This exploratory analysis of pooled data from selected older patients with pretreated metastatic breast cancer in phase II and III clinical trials showed similar efficacy and tolerability for eribulin mesylate among patients who were 70 years of age or older when compared with younger patient subgroups. These data indicate that eribulin may be an effective option for older patients.
Keywords: Eribulin mesylate, Metastatic breast cancer, Age, Chemotherapy
Abstract
Purpose.
Following the demonstrated efficacy and safety of eribulin mesylate in heavily pretreated patients with metastatic breast cancer, an exploratory analysis was performed to investigate the effect of age in these patients.
Methods.
Data were pooled from two single-arm phase II studies and one open-label randomized phase III study in which patients received eribulin mesylate at 1.4 mg/m2 as 2- to 5-minute intravenous infusions on days 1 and 8 of a 21-day cycle. The effect of age on median overall survival (OS), progression-free survival (PFS), overall response rate (ORR), clinical benefit rate (CBR), and incidence of adverse events (AEs) was calculated for four age groups (<50 years, 50–59 years, 60–69 years, ≥70 years).
Results.
Overall, 827 patients were included in the analysis (<50 years, n = 253; 50–59 years, n = 289; 60–69 years, n = 206; ≥70 years, n = 79). Age had no significant impact on OS (11.8 months, 12.3 months, 11.7 months, and 12.5 months, respectively; p = .82), PFS (3.5 months, 2.9 months, 3.8 months, and 4.0 months, respectively; p = .42), ORR (12.7%, 12.5%, 6.3%, and 10.1%, respectively), or CBR (20.2%, 20.8%, 20.4%, and 21.5%, respectively). Although some AEs had higher incidence in either the youngest or the oldest subgroup, there was no overall effect of age on the incidence of AEs (including neuropathy, neutropenia, and leukopenia).
Conclusion.
Eribulin monotherapy in these selected older patients with good baseline performance status led to OS, PFS, ORR, CBR, and tolerability similar to those of younger patients with metastatic breast cancer. The benefits and risks of eribulin appear to be similar across age groups.
Implications for Practice:
Although metastatic breast cancer (MBC) affects women of all ages, the use of sequential single-agent chemotherapy treatment in patients with hormone-refractory MBC can be particularly challenging in the elderly because of patient comorbidities and functional deficits. There is a major unmet need to find new, effective therapies with favorable safety profiles for older patients. This exploratory analysis of pooled data from selected older patients with pretreated MBC in phase II and III clinical trials showed similar efficacy and tolerability for eribulin among patients who were 70 years of age or older when compared with younger patient subgroups. These data indicate that eribulin may be an effective option for selected older patients with MBC.
Introduction
Breast cancer is largely a disease of older women. In the U.S., the median age at diagnosis is approximately 61 years [1], and most deaths occur in women aged 65 years and older [2]. The probability of a woman developing invasive breast cancer within the next 10 years increases from 0.06% for a 20-year-old to 3.74% for a 70-year-old [1, 3]. Owing to increased life expectancy in developed countries, the lifetime risk of being diagnosed with breast cancer is also increasing [1], underscoring the need for effective therapies in older patients.
Metastatic breast cancer (MBC) is incurable, so the goals of treatment are to control symptoms, to improve or maintain quality of life, and to prolong survival. However, few agents have demonstrated an overall survival (OS) benefit in the metastatic setting, and the optimal treatment sequence has yet to be established [1, 4]. Consequently, the median OS time for patients with MBC has not improved much in the last decade and is still between 2 years and 3 years when all subtypes are considered together [5, 6]. For the majority of patients, sequential use of single chemotherapeutic agents represents the best strategy if patients are or become refractory to endocrine therapy [7]. For older patients, comorbidities and functional deficits can complicate chemotherapy, resulting in increased toxicity and further loss of function [8–10]. Consequently, there is an unmet need among older patients for therapies that demonstrate efficacy and a favorable safety profile [11]. In particular, there is generally a lack of evidence to support the use of currently available chemotherapeutic agents in older patients with pretreated MBC.
Eribulin mesylate (E7389, Halaven; Eisai Inc., Woodcliff Lake, NJ, http://www.halaven.com) is a structurally modified synthetic analog of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai [12]. Eribulin is a nontaxane microtubule inhibitor with a novel mode of action distinct from other tubulin-targeting agents such as paclitaxel [13–15]. Preclinical studies have indicated that eribulin has antitumor activity in cell lines resistant to paclitaxel and induces less neuropathy than paclitaxel or ixabepilone [16, 17].
Eribulin has recently been granted approval as monotherapy in several countries globally based in part on the results of the phase III Eribulin Monotherapy Versus Treatment of Physician’s Choice in Patients With Metastatic Breast Cancer (EMBRACE) study in patients with heavily pretreated locally recurrent breast cancer or MBC [18]. In this large, randomized clinical trial, eribulin demonstrated a significant improvement in median OS of 2.7 months compared with treatment of physician’s choice (TPC) (hazard ratio: 0.81; 95% confidence interval: 0.68–0.96; nominal p = .014) [18]. In addition, two phase II clinical trials (Study 201 [19] and Study 211 [20]) of eribulin in heavily pretreated patients with locally advanced breast cancer or MBC demonstrated therapeutic activity and a manageable tolerability profile. Across all age groups for patients treated with eribulin in Study 201, Study 211, and EMBRACE, respectively, OS was 9.0 months, 10.4 months, and 13.2 months and progression-free survival (PFS) was 2.6 months, 2.6 months, and 3.7 months [18–20]. Objective response rates (ORRs) were 11.5%, 9.3%, and 12% for Study 201, Study 211, and EMBRACE, respectively, and clinical benefit rates (CBRs) were 17.2%, 17.1%, and 23%, respectively [18–20].
Owing to the observed efficacy of eribulin in heavily pretreated patients with MBC, this exploratory analysis was conducted to determine whether similar therapeutic benefits and tolerability profiles were observed among female patients 70 years and older versus younger patient cohorts using pooled data from these three clinical trials [18–20].
Methods
Study Design and Patients
Full descriptions of the individual study designs and patient inclusion and exclusion criteria have been published previously [18–20]. All three studies enrolled female patients (≥18 years) with histologically or cytologically confirmed breast cancer who had measurable and/or evaluable metastatic disease, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 (0–1 for Study 201), and life expectancy of ≥3 months. All patients provided written informed consent before undergoing any study-specific procedures. The studies were conducted in accordance with the Declaration of Helsinki, and approval was obtained from the relevant ethics committees.
The EMBRACE trial (ClinicalTrials.gov identifier NCT00388726) was a phase III, open-label, randomized, global, multicenter study of eribulin versus TPC (any single-agent chemotherapy, approved hormonal or biologic treatment, radiotherapy, or symptomatic treatment alone) involving patients with heavily pretreated locally recurrent breast cancer or MBC, with previous treatment including an anthracycline and a taxane. Patients with pre-existing grade >2 neuropathy were excluded. The primary endpoint was OS; secondary endpoints included PFS, ORR, and tolerability [18].
Study 201 (ClinicalTrials.gov identifier NCT00097721) was a phase II, open-label, single-arm study involving patients with MBC who had received prior treatment that included at least an anthracycline and a taxane [19]. Study 211 (ClinicalTrials.gov identifier NCT00246090) was a phase II, open-label, single-arm, multicenter study involving patients with locally advanced breast cancer or MBC previously treated with an anthracycline, taxane, and capecitabine [20]. For both studies, patients had documented progression on chemotherapy or within 6 months of their last chemotherapy, and patients with pre-existing grade >2 neuropathy were excluded [19, 20]. The primary endpoint for both studies was ORR; secondary endpoints included duration of response, PFS, OS, and tolerability [19, 20].
In all three studies, eribulin mesylate at 1.4 mg/m2 (equivalent to eribulin at 1.23 mg/m2) was administered intravenously over 2–5 minutes on days 1 and 8 of a 21-day cycle. In Study 201, eribulin mesylate at 1.4 mg/m2 was initially administered intravenously over 2–5 minutes on days 1, 8, and 15 of a 28-day cycle [19]; however, owing to the incidence of neutropenia, the dosing schedule was amended to days 1 and 8 of a 21-day cycle, and only patients who received the 21-day dosing schedule have been included in this analysis.
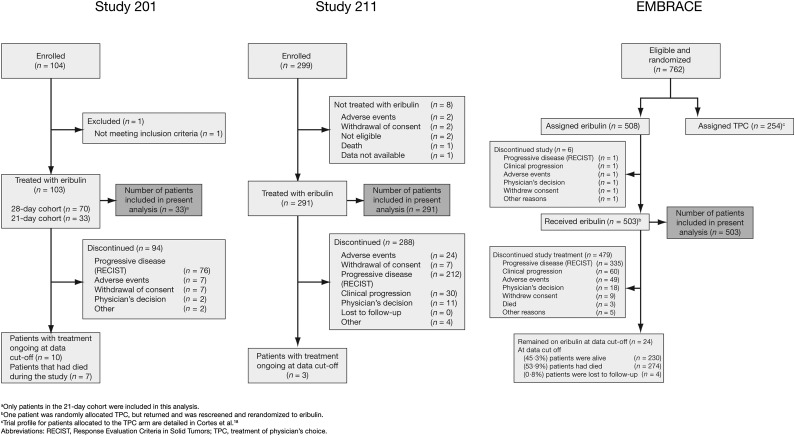
A CONSORT diagram representing the patients included in the present analysis is depicted in Figure 1.
Figure 1.
CONSORT diagram. Reprinted with permission from © 2010 American Society of Clinical Oncology (Cortes J, et al.: J Clin Oncol Vol. 28, 2010: 3922–3928). All rights reserved. Reprinted from The Lancet, Vol. 377, Cortes J, et al., Eribulin Monotherapy Versus Treatment of Physician’s Choice in Patients With Metastatic Breast Cancer (EMBRACE): A phase 3 open-label randomised study, pages 914–923, © 2011, with permission from Elsevier.
Abbreviations: RECIST, Response Evaluation Criteria in Solid Tumors, TCP, treatment of physician’s choice.
Exploratory Analysis
Data from these three studies were pooled and compared across four age cohorts based on patient age at study recruitment: <50 years, 50–59 years, 60–69 years, and ≥70 years. OS and PFS were analyzed using a Cox proportional hazards model with 1 degree of freedom. OS was defined from the date of randomization to death from any cause or last known date alive (censored). PFS was defined from the date of randomization to the earliest date of disease progression or death from any cause or was censored (as for OS).
ORR, defined as complete response (CR) plus partial response (PR), and CBR, defined as CR plus PR plus stable disease ≥6 months, were analyzed using the Cochran-Mantel-Haenszel test.
Categorical model covariates were applied using a stepwise model-selection process (entry, p ≤ .20, and removal, p > .05). Categorical covariates that were considered during the selection process in addition to age were race; prior vinorelbine treatment; prior capecitabine treatment; refractory to anthracycline, taxane, and capecitabine (defined as those who progressed on therapy or within 6 months of receiving the therapy); number of total prior chemotherapy regimens (≤3 vs. >3); number of prior chemotherapy regimens for metastatic disease (≤3 vs. >3); hormone receptor status (ER, PGR); HER-2/neu status; triple-negative tumor status (ER-negative/PGR-negative/HER-2/neu-negative); and tumor stage at initial diagnosis.
A further sensitivity analysis fitted age, baseline body surface area (BSA), and baseline ECOG performance status as continuous covariates with stratification similar to the primary analysis.
Adverse events (AEs) were summarized for each age cohort according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Treatment-related AEs of special interest included asthenia or fatigue, peripheral neuropathy, nausea, arthralgia or myalgia, and vomiting.
Results
Patients
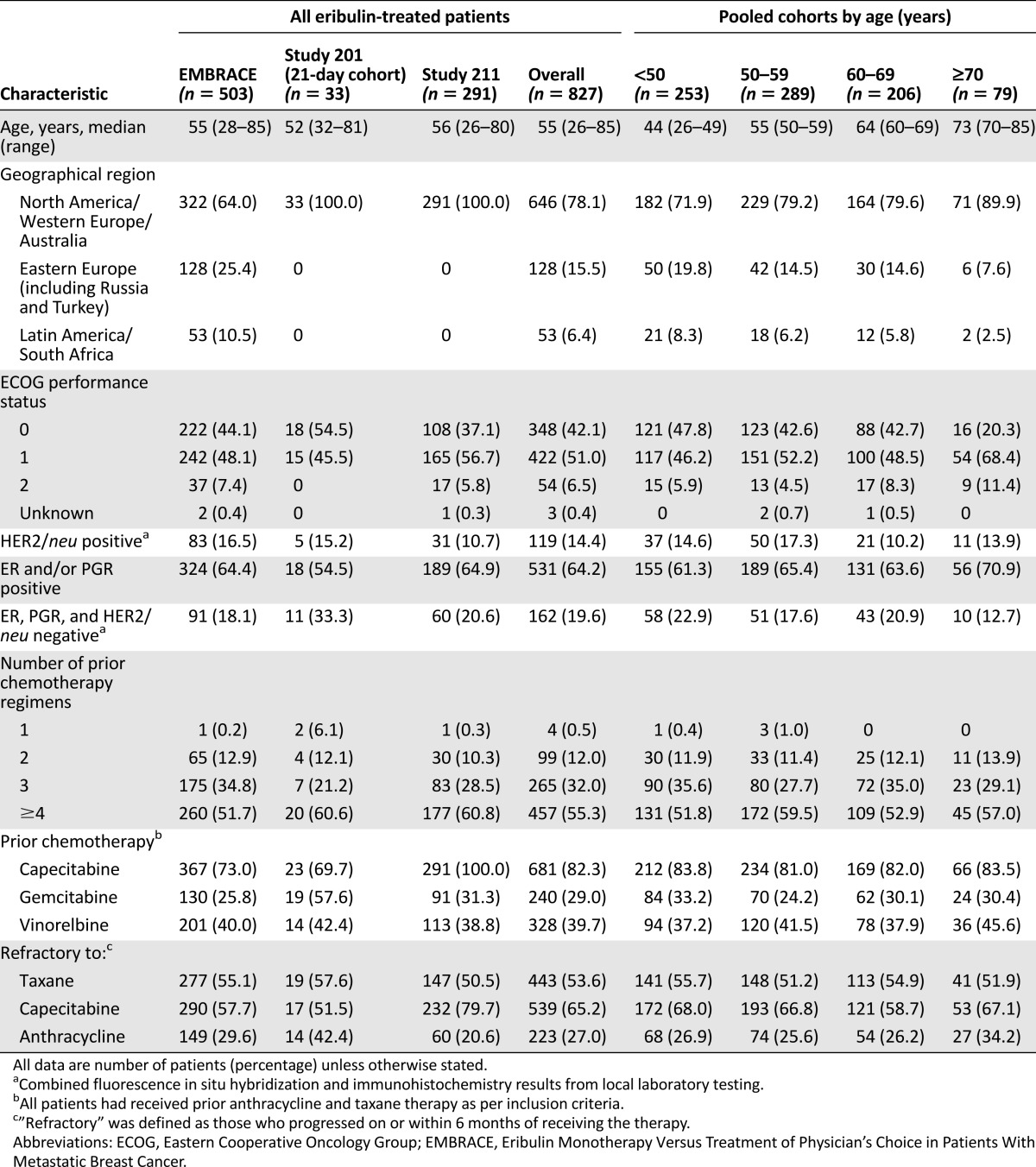
A total of 827 patients received eribulin mesylate on the 21-day treatment cycle and were included in this pooled analysis (Table 1). Of these, 253 patients (30.6%) were <50 years old, 289 (34.9%) were 50–59 years old, 206 (24.9%) were 60–69 years old, and 79 (9.6%) were ≥70 years old. Baseline demographic characteristics were balanced between the age groups, although a smaller proportion of patients in the ≥70 years subgroup exhibited ECOG performance scores of 0 compared with the other age groups (20.3% vs. 42.6%–47.8%) (Table 1).
Table 1.
Patient demographics and baseline characteristics
Efficacy
Overall Survival
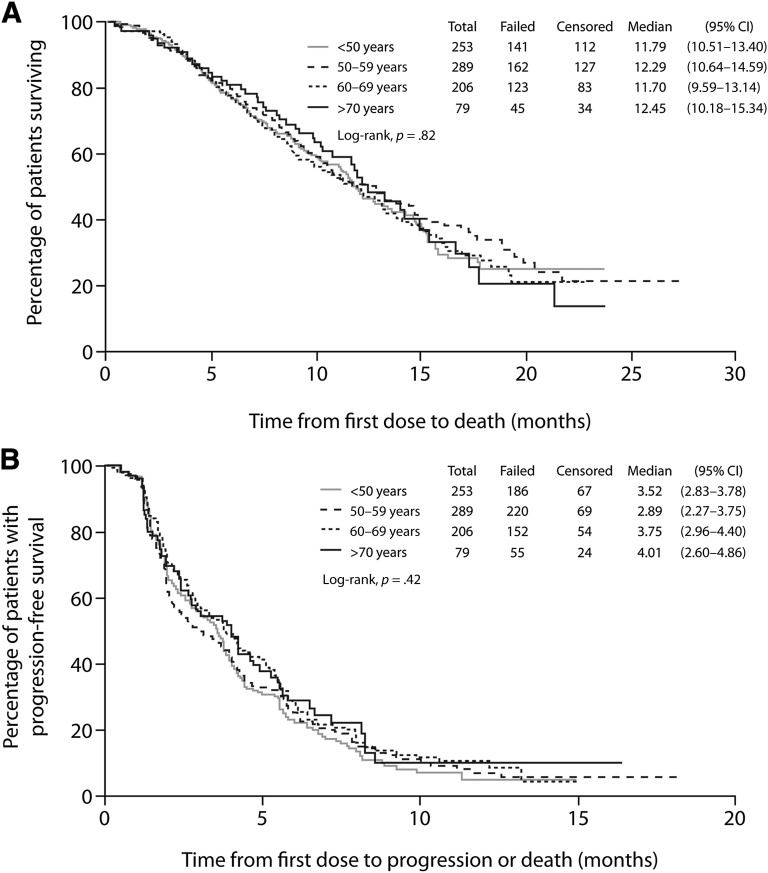
For the cohorts of patients aged <50 years, 50–59 years, 60–69 years, and ≥70 years, median OS was 11.8 months, 12.3 months, 11.7 months, and 12.5 months, respectively (p = .82) (Fig. 2A, Table 2). No relationship was observed between age and OS when analyzed by cohort or as a continuous variable (Table 3). Of the clinical variables examined, those that had a significant impact favoring improved OS were having three or more prior chemotherapy regimens, not being refractory to taxane therapy, and having an ER-positive tumor. In the sensitivity analyses using continuous covariates, higher BSA and better baseline ECOG performance status also predicted improved OS (Table 3).
Figure 2.
Kaplan-Meier graph of overall survival (A) and progression-free survival (B) by independent review.
Abbreviation: CI, confidence interval.
Table 2.
Efficacy outcomes by age cohort
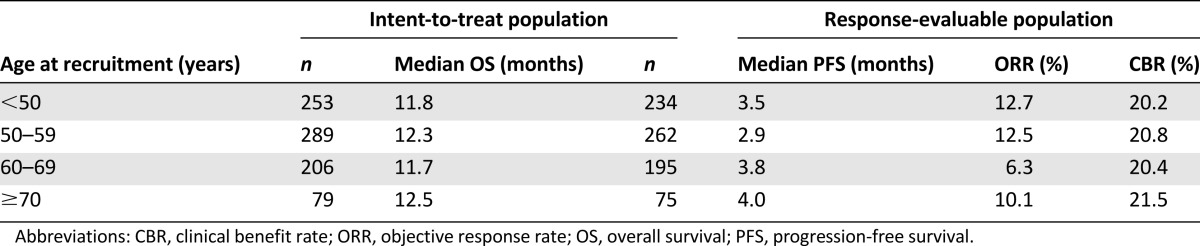
Table 3.
Multivariate analysis of efficacy and OS with age as continuous covariate

Progression-Free Survival
For the cohorts of patients aged <50 years, 50–59 years, 60–69 years, and ≥70 years, median PFS assessed by independent review was 3.5 months, 2.9 months, 3.8 months, and 4.0 months, respectively (p = .42) (Fig. 2B, Table 2). PFS assessed by investigators was similar (data not shown). No significant differences in PFS were observed between the four age cohorts (Table 3). Comparable PFS results were also obtained when age was fitted as a continuous variable (data not shown). Clinical categorical variables that had a favorable impact on PFS as determined by independent review were not being refractory to capecitabine therapy and having an ER-positive tumor; higher baseline BSA and better baseline ECOG performance status also favored longer PFS (Table 3).
ORR and CBR
Age did not have a significant impact on either ORR (independent or investigator assessed) or CBR (Table 2). Positive ER status and higher baseline BSA were the only significant predictors of both ORR and CBR improvement (Table 3).
Safety
Compared with the overall population, a similar proportion of patients within the different age cohorts experienced at least one AE of any grade (<50 years, 98.8%; 50–59 years, 99.3%; 60–69 years, 99.0%; ≥70 years, 100%). The numbers of patients reporting one treatment-related AE or more (<50 years, 94.9%; 50–59 years, 95.8%; 60–69 years, 96.6%; ≥70 years, 93.7%) or one serious AE or more (<50 years, 28.5%; 50–59 years, 24.6%; 60–69 years, 29.6%; ≥70 years, 25.3%) were also comparable between the age cohorts. However, AEs leading to dose reduction, delay, or discontinuation increased slightly with age (<50 years, 45.8%; 50–59 years, 49.8%; 60–69 years, 51.5%; ≥70 years, 51.9%). An analysis comparing the cohorts aged <50 years, 50–59 years, and 60–69 years with the cohort aged ≥70 years showed no significant differences in the odds ratios for the occurrence of adverse events leading to dose delays, reductions, or withdrawal from treatment (data not shown).
No differences in concomitant use of growth factors during the study were observed between age groups (granulocyte colony-stimulating factor: <50 years, 6.0%; 50–59 years, 8.1%; 60–69 years, 5.2%; ≥70 years, 2.1%; erythropoiesis-stimulating agent: <50 years, 5.4%; 50–59 years, 6.9%; 60–69 years, 4.2%; ≥70 years, 2.9%).
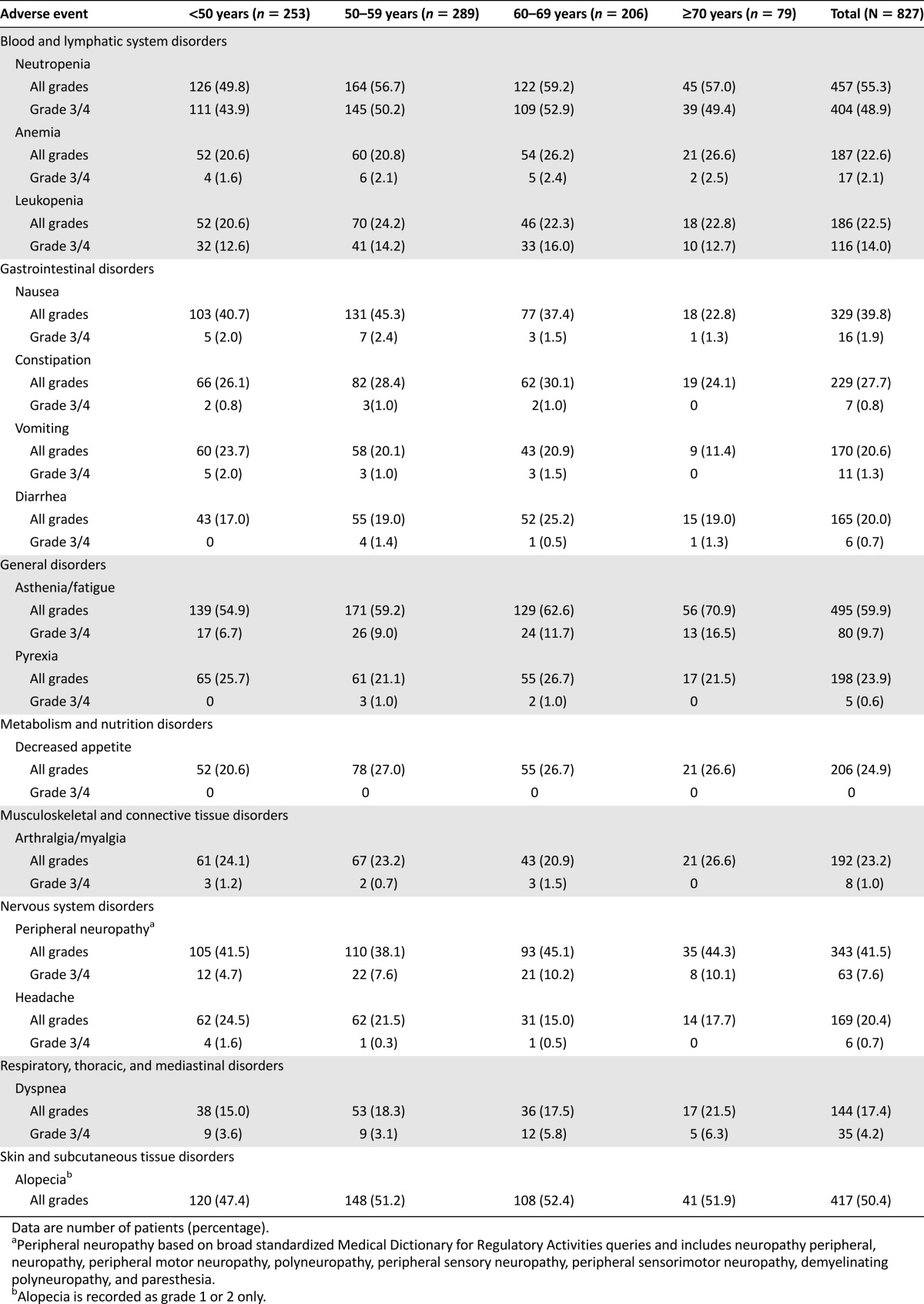
Most AEs (all grades) displayed similar prevalence among the different age cohorts (Table 4). The incidences of alopecia, dyspnea, and peripheral neuropathy were similar across the different age cohorts, as were the rates for neutropenia, febrile neutropenia, and leukopenia (Table 4; data not shown). Certain AEs had higher incidence in older patients compared with younger patients, including asthenia or fatigue (<50 years, 54.9%; ≥70 years, 70.9%), peripheral edema (<50 years, 5.5%; ≥70 years, 19.0%), and dizziness (<50 years, 5.9%; ≥70 years, 12.7%) (Table 4; data not shown). Nausea (<50 years, 40.7%; ≥70 years, 22.8%) and vomiting (<50 years, 23.7%; ≥70 years, 11.4%) tended to be more common in the youngest cohort (Table 4). Similar incidences of grade 3/4 AEs were experienced across the age cohorts and in comparison with the overall population (Table 4). Treatment-related deaths were reported for five patients in the cohort aged 50–59 years (febrile neutropenia, lung infection, bronchopneumonia, dyspnea, and unknown) and for one patient in the cohort aged 60–69 years (dyspnea).
Table 4.
Overall adverse events in each age cohort with an incidence of >20% (all grades) or >5% (grade 3/4) in any cohort
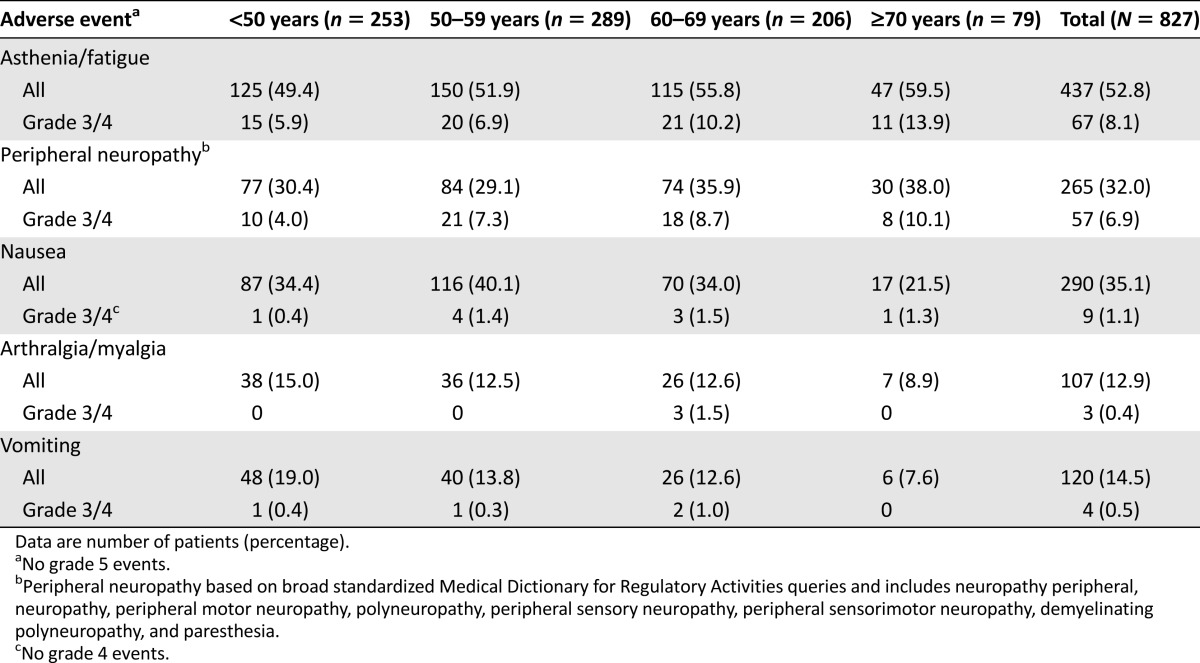
Similar incidences of each treatment-related AE of special interest (asthenia or fatigue, peripheral neuropathy, nausea, arthralgia or myalgia, and vomiting) were reported among the different age cohorts (Table 5). However, some differences were observed in grade 3/4 treatment-related AEs in older patients compared with those in the younger cohort. Of these, asthenia or fatigue (<50 years, 5.9%; ≥70 years, 13.9%) and peripheral neuropathy (<50 years, 4.0%; ≥70 years, 10.1%) were more frequent in the oldest cohort (Table 5).
Table 5.
Treatment-related adverse events of special interest in each age cohort
Discussion
In this patient population that was fit enough to be eligible for clinical trials, eribulin monotherapy showed similar benefits among all age cohorts. Importantly, efficacy benefits were achieved in all age groups associated with a manageable tolerability profile; toxicity appeared to be no greater in older patients. There were some differences in the incidences of individual AEs among the different age cohorts, suggesting that clinical judgment should play a key role in predicting the effect of age on toxicity, particularly in relation to any pre-existing comorbidity. Implementation of geriatric assessment tools in cancer trials, such as that evaluated in the recent Cancer and Leukemia Group B (CALG-B) 360401 study, may assist in identifying factors that contribute to morbidity and mortality in older patients and, in turn, help establish treatment recommendations [21]. In this analysis, most patients had normal or mild declines in performance status prior to trial entry, and the effectiveness of chemotherapy in patients with functional deficits is uncertain, particularly in older populations.
A limitation of this analysis is that the patients were enrolled in clinical trials with stringent entry criteria. Many of the elderly patients in this analysis had previously received multiple lines of chemotherapy, and thus the studied population may manifest above-average fitness in terms of chemotherapy tolerance. Although this study included a substantial number of older adults with heavily pretreated MBC—approximately 10% (79 of 827patients) who were aged ≥70 years—only a large prospective study including prospectively performed geriatric assessments would more precisely define the efficacy and tolerability of eribulin in older patients.
Establishing the optimal treatment strategy for older patients with breast cancer is challenging, and this is highlighted by the fact that although the overall mortality rate for patients with breast cancer has generally decreased, this outcome has not been achieved equally across age groups [22–24]. Using data from National Vital Statistics Reports spanning 1990–2007, Smith et al. recently showed that although the 10-year absolute risk of breast cancer death has decreased by 15.3% for women aged 50–64 years, this risk has been reduced by only 7.5% for women aged ≥75 years [24]. This may, in part, be due to the observation that effective screening and treatments are less likely to be offered to older women. Moreover, prior exclusion of older patients from clinical trials and poor accrual of older, eligible patients to more current trials limits our knowledge concerning optimal therapy for older patients. Consequently, appropriate and effective therapy may be underused in older women because of concerns about the potential complications caused by multiple comorbidities as well as the potential for increased toxicity [11, 25, 26].
In line with the results of this study, other retrospective analyses of the benefits of chemotherapy in MBC have also shown no detrimental interaction of age and outcome in generally fit patients [2]. CALG-B showed that the efficacy of paclitaxel (80 mg/m2 weekly vs. 175 mg/m2 every 3 weeks) in 1,048 older patients with MBC was not related to age group (<55 years, 55–64 years, and ≥65 years) [27]; better performance status and first-line therapy were associated with greater treatment benefit. However, the incidence of grade 3 neurotoxicity increased with age, with the shortest time to onset seen in patients aged ≥65 years receiving paclitaxel as second-line treatment.
Similar results were observed in a retrospective analysis of two European Organization for Research and Treatment of Cancer trials involving pegylated liposomal doxorubicin administered at either 60 mg/m2 every 6 weeks or 50 mg/m2 every 4 weeks to older patients with MBC (48% [65 of 136] of patients were aged ≥70 years). Efficacy outcomes were not affected by age; however, the incidences of hematologic toxicity, anorexia, asthenia, and stomatitis were higher for older patients compared with younger patients who received the 6-week schedule but not the 4-week schedule [28].
The effect of age on capecitabine effectiveness has also been investigated recently in patients with MBC. The pooled results of five phase II/III pivotal trials of capecitabine (monotherapy or combination therapy) in 570 patients showed that OS, CBR or ORR, and the incidence of AEs were not related to age (cohorts were aged 18–49 years, 50–64 years, and ≥65 years) [29]. However, women aged ≥65 years were more likely to withdraw from treatment because of an AE [29]. A recent review of capecitabine in elderly patients with MBC concluded that capecitabine monotherapy is suitable for such patients but that reductions from the labeled dose should be considered [22].
In a retrospective population-based analysis of women aged ≥66 years who had stage IV ER-negative MBC (n = 1,519; 33% had received chemotherapy), age did not affect the significant survival benefit associated with chemotherapy [30]. Similarly, a recent review of retrospective evidence suggested that although chemotherapy is probably more toxic in older patients with ER-negative MBC, they can derive the same benefits from chemotherapy treatment as younger women [31]. The association between higher BSA and better outcomes seen in this study was unanticipated. A similar trend has also been observed in an analysis of patients in a German cancer registry [32] who received chemotherapy. Further analysis of eribulin pharmacokinetic data may provide more insight into these observations.
Conclusion
Our data show that eribulin monotherapy in older women with MBC who are sufficiently fit to enter clinical trials leads to similar response rates, times to progression, and OS benefits as experienced those by younger patients. These data are similar to retrospective analyses of other agents in this setting and show that in generally fit patients, age is not related to outcome. Of note, eribulin was used in heavily treated patients and in the EMBRACE trial showed a significant improvement in survival compared with other agents routinely used in the metastatic setting. This survival advantage, coupled with a manageable safety profile, demonstrates that eribulin may be an effective option for selected older patients with MBC.
Acknowledgments
We thank all the patients and investigators who participated in these studies. Editorial support was provided by Emma Robinson and Haley Bennett, of Complete Medical Communications. Editorial support for manuscript revisions after journal review was provided Steven Inglis, of Oxford PharmaGenesis Ltd UK. Funding for all editorial support was provided by Eisai Inc. This study was funded by Eisai Inc. This pooled analysis has not been presented previously; however, data from an analysis of the effect of age in the EMBRACE trial were presented at the 2011 annual meeting of the American Society of Clinical Oncology (J Clin Oncol 2011;29(Suppl):abstract 1060), and results of the individual studies have been published (J Clin Oncol 2009;27:2954–2961; J Clin Oncol 2010;28:3922–3928; Lancet 2011;377:914–923). Fatima Cardoso is currently affiliated with Champalimaud Cancer Center, Lisbon, Portugal.
Footnotes
For Further Reading: Willemien van de Water, Caroline Seynaeve, Esther Bastiaannet et al. Elderly Postmenopausal Patients With Breast Cancer Are at Increased Risk for Distant Recurrence: A Tamoxifen Exemestane Adjuvant Multinational Study Analysis. The Oncologist 2013;18:8–13.
Implications for Practice: This study analyzed 9,766 postmenopausal breast cancer patients with hormone-sensitive disease who were included in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. The authors demonstrated a higher incidence of distant breast cancer recurrence with increasing age at diagnosis. Thus, the common belief that the clinical course of breast cancer in older women may be more indolent is rejected in this study. All patients received endocrine therapy, while radiotherapy after breast-conserving surgery and administration of chemotherapy decreased with increasing age. As distant recurrence may reflect underuse of systemic therapy, these findings hint at undertreatment of systemic therapy, and chemotherapy in particular. Consequently, chemotherapy may be considered more often in relatively fit elderly breast cancer patients with hormone-sensitive disease.
Author Contributions
Conception/Design: Hyman Muss, Javier Cortes, Linda Vahdat, Fatima Cardoso, Chris Twelves, Jantien Wanders, Seth Seegobin
Provision of study material or patients: Javier Cortes, Linda Vahdat, Fatima Cardoso, Chris Twelves, Jantien Wanders, Corina E. Dutcus
Collection and/or assembly of data: Hyman Muss, Linda Vahdat, Corina E. Dutcus, Seth Seegobin
Data analysis and interpretation: Hyman Muss, Javier Cortes, Linda Vahdat, Fatima Cardoso, Jantien Wanders, Corina E. Dutcus, Jay Yang, Seth Seegobin, Joyce O’Shaughnessy
Manuscript writing: Hyman Muss, Javier Cortes, Linda Vahdat, Fatima Cardoso, Chris Twelves, Jantien Wanders
Final approval of manuscript: Hyman Muss, Javier Cortes, Linda Vahdat, Fatima Cardoso, Chris Twelves, Jantien Wanders, Corina E. Dutcus, Jay Yang, Seth Seegobin, Joyce O’Shaughnessy
Disclosures
Hyman Muss: Pfizer (C/A); Fatima Cardoso: Pfizer (C/A); Javier Cortes: Eisai (H); Linda Vahdat: Eisai (H, speaker program, RF, trial funding to institution); Chris Twelves: Eisai (C/A, H, other); Corina E. Dutcus: Eisai (E); Jantien Wanders: Eisai (E, former); Seth D. Seegobin: Eisai (E, former); Joyce O'Shaughnessy: Eisai (C/A, H). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Breast cancer facts & figures 2011-2012. Available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf Accessed March 4, 2014.
- 2.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER cancer statistics review, 1975–2008. Available at http://seer.cancer.gov/csr/1975_2008/ Accessed March 4, 2014.
- 4.Oostendorp LJ, Stalmeier PF, Donders AR, et al. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: A systematic review. Lancet Oncol. 2011;12:1053–1061. doi: 10.1016/S1470-2045(11)70045-6. [DOI] [PubMed] [Google Scholar]
- 5.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: A review of recent randomized clinical trials. J Clin Oncol. 2010;28:1958–1962. doi: 10.1200/JCO.2009.25.5414. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso F, Bedard PL, Winer EP, et al. International guidelines for management of metastatic breast cancer: Combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174–1181. doi: 10.1093/jnci/djp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cashman J, Wright J, Ring A. The treatment of co-morbidities in older patients with metastatic cancer. Support Care Cancer. 2010;18:651–655. doi: 10.1007/s00520-010-0813-1. [DOI] [PubMed] [Google Scholar]
- 9.Ring A. The influences of age and co-morbidities on treatment decisions for patients with HER2-positive early breast cancer. Crit Rev Oncol Hematol. 2010;76:127–132. doi: 10.1016/j.critrevonc.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Ring A, Reed M, Leonard R, et al. The treatment of early breast cancer in women over the age of 70. Br J Cancer. 2011;105:189–193. doi: 10.1038/bjc.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muss HB. Coming of age: Breast cancer in seniors. The Oncologist. 2011;16(suppl 1):79–87. doi: 10.1634/theoncologist.2011-S1-79. [DOI] [PubMed] [Google Scholar]
- 12.Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 13.Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 14.Okouneva T, Azarenko O, Wilson L, et al. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther. 2008;7:2003–2011. doi: 10.1158/1535-7163.MCT-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JA, Wilson L, Azarenko O, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuznetsov G, TenDyke K, Yu MJ, et al. Antiproliferative effects of halichondrin B analog eribulin mesylate (E7389) against paclitaxel-resistant human cancer cells in vitro. Proc Am Assoc Cancer Res. 2007;48:275. [Google Scholar]
- 17.Wozniak KM, Nomoto K, Lapidus RG, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res. 2011;71:3952–3962. doi: 10.1158/0008-5472.CAN-10-4184. [DOI] [PubMed] [Google Scholar]
- 18.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 19.Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–2961. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28:3922–3928. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 21.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290–1296. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedard PL, Bernard-Marty C, Raimondi C, et al. The role of capecitabine in the management of breast cancer in elderly patients. J Geriatr Oncol. 2011;2:72–81. [Google Scholar]
- 23.Muss HB, Busby-Whitehead J. Older women with breast cancer: Slow progress, great opportunity, now is the time. J Clin Oncol. 2011;29:4608–4610. doi: 10.1200/JCO.2011.38.6888. [DOI] [PubMed] [Google Scholar]
- 24.Smith BD, Jiang J, McLaughlin SS, et al. Improvement in breast cancer outcomes over time: Are older women missing out? J Clin Oncol. 2011;29:4647–4653. doi: 10.1200/JCO.2011.35.8408. [DOI] [PubMed] [Google Scholar]
- 25.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: Importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 26.Lavelle K, Todd C, Moran A, et al. Non-standard management of breast cancer increases with age in the UK: A population based cohort of women > or =65 years. Br J Cancer. 2007;96:1197–1203. doi: 10.1038/sj.bjc.6603709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtman SM, Hurria A, Cirrincione CT, et al. Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: Combined analysis of CALGB 9342 and 9840. Ann Oncol. 2012;23:632–638. doi: 10.1093/annonc/mdr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biganzoli L, Coleman R, Minisini A, et al. A joined analysis of two European Organization for the Research and Treatment of Cancer (EORTC) studies to evaluate the role of pegylated liposomal doxorubicin (Caelyx) in the treatment of elderly patients with metastatic breast cancer. Crit Rev Oncol Hematol. 2007;61:84–89. doi: 10.1016/j.critrevonc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Blum JL, Kohles J, McKenna E, et al. Association of age and overall survival in capecitabine-treated patients with metastatic breast cancer in clinical trials. Breast Cancer Res Treat. 2011;125:431–439. doi: 10.1007/s10549-010-1222-3. [DOI] [PubMed] [Google Scholar]
- 30.Schneider M, Zuckerman IH, Onukwugha E, et al. Chemotherapy treatment and survival in older women with estrogen receptor-negative metastatic breast cancer: A population-based analysis. J Am Geriatr Soc. 2011;59:637–646. doi: 10.1111/j.1532-5415.2011.03351.x. [DOI] [PubMed] [Google Scholar]
- 31.Barni S, Cabiddu M, Petrelli F. Benefit of adjuvant chemotherapy in elderly ER-negative breast cancer patients: Benefits and pitfalls. Expert Rev Anticancer Ther. 2010;10:185–198. doi: 10.1586/era.09.188. [DOI] [PubMed] [Google Scholar]
- 32.Marschner N, Dörfel S, Meyer D, et al. Effectiveness of taxane- or anthracyline-based compared to taxane- and anthracyline-free first-line treatments of patients with metastatic breast cancer treated by German office-based medical oncologists. Data from the TMK registry group [abstract 41] Breast. 2013;22:S33. [Google Scholar]