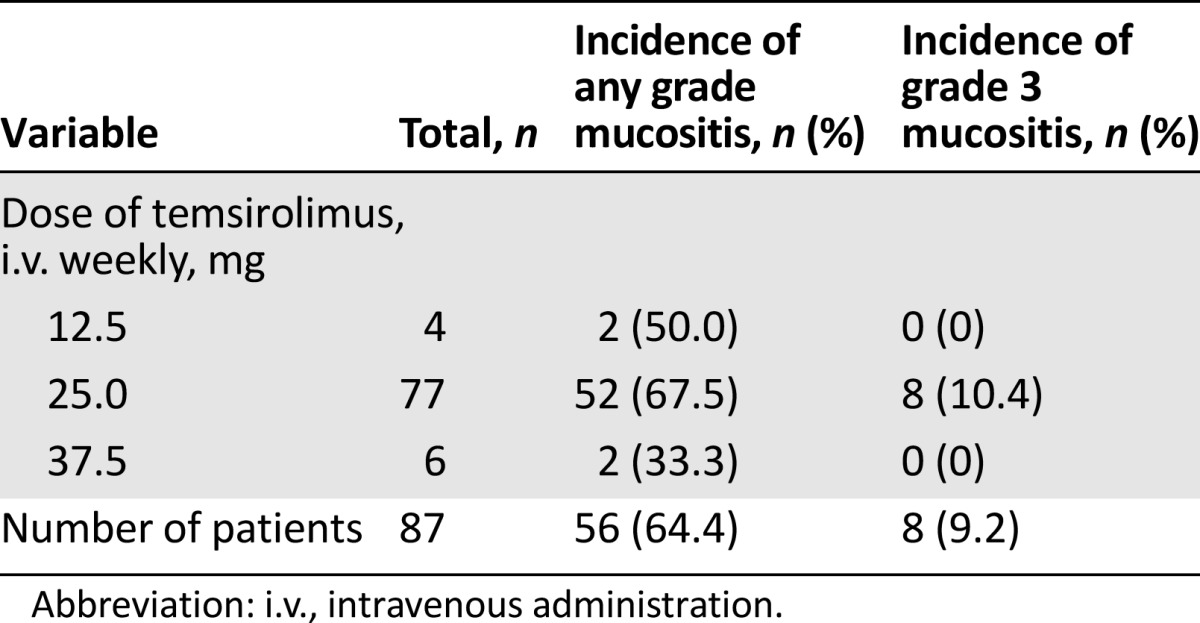
Although we had previously suggested that more severe mucositis may be correlated with a better response to temsirolimus-based cancer treatment [9], our current results suggest that response to the temsirolimus-based treatment increased with a higher grade of mucositis. These results, however, are statistically inconclusive (odds ratio: 1.4; 95% confidence interval: 0.9–2.4; p = .17). Furthermore, neither incidence nor severity correlated with dose levels of temsirolimus. One limitation of this analysis was that only 10 patients received temsirolimus at 12.5 mg by i.v. weekly (n = 4) or 37.5 mg by i.v. weekly (n = 6), creating insufficient statistical power because of cohort sample size imbalance (Table 2).
Table 2.
Incidence of mucositis stratified by temsirolimus dose level
With aggressive management with the mucositis regimen at initial presentation of mucositis, we achieved an 83% response rate for mucositis, and eight patients (9.2%) eventually developed grade 3 mucositis that required treatment hold or dose reduction. The median time to improvement of mucositis treatment was 14 days; however, no standard protocol was used to treat mucositis. Based on physician discretion, some patients received one of our mucositis regimens or more [7]. Better regimens for mucositis are indeed warranted.
Conclusion
Mucositis may limit the therapeutic window for mTOR inhibitor-based combination therapy, necessitating treatment interruptions and/or dose reductions. Optimizing treatment or prophylactic interventions for mucositis will enable patients to continue effective treatment while maintaining good quality of life. It is of great importance to devise a means of early detection, an optimal regimen, and supportive care in both prophylactic and treatment settings.
Acknowledgments
We thank Kristie Lawhorn and Sylvia Barrientes for coordinating and the collection of data. This study was supported by R21CA13763301A1 (A.N.), U01CA62461 (R.K.), and U01CA62487 (P.L.).
The data were presented in part at the American Association for Cancer Research, the National Cancer Institute, and the European Organisation for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics meeting, November 15–19, 2009, Boston, Massachusetts, USA; at the National Cancer Institute Cancer Therapy Evaluation Program Early Drug Development meeting, October 2009, Bethesda, Maryland, USA; at the National Cancer Institute Cancer Therapy Evaluation Program Early Drug Development meeting, October 2011, Bethesda, Maryland, USA; at the 46th annual meeting of the American Society of Clinical Oncology, June 4–8, 2010, Chicago, Illinois, USA; at the 47th annual meeting of the American Society of Clinical Oncology, June 3–7, 2011, Chicago, Illinois, USA; and at the American Association for Cancer Research, the National Cancer Institute, and the European Organization for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics meeting, November 6–9, 2012, Dublin, Ireland.
Disclosures
Ekaterine Asatiani: Merck, EMD Serono (E); Patricia LoRusso: Genentech, Novartis, and Astellas (C/A); Genentech (H); National Cancer Institute, Amgen, Array Biopharma, Ariad, AstraZeneca, Boehringer-Ingelheim, Endocyte (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 2.Kwitkowski VE, Prowell TM, Ibrahim A, et al. FDA approval summary: Temsirolimus as treatment for advanced renal cell carcinoma. The Oncologist. 2010;15:428–435. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gridelli C, Maione P, Rossi A. The potential role of mTOR inhibitors in non-small cell lung cancer. The Oncologist. 2008;13:139–147. doi: 10.1634/theoncologist.2007-0171. [DOI] [PubMed] [Google Scholar]
- 4.Fujisaka Y, Yamada Y, Yamamoto N, et al. A phase 1 clinical study of temsirolimus (CCI-779) in Japanese patients with advanced solid tumors. Jpn J Clin Oncol. 2010;40:732–738. doi: 10.1093/jjco/hyq047. [DOI] [PubMed] [Google Scholar]
- 5.Margolin K, Longmate J, Baratta T, et al. CCI-779 in metastatic melanoma: A phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 6.Mita M, Sankhala K, Abdel-Karim I, et al. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–1954. doi: 10.1517/13543780802556485. [DOI] [PubMed] [Google Scholar]
- 7.Naing A, Kurzrock R, Burger A, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res. 2011;17:6052–6060. doi: 10.1158/1078-0432.CCR-10-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naing A, Lorusso P, Fu S, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108:826–830. doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naing A, LoRusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res. 2012;18:2625–2631. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naing A. Overcoming resistance to mTOR inhibition for enhanced strategies in clinical trials. Expert Opin Investig Drugs. 2013;22:679–685. doi: 10.1517/13543784.2013.795947. [DOI] [PubMed] [Google Scholar]
- 11.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009;20:1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 12.Sonis S, Treister N, Chawla S, et al. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210–215. doi: 10.1002/cncr.24696. [DOI] [PubMed] [Google Scholar]
- 13.Martins F, de Oliveira MA, Wang Q, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49:293–298. doi: 10.1016/j.oraloncology.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.


