In this study, the authors explored baseline prognostic factors in Japanese advanced gastric cancer (AGC) patients. They propose a new prognostic index for patients with AGC. This can be used for more appropriate patient stratification in future clinical trials.
Keywords: Prognostic index, Prognostic factor, Advanced gastric cancer, Chemotherapy
Abstract
Background.
In advanced gastric cancer (AGC), no globally accepted prognostic scoring system has been developed. Therefore, we explored baseline prognostic factors in Japanese AGC patients using the data from a randomized controlled trial, Japan Clinical Oncology Group (JCOG) 9912, which investigated the efficacy of systemic chemotherapy as a first-line treatment.
Patients and Methods.
Prognostic factors and prognostic indices for overall survival were screened and evaluated in patients enrolled in JCOG9912 using the Cox proportional hazard model. The Royal Marsden Hospital prognostic model was also applied to the JCOG9912 trial.
Results.
A total of 650 (92.3%) of the 704 patients randomized in the JCOG9912 trial, for whom complete data were available for multivariate analyses, was included in the present study (5-fluorouracil arm, n = 215; irinotecan plus cisplatin arm, n = 216; S-1 arm, n = 219). The median survival time (MST) for all patients was 11.8 months. To construct a prognostic index, we selected four risk factors by multivariate analysis: performance status ≥ 1, number of metastatic sites ≥ 2, no prior gastrectomy, and elevated alkaline phosphatase. MSTs were 17.0 months for patients categorized into the low-risk group, who had zero or one risk factor (n = 225); 10.4 months for patients in the moderate-risk group, who had two or three risk factors (n = 368); and 5.0 months for patients in the high-risk group, who had all four risk factors (n = 57).
Conclusion.
In the present study, we propose a new prognostic index for patients with AGC. This can be used for more appropriate patient stratification in future clinical trials.
Implications for Practice:
Prognostic indices are useful not only to estimate the prognosis of each patient but are also applicable for stratification of patients for clinical trials. By using patient data from the Japan Clinical Oncology Group (JCOG) 9912 trial, we explored baseline prognostic factors and prognostic index. In the results, a novel prognostic index consisting of four risk factors (performance status ≥1, metastatic sites ≥2, no prior gastrectomy, and elevated ALP), which can classify patients into three risk groups, is proposed. This index can be used for more accurate patient stratification in future clinical trials.
Introduction
Despite a steady decrease in the mortality rate of gastric cancer (GC) in recent years, GC remains a major health problem, causing approximately 738,000 deaths worldwide in 2008 [1]. For advanced gastric cancer (AGC) patients, the primary treatment is systemic chemotherapy, which improves survival and quality of life [2, 3]. Whereas fluoropyrimidine plus platinum has been regarded as the standard first-line chemotherapy for AGC worldwide, there are some regional variations in chemotherapy regimens. The most popular chemotherapy is epirubicin plus cisplatin plus 5-fluorouracil (5-FU) or epirubicin plus oxaliplatin plus capecitabine [4] in the U.K., docetaxel plus cisplatin plus 5-FU (DCF) [5] or 5-FU, leukovorin, and oxaliplatin (FOLFOX) in Europe, cisplatin plus 5-FU or DCF in the U.S., and S-1 plus cisplatin in Japan [6].
Recently, new drugs have been developed globally, and a multinational phase III trial named AVAGAST has been conducted [7] to evaluate the efficacy of adding bevacizumab to capecitabine plus cisplatin as a first-line chemotherapy for AGC. In this trial, substantial differences in the prognosis of AGC patients from Western and Asian countries, especially Japan, were observed. These results suggest some interaction between treatment effects and regions. However, before investigating the reasons for regional differences, it is first necessary to identify common prognostic factors between Asian and Western populations and to compare them after adjusting for the patients’ backgrounds.
Prognostic indices are now available for several cancer types, including non-Hodgkin lymphoma [8], multiple myeloma [9], breast cancer [10], prostate cancer [11], renal cancer [12], and colorectal cancer [13]. In several cancers, such as non-Hodgkin lymphoma and renal cancer, prognostic indices are not only useful to estimate the prognosis of each patient but also are applicable for determination of the optimal treatment strategy and stratification of patients for clinical trials. In AGC, a prognostic index based on clinical trials conducted in the 1990s was proposed by Royal Marsden Hospital (RMH) in 2004; this index consists of four independent risk factors for survival: Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥ 2, liver metastasis, peritoneal metastasis, and serum alkaline phosphatase (ALP) ≥ 100 μ/L [14]. To formulate this index, patients were classified into three groups by the number of risk factors: low risk (no risk factors), moderate risk (one or two risk factors), and high risk (three or four risk factors), resulting in significant survival differences across the groups. However, the RMH index was developed using only data from Western patients, and 30% of the patients had esophageal cancer. In Asia, a few reports have investigated the prognostic factors and indices in Korean populations [15–17]; however, all of these studies were based on retrospective data. From Japan, prognostic factors based on clinical trials conducted in the 1990s have been reported [18]. However, recent clinical trials have been conducted globally, and regional differences, such subsequent chemotherapy, are recognized as a substantial problem. Recently, active new agents for gastric cancer have contributed to the prognosis not only in the first-line but also in the subsequent lines. Thus, new prognostic scoring systems for AGC, including Asian patients, should be proposed.
Japan Clinical Oncology Group (JCOG) 9912 was a large randomized trial investigating the superiority of irinotecan plus cisplatin (IP) and the noninferiority of oral S-1 compared with continuous infusion of 5-FU for patients with metastatic or recurrent gastric cancer [19]. In this trial, it was demonstrated that S-1 was not inferior to 5-FU (hazard ratio [HR]: 0.83 [95% confidence interval (CI): 0.68–1.01]; p = .0005 for noninferiority) in terms of overall survival (OS), but IP was also not superior (HR: 0.85 [95% CI: 0.70–1.04]; p = .0552 for superiority).
In the present study, we first investigated whether the RMH index could be applicable to Japanese patients with AGC. Next, we tried to establish a new prognostic index in AGC using the data from JCOG9912.
Patients and Methods
Between 2000 and 2006, 704 patients were enrolled in JCOG9912, which was registered with ClinicalTrials.gov, number NCT00142350. The details of the inclusion/exclusion criteria and treatment regimen for patients enrolled in JCOG9912 were published previously [19]. The patients analyzed in the present study were those having complete data available for multivariate analyses using the Cox proportional hazard model. Metastatic sites were reported by each investigator according to the Response Evaluation Criteria in Solid Tumors version 1.0, specifying all target and nontarget lesions in the case report form of each enrolled patient, in which the investigator checked prospectively the presence or absence of the metastatic sites, such as cervical, mediastinal, abdominal and superficial lymph nodes, lung, liver, peritoneum, ovary, adrenal gland, bone, skin, and others listed. For the total number of metastatic sites of each patient, each organ was counted separately; all lymph node metastases, regardless the regions, were counted as one site.
Statistical Analysis
OS was measured from the date of randomization to the date of death and censored at the date of last contact for a surviving patient.
To investigate whether the RMH index could be applicable to Japanese patients with AGC, regression analysis was performed using the Cox proportional hazard model, including the same factors as those proposed by the RMH index.
An exploration of the potential prognostic index model was carried out within the model, including four factors. The number of factors was determined by taking into account the applicability of the results to clinical practice and to avoid an over-fit model. To construct a prognostic index, we performed multivariate analysis with the Cox proportional hazard model by using PROC PHREG in SAS 9.1 (SAS Institute, Cary, NC, http://www.sas.com) and selected five models based on their score χ2 values from all possible models, which included four factors by specifying the SELECTION = SCORE option in the MODEL statement. When there were substantial differences among those five possible models in terms of statistical adequacy, that is, score χ2 values, the model with the largest score χ2 values was to be selected. Otherwise, model selection was to be performed based on clinical aspects.
Factors included in these analyses were as follows: age (<65/≥65), sex (male/female), PS (0/1, 2), disease status (metastatic/recurrent), number of metastatic sites (0, 1/≥2), target lesion (−/+), macroscopic type (0, 1, 2/3, 4, 5) [20], histological type (intestinal/diffuse), prior gastrectomy (−/+), and laboratory data at the date of enrollment in the trial, such as hemoglobin (Hb), white blood cell (WBC), platelets (Plt), Na, K, Ca, albumin, ALP, total bilirubin, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), C-reactive protein (CRP), carcinoembryonic antigen, and creatinine clearance (CCr). Each of these laboratory variables, except for Hb, WBC, Plt, and CCr, was dichotomized with the cutoff point at the limit of its normal range at each institution. Hb, WBC, Plt, and CCr were dichotomized with the cutoff points at 11 g/dL, 4000/μL, 10.0 × 104/μL, and 60 mL/min, corresponding to grade 1 adverse events in the National Cancer Institute Common Toxicity Criteria (version 2.0).
Survival curves were estimated by the Kaplan-Meier method and compared for statistical differences using the log-rank test. All p values are two-sided.
Results
Data Collection
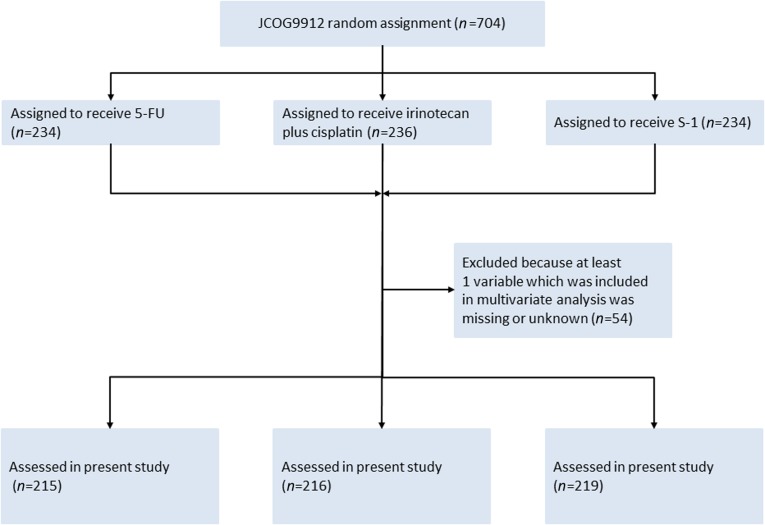
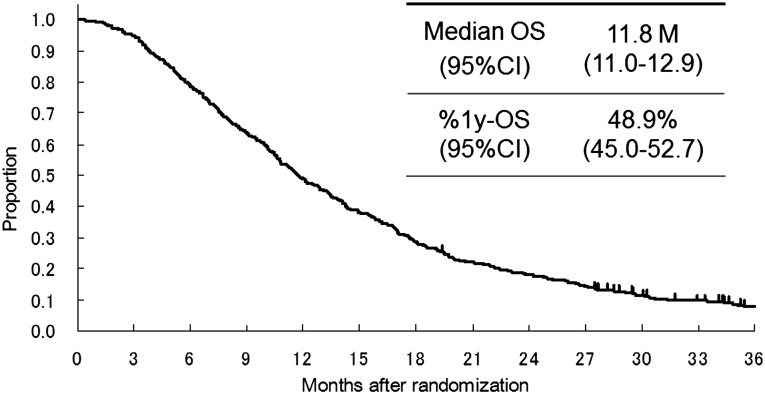
All data for baseline factors and laboratory tests for the multivariate analysis were available in 650 (5-FU arm, n = 215; irinotecan plus cisplatin arm, n = 216; S-1 arm, n = 219) of 704 patients enrolled in JCOG9912 (Fig. 1). Table 1 shows the baseline characteristics of the subjects in the present study. A total of 417 patients (64%) showed PS 0, 283 patients (44%) had 0 or 1 metastatic sites, and 123 (19%) had recurrent disease after curative surgery. A total of 607 (93%) of 650 patients did not survive until the final data cutoff in April 2008. The median survival time (MST) for all analyzed patients was 11.8 months (Fig. 2).
Figure 1.
CONSORT diagram.
Abbreviations: 5-FU, 5-fluorouracil; JCOG, Japan Clinical Oncology Group.
Table 1.
Patient characteristics
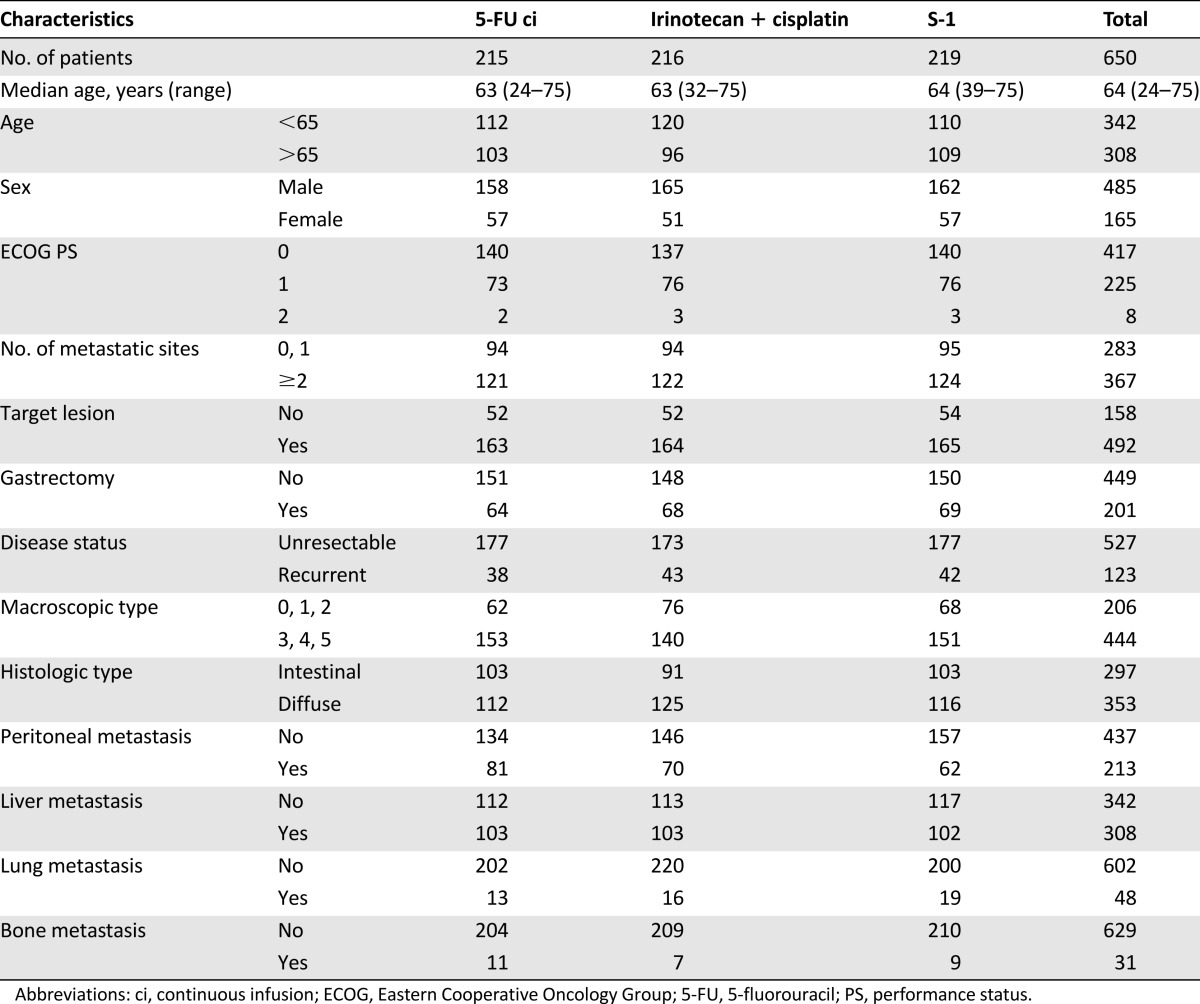
Figure 2.
Survival curve of the 650 patients with complete data for baseline factors and laboratory tests for the multivariate analysis.
Abbreviations: %1y-OS, 1 year overall survival; CI, confidence interval; OS, overall survival.
RMH Prognostic Index
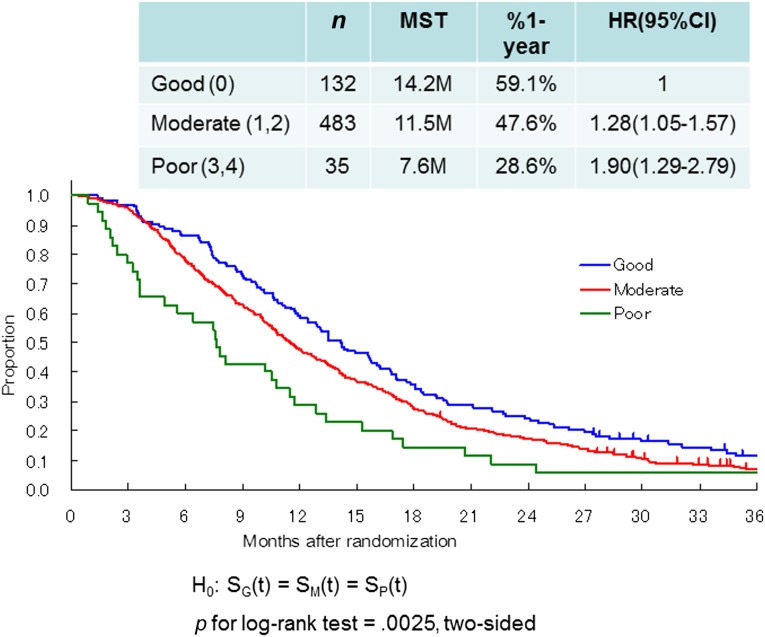
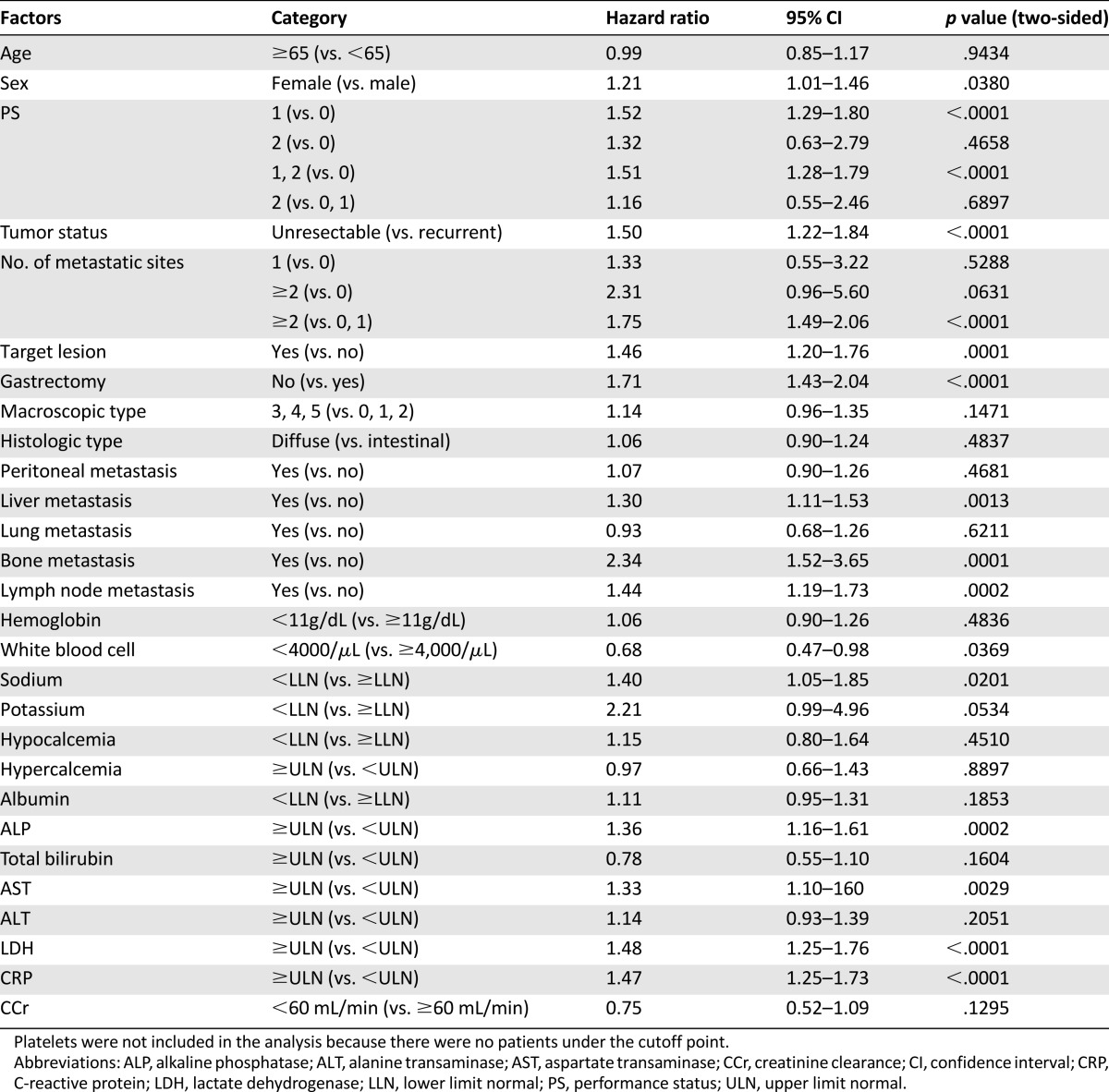
First, we applied the RMH index to our data. Of the patients in the present study, only 35 (5%) were classified in the poor-risk group, 483 patients (74%) were classified in the moderate-risk group, and 132 (21%) were classified in the good-risk group. Survival differences were also significant (log-rank p = .0025, two-sided; moderate-risk group, HR = 1.28, 95% CI = 1.05–1.57; high-risk group, HR = 1.90, 95% CI = 1.29–2.79; Fig. 3).
Figure 3.
Survival curves of the three groups in the present study classified according to the Royal Marsden Hospital prognostic index. Good (0), no risk factors; moderate (1,2), 1 or 2 risk factors; poor (3,4), 3 or 4 risk factors.
Abbreviations: CI, confidence interval; HR, hazard ratio; MST, median survival time.
JCOG Prognostic Index
Table 2 shows the results of the univariate analyses for survival using baseline characteristics and laboratory tests. The following parameters were strongly related to poor prognosis: PS ≥1, unresectable disease, number of metastatic sites ≥2, having target lesions, no prior gastrectomy, metastasis of bone and lymph nodes, elevated ALP, elevated LDH, and elevated CRP (p < .001 for each factor).
Table 2.
Univariate analyses of survival
To construct the prognostic index, we proposed five models whose χ2 values were the highest in all possible models (Table 3). Because the six risk factors in the five selected models (PS ≥1, number of metastatic sites ≥2, no prior gastrectomy, elevated ALP, LDH, and CRP) had similar HRs, risk scores were assigned based on HRs with one point for each factor. Moreover, because score χ2 values of these five models were statistically nearly equal, taking into account clinical aspects, we selected the fifth model as the JCOG prognostic index (JCOG index), which included PS ≥1, number of metastatic sites ≥2, no prior gastrectomy, and elevated ALP as prognostic factors.
Table 3.
The five best models to construct a prognostic index
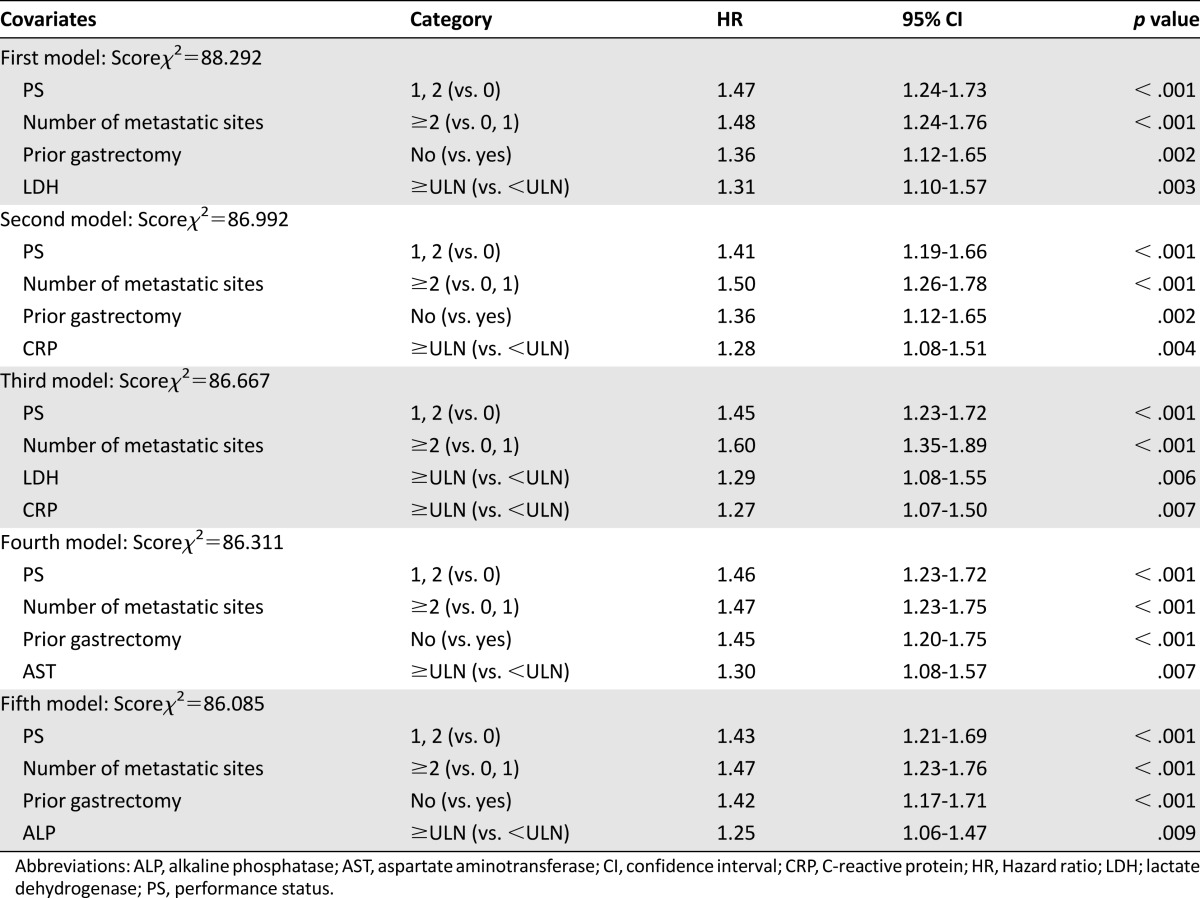
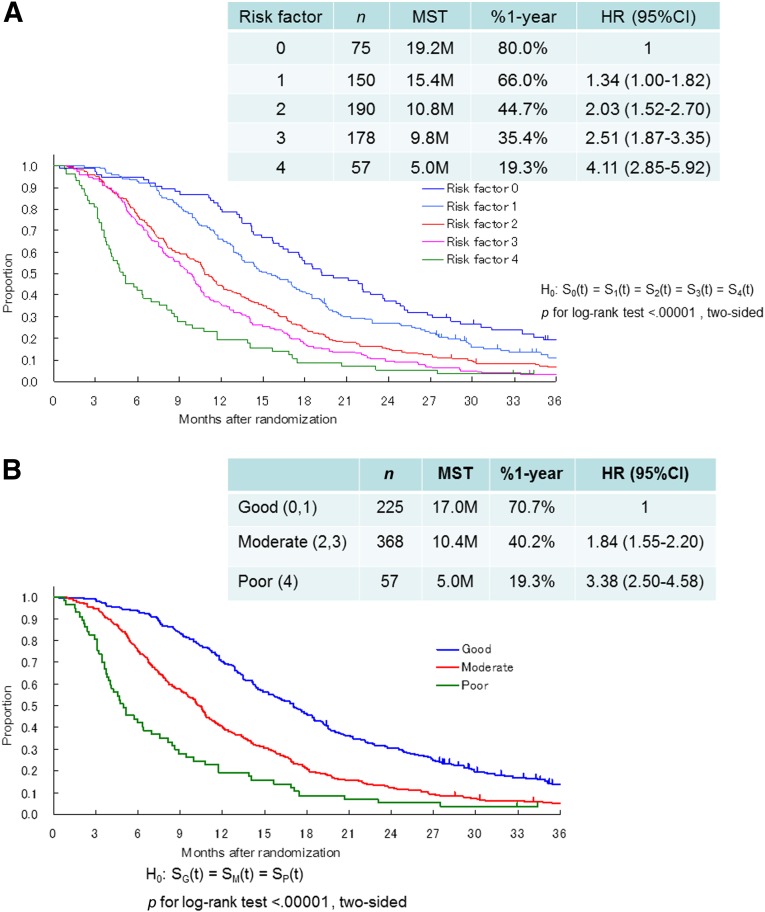
Figure 4A shows survival according to the number of risk factors, from 0 to 4, as determined by the JCOG index. There were significant survival differences among five groups (log-rank p < .0001, two-sided). Furthermore, for clinical convenience, we divided patients into three groups, rather than the five groups proposed by the authors of the RMH index. Patients with zero or one risk factor were categorized into the low-risk group (n = 225), those with two or three risk factors were categorized into the moderate-risk group (n = 368), and those with four risk factors were categorized into the high-risk group (n = 57). MSTs for the low-, moderate-, and high-risk groups were 17.0, 10.4, and 5.0 months, respectively. Compared with the low-risk group, the moderate-risk group had a nearly 2-fold increased risk of death (HR = 1.84, 95% CI = 1.55–2.20), and the high-risk group had a 3.4-fold increased risk of death (HR = 3.38, 95% CI = 2.50–4.58; Fig. 4B). Although statistically significant interaction between prognostic index and treatment was shown (p = .0002), a similar trend was observed in each of the three treatment arms. HRs of the moderate and high-risk groups compared with the low-risk group were 1: 2.04 (95% CI = 1.51–2.76): 10.00 (95% CI = 5.69–17.59 in the 5-FU arm); 1: 1.99 (95% CI = 1.46–2.72): 2.24 (95% CI = 1.39–3.59 in the irinotecan and cisplatin arm); 1: 1.65 (95% CI = 1.22–2.24): 4.66 (95% CI = 2.54–8.56) in the S-1 arm.
Figure 4.
Survival curves according to the Japan Clinical Oncology Group prognostic index. (A): Survival according to the number of risk factors, from 0 to 4. (B): Survival was divided into three groups, good (0, 1), moderate (2, 3), and poor (4). Risk factors consist of performance status ≥1, number of metastatic sites ≥2, no prior gastrectomy, and elevated alkaline phosphatase. Good (0,1), low risk (0 or 1 risk factors); moderate (2,3), moderate risk (2 or 3 risk factors); poor (4), high risk (4 risk factors).
Abbreviations: %1-year, 1-year survival; CI, confidence interval; HR, hazard ratio; MST, median survival time.
Discussion
To the best of our knowledge, this is the first report of a prognostic index limited to patients with only AGC in an Asian population based on data from a single large prospective randomized controlled trial. We adopted four risk factors for survival (PS ≥1, metastatic sites ≥2, no prior gastrectomy, and elevated ALP) and used these factors to develop the JCOG index. By classifying patients into three risk groups (low, zero to one risk factor; moderate, two to three risk factors; high, four risk factors), OS curves for our three risk groups indicated significantly good separation in JCOG9912. We believe that the JCOG index can be used for more accurate patient stratification in future clinical trials.
We selected these four prognostic factors to construct the prognostic index because there were no remarkable differences in the score χ2 values between the prognostic indices consisting of four and five factors. In terms of metastatic sites, to avoid confounding with other factors (such as bone metastasis and ALP), we adopted a factor that considered the number of metastatic sites rather than selecting each metastatic site individually. It seems reasonable to consider that the number of metastatic sites can reflect the tumor burden in the entire body.
To select the most optimal of our five candidate models, three of the four factors (PS ≥1, number of metastatic sites ≥2, and no prior gastrectomy), excluding elevated ALP and LDH, were included in all models, and we selected elevated ALP from the point of consistency in previous reports [14, 15, 17]. Both LDH and ALP commonly represent liver function, bone metastasis, and other abnormal conditions; however, there were no previous reports of prognostic models, including LDH. Finally, we decided to select the fifth model, which included the risk factors PS ≥1, number of metastatic sites ≥2, no prior gastrectomy, and elevated ALP, for the JCOG index.
In the late 1990s, Yoshida et al. [18] also reported prognostic factors from the old JCOG trials; PS, number of metastatic sites, and scirrhous-type tumor were found to be prognostic factors. Moreover, a few reports have described prognostic factors for GC from Korean patients [15–17]. Lee et al. [15] reported ECOG PS ≥2, no prior gastrectomy, peritoneal metastasis, bone metastasis, elevated ALP, and decreased albumin as independent prognostic factors; Kim et al. [16] reported ECOG PS ≥2, peritoneal metastasis, bone metastasis, metastatic sites ≥2, and elevated total bilirubin as prognostic factors; and Koo et al. [17] reported ECOG PS ≥2, no prior gastrectomy, peritoneal metastasis, bone metastasis, lung metastasis, elevated ALP, decreased albumin, and elevated total bilirubin as prognostic factors. Only PS was a common prognostic factor in all four studies, and peritoneal and bone metastasis were shared among three studies. Whereas the Korean reports were retrospective studies based on data from clinical practice populations that contained patients who were in poor condition, the present study focused only on patients eligible for a specific clinical trial. In JCOG9912, for example, there were very few patients with PS = 2, and those with severe peritoneal metastasis were excluded. Indeed, the cutoff value of PS was set at PS = 1 in the present study, but was set at PS = 2 in the Korean studies. Thus, the patient population, such as patients enrolled in a clinical trial and patients in clinical practice, may have some influence on the prognostic factors.
Chau et al. [14] proposed the RMH prognostic index based on clinical trial data. When we applied the RMH index to our data, about three-quarters (74%) of the patients were classified into the moderate-risk group, and only 5% of the patients were classified into the poor-risk group. Whereas the criteria for the poor-risk group in the JCOG index covered more patients (9%) than the RMH index did (5%) in the present study, the survival of the poor-risk group in the JCOG index was worse than that in the RMH index, even although the overall survival was much better in the present study than that of the subjects of the RMH index [14]. In contrast, although the good-risk group in the JCOG index included more patients (35%) than the RMH index did (20%) in the present study, the survival of the good-risk group in the JCOG index was better than that of the RMH index. Furthermore, the impact on the survival difference was smaller by the RMH index than that observed after application of the JCOG index. These results suggest that the JCOG index may be a better indicator for survival than the RMH index on the points of proportion of the three risk groups and differences in survival.
Except for PS and ALP, the factors used in the JCOG index were substantially different from those used in the RMH index. This may be because of the following three reasons. First, there may be differences in the disease entities, because the studies used to formulate the RMH index included patients with esophageal cancer (27.3% vs. 0% in our study) and those with locally advanced disease (22.2% vs. 0% in our study). Actually, few patients with gastric cancer have locally advanced disease, whereas some patients with esophageal cancer have. Second, there may be differences in severity of peritoneal metastasis. There are two types of peritoneal metastasis: one, such as ascites, is associated with a poor prognosis and can be diagnosed by imaging, and the other can be diagnosed only at laparotomy with small tumor burden, which has a small impact on survival. In JCOG9912, although there were few patients with peritoneal metastasis detected by imaging, peritoneal metastasis was diagnosed at laparotomy in many cases, because many gastric cancer patients go through surgical procedures in Japan. It is considered that this is why peritoneal metastasis was not adopted as a prognostic factor in the present study. The final reason is that there seemed to be some differences in PS between the RMH index and the JCOG index. The cutoff value for the RMH index was PS ≥2 as a risk factor of survival, whereas the cutoff value for PS was ≥1 in our study. This difference may have resulted from the difference in the proportion of patients with PS ≥2 between these studies (23.4% in the RMH studies vs. 1% in our study). Recently, the Global Advanced/Adjuvant Stomach Tumor Research through International Collaboration (GASTRIC) project reported that PS ≥1, disease status, number of metastatic organs, location of metastasis, and prior gastrectomy were prognostic factors for AGC patients treated with systemic chemotherapy as a result of meta-analyses of previous randomized trials, which included both Eastern and Western populations [21]. Notably, this GASTRIC study identified that not only PS = 2 but also PS = 1 were significantly associated with poor prognosis (HRs = 2.17 and 1.36, respectively), which showed the same trend as the present study. In recent phase III trials of gastric cancer, the proportion of patients with PS = 2 has decreased, because patient selection criteria have become more stringent. It can be proposed that the cutoff value for PS should be set between one and two for prognostic analysis in future clinical trials.
The JCOG index proposed in the present study has some limitations. First, whereas number of metastatic sites was an important prognostic factor, metastatic sites were designated by each investigator, and radiological images showing metastatic sites were not reviewed independently for this study. However, because metastatic sites were reported prospectively by checking the list of common metastatic sites in the case report form, the variability is relatively small. Second, it was not validated on other cohorts, especially those including Western patients. Therefore, we plan to validate this JCOG index using the data from other phase III trials. Third, the condition of the subjects in the present study was much better than those often encountered in clinical practice, such as those having good PS and fewer peritoneal metastases. Therefore, the JCOG index may not be applicable to the general patient population in clinical practice. Recently, however, oral fluoropyrimidines, such as capecitabine and S-1, have been replacing the continuous infusion of fluorouracil, and global trials of first-line chemotherapies for AGC have been based on the use of oral agents. Thus, future trials may also tend to exclude patients with severe peritoneal metastasis, which often impairs oral intake, and it is anticipated that exclusion of patients with severe peritoneal metastasis will lead to enrollment of good conditioned patients to the future clinical trials. Therefore, it is expected that this JCOG index may be useful for adjusting and/or balancing prognostic backgrounds even of patients in good condition regardless of their region of origin.
In conclusion, we propose a novel prognostic index (the JCOG index) consisting of four risk factors (PS ≥1, number of metastatic sites ≥2, no prior gastrectomy, and elevated ALP), which classified patients into three risk groups. Although further validation of this index using other trials for AGC is required, it is expected that the JCOG index will be useful in future clinical trials and studies investigating treatment options in AGC patients.
Acknowledgments
This study was supported by the National Cancer Center Research and Development Fund (23-A-16 and 23-A-19), a Grant-in-Aid for Cancer Research (20-S-3, 20-S-6), and a Grant-in Aid for Clinical Cancer Research from the Ministry of Health, Labour, and Welfare, Japan.
This study was previously presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, June 3–7, 2011, Chicago, Illinois, USA.
Footnotes
For Further Reading: Noman Ashraf, Sarah Hoffe, Richard Kim. Adjuvant Treatment for Gastric Cancer: Chemotherapy Versus Radiation. The Oncologist 2013;18:1013–1021.
Implications for Practice: Adjuvant treatment in gastric cancer plays a big role after surgical resection in order to decrease tumor reoccurrence and improve overall survival. However, there is no globally accepted standard of care. Adjuvant chemotherapy after surgical resection has shown benefit in several trials. These trials, however, were mostly conducted in Asia and have not been duplicated in the Western hemisphere. Therefore, the adjuvant treatment in the Western population with gastric cancer is somewhat different. Some patients will receive chemotherapy before and after the surgery and others will receive concurrent chemotherapy and radiation after surgery. This article reviews the current data in adjuvant treatment of gastric cancer and will attempt to clarify the current controversy.
Author Contributions
Conception/Design: Daisuke Takahari, Narikazu Boku, Junki Mizusawa, Atsuo Takashima, Yasuhide Yamada, Takayuki Yoshino, Kentaro Yamazaki, Wasaburo Koizumi, Kensei Yamaguchi, Masahiro Goto, Tomohiro Nishina, Takao Tamura, Akihito Tsuji, Atsushi Ohtsu, Kazutoshi Fukase
Provision of study material or patients: Daisuke Takahari, Narikazu Boku, Junki Mizusawa, Atsuo Takashima, Yasuhide Yamada, Takayuki Yoshino, Kentaro Yamazaki, Wasaburo Koizumi, Kensei Yamaguchi, Masahiro Goto, Tomohiro Nishina, Takao Tamura, Akihito Tsuji, Atsushi Ohtsu, Kazutoshi Fukase
Collection and/or assembly of data: Daisuke Takahari, Narikazu Boku, Junki Mizusawa, Atsuo Takashima
Data analysis and interpretation: Daisuke Takahari, Narikazu Boku, Junki Mizusawa, Atsuo Takashima
Manuscript writing: Daisuke Takahari, Narikazu Boku, Junki Mizusawa, Atsuo Takashima, Yasuhide Yamada, Takayuki Yoshino, Kentaro Yamazaki, Wasaburo Koizumi, Kensei Yamaguchi, Masahiro Goto, Tomohiro Nishina, Takao Tamura, Akihito Tsuji, Atsushi Ohtsu, Kazutoshi Fukase
Final approval of manuscript: Daisuke Takahari, Narikazu Boku, Junki Mizusawa, Atsuo Takashima, Yasuhide Yamada, Takayuki Yoshino, Kentaro Yamazaki, Wasaburo Koizumi, Kensei Yamaguchi, Masahiro Goto, Tomohiro Nishina, Takao Tamura, Akihito Tsuji, Atsushi Ohtsu, Kazutoshi Fukase
Disclosures
Narikazu Boku: Taiho (RF, H); Yakult, Daiichi-Sankyo (H); Yasuhide Yamada: Taiho (RF, H); Daiichi-Sankyo, Yakult (RF); Takayuki Yoshino: Takeda (C/A, H); Bayer, Taiho, Daiichi-Sankyo, ImClone (RF); Chugai, Bristol-Meyers Squibb, Yakult, Merck Serono (H); Kentaro Yamazaki: Taiho, Yakult Honsya (H); Atsushi Ohtsu: Taiho, Yakult, Daiichi-Sankyo (H); Kensei Yamaguchi: Chugai, Takeda, Merck Serono, Bristol (H, Speakers Bureau). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Glimelius B, Hoffman K, Haglund U, et al. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol. 1994;5:189–190. doi: 10.1093/oxfordjournals.annonc.a058778. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Starling N, Rao S, et al. Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. V325 Study Group Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 8.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 9.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 10.Goldhirsch A, Wood WC, Gelber RD, et al. 10th St. Gallen Conference Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 13.Köhne CH, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 14.Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Lim T, Uhm JE, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886–891. doi: 10.1093/annonc/mdl501. [DOI] [PubMed] [Google Scholar]
- 16.Kim JG, Ryoo B-Y, Park YH, et al. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301–307. doi: 10.1007/s00280-007-0476-x. [DOI] [PubMed] [Google Scholar]
- 17.Koo DH, Ryoo B-Y, Kim HJ, et al. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: Validation and comparison with previous models. Cancer Chemother Pharmacol. 2011;68:913–921. doi: 10.1007/s00280-011-1561-8. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Ohtsu A, Boku N, et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol. 2004;34:654–659. doi: 10.1093/jjco/hyh120. [DOI] [PubMed] [Google Scholar]
- 19.Boku N, Yamamoto S, Fukuda H, et al. Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: A randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 20.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 21.Pozzo C, Ohashi Y; on behalf of the GASTRIC project. Meta-analysis of randomized trials assessing the influence of chemotherapy and prognostic factor in advanced/adjuvant gastric cancer. J Clin Oncol 2009; 27: 15s(supple; abstr 4550).