A cross-sectional survey was conducted of 72 health care providers from five of six of the U.S. Affiliated Pacific Island Jurisdictions in 2011 to assess knowledge, beliefs, practices, and perceived barriers regarding routine cervical cancer screening. Although cervical cancer screening is a priority in clinical practice, beliefs about annual screening, costs associated with screening, and varying levels of support for alternative screening tests pose barriers to these providers.
Keywords: Uterine cervical neoplasms, Cancer screening, Pacific Islands, Female, Early detection of cancer, Papillomavirus infections, Diagnosis, Prevention and control
Abstract
Background.
Cervical cancer is a leading cause of cancer mortality in nearly all U.S. Affiliated Pacific Island Jurisdictions (USAPIJ); however, most jurisdictions are financially and geographically limited in their capacity to deliver routine screening.
Methods.
We conducted a cross-sectional survey of 72 health care providers from five of the six USAPIJ in 2011 to assess knowledge, beliefs, practices, and perceived barriers regarding routine cervical cancer screening. We compared the responses of providers from jurisdictions that were funded by the Centers for Disease Control and Prevention’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP) with those that were not funded.
Results.
Most providers reported cervical cancer prevention as a priority in their clinical practices (90.3%) and use the Papanicolaou test for screening (86.1%). Many providers reported knowledge of screening guidelines (76.4%); however, more than half reported that annual screening is most effective (56.9%). Providers in non-NBCCEDP-funded jurisdictions reported greater acceptance of visual inspection with acetic acid (93.9%) and self-sampling for human papillomavirus testing (48.5%) compared with NBCCEDP-funded jurisdictions (15.4% and 30.8% respectively). Providers from non-NBCCEDP-funded jurisdictions reported inadequate technological resources for screening women (42.4%), and approximately 25% of providers in both groups believed that screening was cost-prohibitive.
Conclusion.
Although cervical cancer screening is a priority in clinical practice, beliefs about annual screening, costs associated with screening, and varying levels of support for alternative screening tests pose barriers to providers throughout the USAPIJ. Further exploration of using evidence-based, lower cost, and sustainable screening technologies is warranted in addition to emphasizing timely follow-up of all positive cases.
Implications for Practice:
The U.S. Affiliated Pacific Island Jurisdictions (USAPIJ) are located in a geographically disparate region with a high burden of cervical cancer. Although cervical cancer screening providers in the USAPIJ stated that screening is a priority in clinical practice, costs associated with screening and varying levels of support for alternative screening tests pose barriers to screening throughout the USAPIJ. Use of alternative screening tests and routine monitoring and quality assurance to ensure all eligible women are reached may be needed to reduce the cervical cancer burden in the USAPIJ and to ensure effective use of limited resources.
Introduction
Cytology-based screening with the Papanicolaou (Pap) test has substantially reduced cervical cancer mortality in the U.S. and other developed countries; however, that has not been the case in low- and middle-income countries (LMICs), where death rates from this disease have increased or remained unchanged [1]. Effective cytology-based screening requires considerable infrastructure and coordination within the health care system to ensure that women receive screening as well as diagnosis and treatment services if necessary. This infrastructure is absent in many resource-constrained settings, including some of those in the U.S. Affiliated Pacific Island Jurisdictions (USAPIJ) [2, 3].
Cervical cancer is one of the leading causes of cancer death in women in the USAPIJ. Although cervical cancer screening programs exist in the USAPIJ, some islands have limited access to infrastructure necessary to provide care to women with abnormal Pap test results [3], resulting in presentation of advanced stage cervical cancer and substantial morbidity after treatment.
Some cervical cancer screening programs in the USAPIJ generally follow guidelines from the U.S. Preventive Services Task Force, the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), and the American Society for Colposcopy and Cervical Pathology [4–6]. U.S. screening guidelines have evolved considerably over the past decade to include longer screening intervals for cytology-based approaches, a later age for young women to initiate screening, use of the human papillomavirus (HPV) cotest for women aged 30 years and older that extends screening to 5-year intervals, and a recommendation to cease screening in women with a history of normal test results at age 65 [4–7]. In addition, guidelines for management of young women with abnormal cytology results have become more conservative because most HPV infections resolve spontaneously without long-term adverse effects, and overtreatment of precancerous lesions may lead to adverse reproductive outcomes [8, 9].
In 2013, the World Health Organization (WHO) recommended, at minimum, screening women at least once in their lifetime, between 30 and 49 years of age [10]. Other cervical cancer screening programs in USAPIJ, such as the Federated States of Micronesia (FSM) and the Republic of the Marshall Islands (RMI), have created their own national standards for cervical cancer screening that support the use of visual inspection with acetic acid (VIA), which were developed in 2009 and 2010, respectively [11, 12]. Demonstration projects and randomized controlled screening trials in LMICs have found that the effectiveness and efficiency of a single visit screen-and-treat strategy using VIA or HPV testing followed by treatment with cryotherapy is high [13–18].
The Cancer Council of the Pacific Islands (CCPI), the indigenous body that advises the Pacific Regional Comprehensive Cancer Control Program and other cancer-related initiatives in the USAPIJ, has had a long-term goal of working with the ministries and departments of health in the USAPIJ to develop minimum regional guidelines for cervical cancer screening and prevention [19, 20]. A comprehensive description of the current cervical cancer screening practices in the USAPIJ was needed to inform health departments and local partners on ways to improve existing cervical cancer prevention strategies and to develop new ones. In 2009, the Pacific Island Health Officers Association, an organization that represents the collective health interests of the USAPIJ, stated its support for a project to assess cervical cancer screening practices in the USAPIJ. The purpose of this study was to assess providers’ cervical cancer-related knowledge, screening and referral practices, awareness of new or alternative screening technologies, and perceptions of barriers to care. The most senior-ranking health official (minister, secretary, or director of health) in each country endorsed the project and encouraged full support in April 2011. Providers were surveyed as part of a larger program evaluation study that included separate assessment of program staff and community members to study awareness, support, and barriers to cervical cancer screening and HPV vaccination.
Materials and Methods
USAPIJ
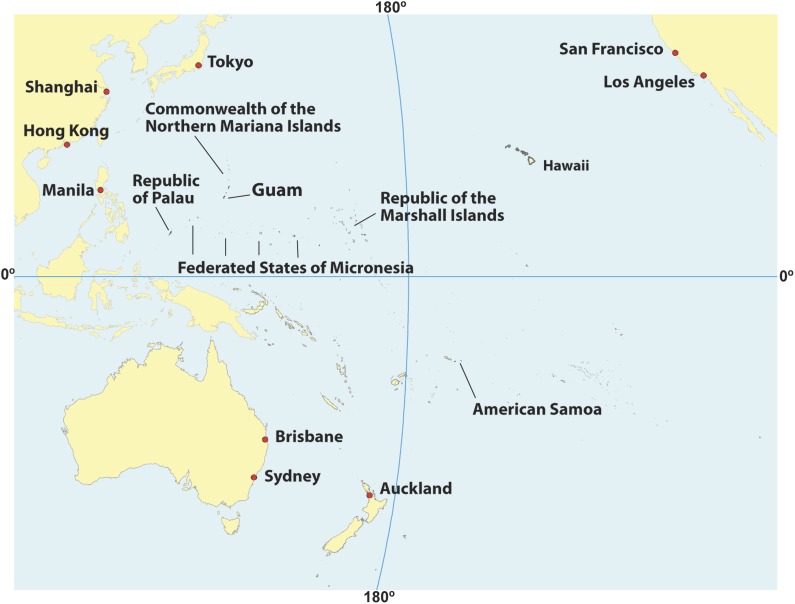
The USAPIJ includes American Samoa, the Commonwealth of the Northern Mariana Islands (CNMI), Guam, the FSM, the Republic of Palau, and the RMI (Fig. 1) [3]. The USAPIJ is part of a geographically, culturally, politically, and economically diverse region, with considerable variations in public health infrastructure and approaches to cervical cancer prevention (supplemental online Table 1). All jurisdictions of the USAPIJ have cervical cancer screening services available, but jurisdictional cancer programs to support screening vary with respect to organization and resources. The screening programs in American Samoa, Guam, the CNMI, and Palau are funded by the Centers for Disease Control and Prevention’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP; http://www.cdc.gov/cancer/nbccedp/). These programs rely solely on cytology-based screening tests (either conventional or liquid based) to conduct cervical cancer screening and have not yet incorporated HPV cotesting into their screening protocols. The RMI and the FSM do not receive NBCCEDP funding. Both jurisdictions developed guidelines that outline a tiered approach (core, expanded, desirable) to screening based on availability of resources. Briefly, RMI's core resource level standard includes screening with VIA women aged 21–50 years at 2-year intervals with referral for Pap test if precancerous lesions are detected. The Pap test is also recommended for screening women aged 50–60 years, as resources permit. FSM's core standard includes screening with VIA women aged 25–45 years at least twice in a lifetime with referral for Pap test if precancerous lesions are detected. Core standards also include opportunistic screening with Pap test, as resources permit. In both jurisdictions, expanded standards include treating precancerous lesions using cryotherapy rather than Pap test follow-up. If resources were available, desirable standards in both jurisdictions include expanding routine screening up to age 60, shortening screening intervals, and using the Pap test and HPV DNA testing. The U.S. Department of Health and Human Services Office of Population Affairs Title X Family Planning Program also funds cytology-based screening in the USAPIJ (http://www.hhs.gov/opa/title-x-family-planning/).
Figure 1.
The U.S. Affiliated Pacific Island Jurisdictions.
Study Participants and Data Collection
The University of Hawaii administered the survey using jurisdictional health department staff to distribute the survey to all cervical cancer screening providers (physicians, nurse practitioners, public health nurses) participating in the NBCCEDP program, the Title X Family Planning program, and providers on outer islands and in private clinics, using various formats (e.g., mail, in person, phone). For larger jurisdictions (e.g., Guam) with more than 20 providers, staff was instructed to survey 20% of physicians as a convenience sample. The final convenience sample was an estimated 48.3% of all USAPIJ providers.
Survey Instrument
A cross-sectional survey tool was developed in collaboration with the University of Hawaii, the CCPI, and their partners that focused on knowledge, awareness, practices, and barriers to providing routine screening and vaccination. Most questions were selected and adapted from existing U.S. or international program assessment tools, such as tools used by PATH (http://www.path.org/about/index.php). The survey included definitions of various cervical cancer screening tests, including the rapid HPV test (referred to as the “point-of-care HPV test”) (careHPV; Qiagen, Gaithersburg, MD, http://www.qiagen.com) and the HPV DNA test, which is currently available for use in the U.S. (digene hybrid capture 2 [HC2]; Qiagen) but is rarely used in the USAPIJ. Members of the CCPI reviewed the survey and offered advice on restructuring and tailoring of survey questions.
The provider survey included questions to assess knowledge and awareness of cervical cancer prevention in general, guidelines for cervical cancer screening, and alternative screening technologies. Response options included “true,” “false,” and “don’t know/not sure.” A set of questions assessed barriers to screening such as cost and infrastructure, and providers were asked to answer “agree,” “disagree,” or “don’t know/not sure.” Multiple-choice questions were used to assess current screening practices, guidelines followed, and referral practices for women with abnormal test results. The survey included open-ended and multiple-choice questions on the use of VIA and knowledge, attitudes, and practice of other screening technologies. Demographic information on the providers and their patient populations were also collected.
The University of Hawaii Committee on Human Subjects determined that this survey was exempt from review in March 2011.
Data Management and Analysis
Completed surveys were sent to the University of Hawaii for entry into a secure Access database. Although personal identifiers were included on these surveys, these were not entered into the Access database. Original surveys were stored on a secure network with restricted access and paper copies were stored in a locked filing cabinet. Deidentified data were analyzed by the Centers for Disease Control and Prevention.
Descriptive statistics were calculated for each survey item analyzed. Survey items were analyzed by funding jurisdictions, grouped as either NBCCEDP-funded or non-NBCCEDP-funded, and differences were assessed using Fisher’s exact test because of the small sample sizes for some survey items. Some NBCCEDP-funded jurisdictions included providers who were not affiliated with the NBCCEDP. Values of p < .05 were considered statistically significant.
”No” or “not sure” responses were included in the denominator in calculating percentages, and the “not applicable” category was excluded. All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Participants’ Professional and Demographic Characteristics
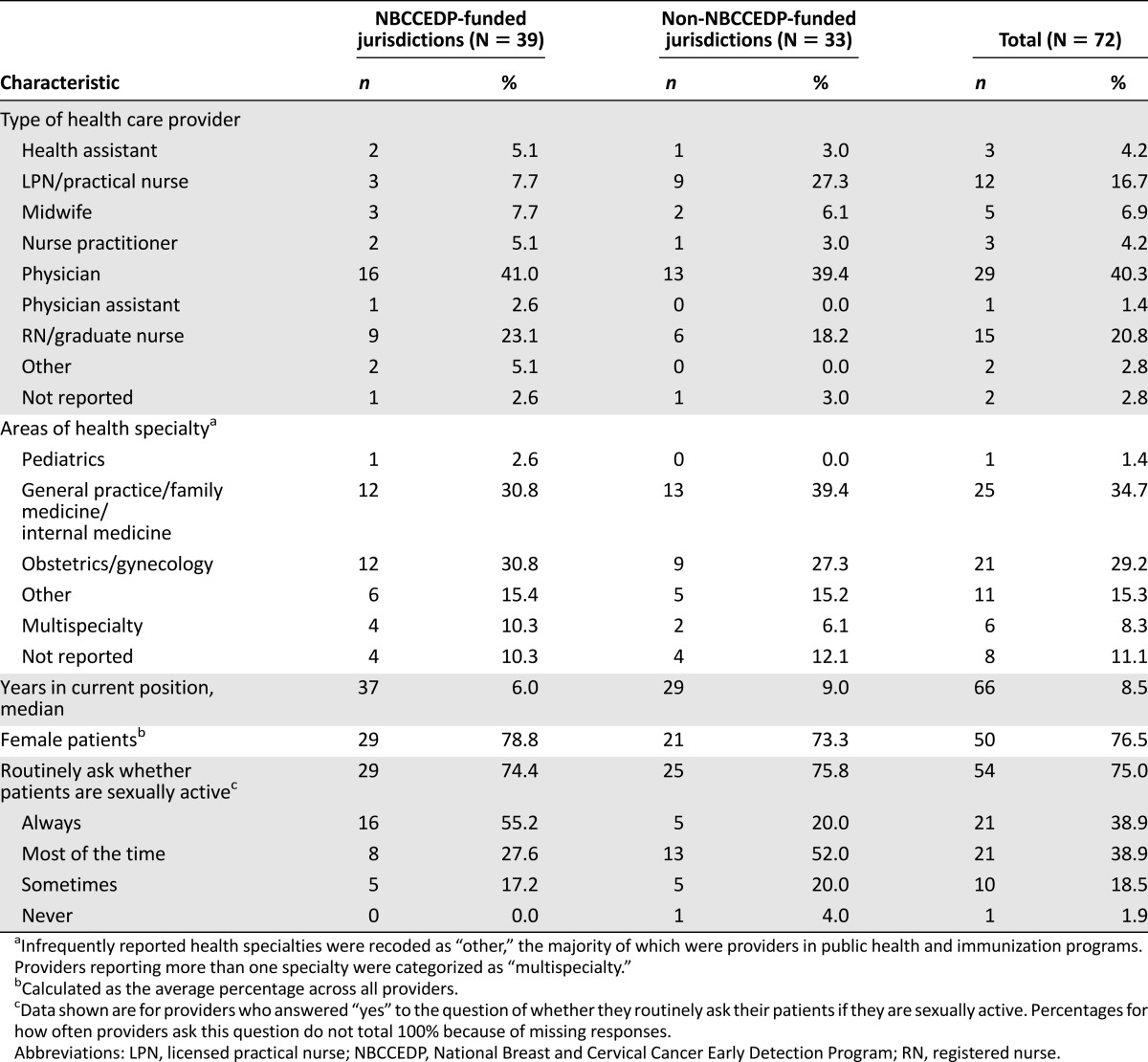
A total of 72 providers from five of the six jurisdictions (excluding American Samoa) completed the survey. Thirty-nine providers were from NBCCEDP-funded jurisdictions, whereas the remaining 33 were from non-NBCCEDP-funded jurisdictions (Table 1). More than 40% of providers were physicians, followed by registered nurses (20.8%) and licensed practical nurses (16.7%; Table 2). Nearly 35% of providers practiced in general practice, family medicine, or internal medicine settings, and 29% practiced in obstetrics and gynecology.
Table 1.
Cervical cancer screening providers by U.S. Affiliated Pacific Island Jurisdictions

Table 2.
Demographic and practice characteristics of cervical cancer screening providers in the U.S. Affiliated Pacific Island Jurisdictions by type of jurisdiction
Knowledge and Beliefs Regarding Screening Tests
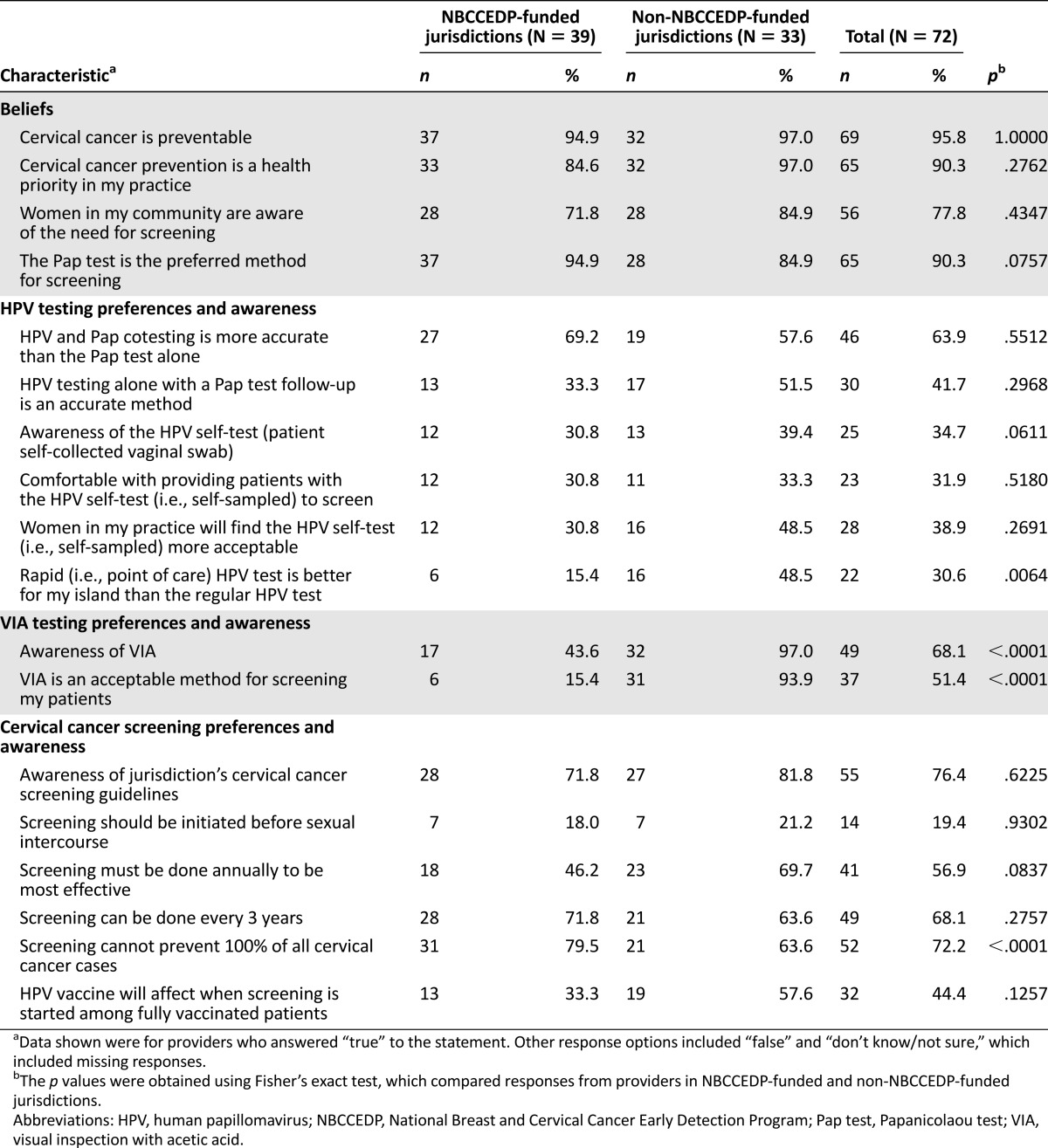
Nearly all providers believed that cervical cancer prevention was a health priority in their practices (90.3%; Table 3), and the Pap test was their preferred method for screening (90.3%). Most also believed that women in their community were aware of the need for cervical cancer screening (77.8%). Nearly 64% of providers believed that HPV cotesting (i.e., testing conducted at the same time that a cytology specimen is collected) is more accurate than the Pap test alone.
Table 3.
Provider knowledge and beliefs regarding cervical cancer screening in the U.S. Affiliated Pacific Island Jurisdictions by type of jurisdiction
Awareness of Alternative Screening Technologies
Only 34.7% of providers were aware of the HPV self-sampling test for cervical cancer screening, and 31.9% answered that they would be comfortable providing patients with the self-sampled HPV test. More than 30% of providers felt that the point-of-care HPV test would be a better screening test for their specific jurisdictions than the conventional HPV test, if the point-of-care HPV test were to become available in the USAPIJ. This view was held by more providers in non-NBCCEDP-funded jurisdictions than providers in NBCCEDP-funded jurisdictions (48.5% vs. 15.4%, respectively; p = .0064).
Overall, 68.1% of providers were aware of VIA for cervical cancer screening, and 51.4% of providers reported that VIA is an acceptable method of screening patients. Among providers in NBCCEDP-funded jurisdictions, 43.6% reported awareness of VIA, whereas 97.0% of providers in non-NBCCEDP-funded jurisdictions were aware of this method (p < .0001). Only 15.4% of providers in NBCCEDP-funded jurisdictions believed that VIA is an acceptable method compared with 93.9% of providers in non-NBCCEDP-funded jurisdictions (p < .0001).
Knowledge of Guidelines and Current Practices
Overall, 76.4% of providers were aware of cervical cancer screening guidelines used or adopted in their specific jurisdictions. Nearly 20% of providers believed that cervical cancer screening should begin before sexual initiation. Although 68.1% of providers agreed that cervical cancer screening can be done every 3 years, 56.9% answered that cervical cancer screening must be done annually to be most effective.
Providers’ Own Practices
Eighty-six percent of providers used the Pap test to screen patients for cervical cancer (Table 4). Nearly two-thirds conduct annual screening of their patients, whereas 25.8% screen every 3 years. More than 45% of providers in non-NBCCEDP-funded jurisdictions used VIA. One-third of providers screen patients annually with VIA, and 26.7% screen patients every 5 years. Thirteen percent reported not using a routine interval.
Table 4.
Cervical cancer screening and follow-up practices of providers in the U.S. Affiliated Pacific Island Jurisdictions by type of jurisdiction
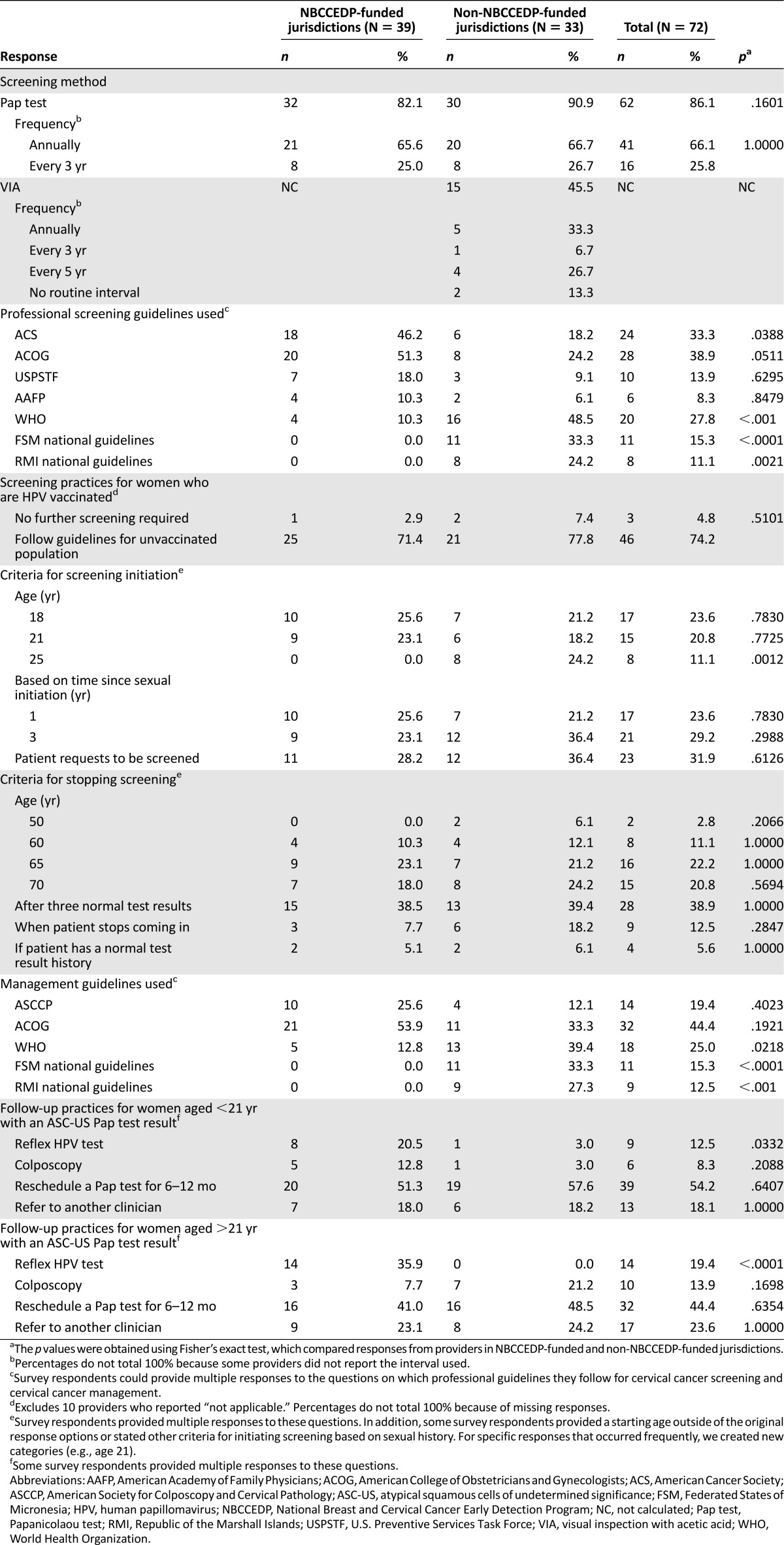
Guidelines for screening from ACOG (38.9%), ACS (33.3%), and WHO (27.8%) were the most commonly followed screening guidelines. Providers in NBCCEDP-funded jurisdictions relied more on ACS guidelines (46.2%; p = .0388), whereas providers in non-NBCCEDP-funded jurisdictions more frequently reported using WHO guidelines (48.5%; p < .001), the FSM national guidelines (33.3%; p < .0001), and the RMI national guidelines (24.2%; p = .0021). Nearly a quarter of providers reported beginning to screen women for cervical cancer at age 18, and 20.8% continue to screen women until age 70.
ACOG management guidelines were most frequently reported (44.4%), followed by WHO guidelines (25%) and American Society for Colposcopy and Cervical Pathology guidelines (19.4%). Similar to screening guidelines, the sources of management guidelines used varied by NBCCEDP funding status of jurisdictions, with providers in non-NBCCEDP-funded jurisdictions more frequently reporting use of WHO guidelines, the FSM national guidelines, and the RMI national guidelines.
Although 54.2% of providers would reschedule a Pap test for 6–12 months in women younger than 21 years of age with an atypical squamous cells of undetermined significance (ASC-US) test result, some would refer women for reflex HPV testing (i.e., as a follow-up to abnormal cytology results; 12.5%) or colposcopy (8.3%).
Approximately one-quarter of providers reported that screening women for cervical cancer is cost prohibitive and that the cost of cervical cancer screening was a barrier to care both for patients and for the provider to provide regular care to patients (Table 5). Nineteen percent of providers reported that their clinics did not have the technology to adequately screen patients. Providers in non-NBCCEDP-funded jurisdictions had greater awareness of the differences in cost among screening tests than providers in NBCCEDP-funded jurisdictions (69.7% vs. 33.3%; p = .0103). A higher proportion of providers in non-NBCCEDP-funded jurisdictions reported that they did not have the technological resources to adequately screen patients (42.4% vs. 0.0%; p < .0001) compared with providers in NBCCEDP-funded jurisdictions. Providers in non-NBCCEDP-funded jurisdictions expressed a greater interest in other methods of cervical cancer screening than what they currently used compared with their counterparts in NBCCEDP-funded jurisdictions (69.7% vs. 25.6%; p < .001). Both types of providers frequently mentioned the HPV test (48.5%), with either provider- or self-collected sampling, as a test they would be interested in using to screen patients in the future. Among all providers, 34.7% reported referring patients to another facility for colposcopy, and 56.9% referred patients for cryotherapy.
Table 5.
Perceived barriers to cervical cancer screening and availability of diagnostic and treatment procedures in the U.S. Affiliated Pacific Island Jurisdictions

Discussion
Providers in the USAPIJ were generally well informed about cervical cancer and prevention strategies and stated that cervical cancer prevention was a priority in their practices; however, some gaps exist in their knowledge and practice of evidence-based guidelines for cervical cancer. A minority of providers believed, for example, that screening should begin before young women are sexually active. Some providers continue to screen women annually or outside of recommended age intervals, which is similar to findings among U.S. providers [21–23]. However, cervical cancer screening coverage is generally low in the USAPIJ [24–27]. Few women are screened through the NBCCEDP or the Title X Family Planning Program in relation to the eligible population (supplemental online Table 1). In Guam, 63.5% of women aged 18 years and older in 2012 reported being screened within the past 3 years compared with 78.0% of women residing in U.S. states and the District of Columbia [28]. Many jurisdictions in the USAPIJ have remote outer islands where access to diagnostic and treatment services is limited, and care is provided by dispensaries staffed by health assistants supported by periodic visits by health care teams; therefore, women residing in these areas may be screened rarely [3].
The vast majority of all providers reported using the Pap test, even in jurisdictions where national screening guidelines permit use of VIA [11, 12]. Lack of health care resources in the USAPIJ makes this region similar to other LMICs [29]. VIA for screening followed by cryotherapy to treat precancerous lesions are suitable procedures for LMICs because both are inexpensive and can be performed by nurses [30]. Currently, Title X Family Planning Program funds may not be applied toward VIA screening. Additional training for providers and program staff may also be needed to fully implement guidelines that support the use of VIA in non-NBCCEDP-funded jurisdictions because use of VIA was only 45.5%. Providers in non-NBCCEDP-funded jurisdictions stated greater awareness of VIA and more frequently reported that VIA was an acceptable method of screening their patients. Open-ended survey items on VIA answered by VIA screening providers generally reflected positive statements (data not shown). Awareness of VIA was considerably lower in NBCCEDP-funded jurisdictions, and few believed it was an acceptable screening method. In general, NBCCEDP policies on cervical cancer screening generally follow U.S. Preventive Services Task Force guidelines, which do not address use of VIA [4, 31].
In LMICs, using primary HPV screening rather than cytology presents numerous advantages and is a feasible strategy [32, 33]. Around 46% of providers expressed interest in alternative screening methods, and many cited the HPV test. Using the conventional HPV test currently available in the U.S. as a primary screening test is an alternative, evidence-based screening strategy that could potentially reach more women [34–38]. Only about 42% of providers across the USAPIJ recognize this as an accurate method. To date, neither the point-of-care HPV test nor the conventional HPV test is approved by the U.S. Food and Drug Administration for primary screening, and the conventional HPV test requires access to a molecular laboratory to process samples. NBCCEDP programs choosing to use reflex HPV testing send their samples to Hawaii for processing, although this may be expensive and cause delays in the time from screening to diagnosis and treatment. Using the HPV test for primary screening may increase the number of referrals for colposcopy [35], and nearly 35% of providers must refer their patients for this procedure. Its lower specificity for detecting transient versus persistent HPV infection may require a separate screening test such as cytology or VIA to triage women with positive results [17, 18, 32, 35–40].
Awareness overall was relatively low for other emerging technologies for screening, such as the self-sampled HPV test and the point-of-care HPV test, given that these tests are not available in the USAPIJ. Providers in non-NBCCEDP-funded jurisdictions were more aware of the point-of-care HPV test than were their counterparts in NBCCEDP-funded jurisdictions. Compared with providers in NBCCEDP-funded jurisdictions, providers in non-NBCCEDP-funded jurisdictions reported more financial and technological barriers to screening, although a sizable minority in both types of jurisdictions reported that screening was cost prohibitive and sometimes a barrier to providing regular care to patients. Many providers refer patients to a hospital or off-island for diagnostic and treatment procedures, including cryotherapy. As more scientific evidence on effectiveness of newer HPV-based methods in LMICs becomes available [2, 33, 41–43], these methods may also become viable screening options in the Pacific Islands. The point-of-care HPV test may be delivered as a self- or provider-administered test, with results available in several hours using laboratory equipment that does not require electricity and highly experienced technicians, making it suitable for low-resource settings [32, 40, 41]. This may be particularly relevant for the USAPIJ, with remote inhabited islands where women may be invited by outreach workers for screening. A woman with a positive HPV result from a point-of-care HPV test could be followed up with VIA performed by either a nurse or a trained health assistant on the same day, and lesions may be treated with cryotherapy immediately afterward. Self-sampling, using either a conventional HPV test or a point-of-care HPV test, is a promising, effective strategy for reaching underserved women in remote settings who have never been screened for cervical cancer and when resources limit screening with a highly sensitive test to only several occasions in a woman’s lifetime [33, 42–45].
Women across the USAPIJ represent diverse cultures and beliefs, so cultural acceptability should be considered as new technologies emerge. Acceptability may not be as much of a problem with the advantages of having test results available immediately or within a couple of hours [46]. Women who are uncomfortable with the Pap test or with male providers conducting these examinations may also find alternative technologies more acceptable, such as the self-sampled HPV test [33, 45, 47, 48]. Although the literature is scarce on USAPIJ women’s attitudes and beliefs regarding cervical cancer prevention, studies have shown that women have concerns regarding confidentiality of test results and respect from health care providers [49, 50]. Fear and shame may also prevent some women from seeking screening [49, 51]. Future health promotion activities may need to continue to address these barriers as alternative screening technologies are deployed.
With the exception of Guam, women diagnosed with stage III (and sometimes stage II) or higher cervical cancer must be referred off-island for treatment because on-island surgical options and chemotherapy are not available. A reduction in these costs may be achieved if early detection is addressed as a comprehensive approach. Regular updates and trainings, including those that focus on routine quality assurance and monitoring and coverage of the target population and that address patient and clinical barriers may be developed by public health professionals. Ministries and departments of health may ensure that equitable access to quality cervical cancer screening services exists for all women and that all women with precancerous lesions receive follow-up and entry into treatment services [52]. Continued programmatic activities on routine quality assurance, population-based surveillance, and evaluation may be continued. The development of referral systems for follow-up treatment for screening programs would increase their effectiveness [52].
This study has inherent limitations. First, this survey was cross-sectional; therefore, our findings reflect only one point in time and predate the latest U.S. cervical cancer screening guidelines in 2012. Second, there was no formal pilot testing of the provider survey, although some members of the CCPI who provided direct input to the survey are providers. Third, because our final sample was a convenience sample, our findings may not be generalizable to all screening providers in some jurisdictions, especially those with large proportions of private providers (Guam and the CNMI). Fourth, the providers who answered the survey may not be aware of the NBCCEDP program and may be providers of other programs (e.g., family planning). Fifth, a variety of health care professionals, including some public health professionals, completed the survey. Some respondents may not have been directly involved in cervical cancer screening. Sixth, nonresponse to sections of the survey was significant; between one-quarter and one-half of providers were unsure or did not provide a response regarding HPV testing preferences and beliefs. Other survey items also had a significant minority of providers not provide an answer. Nonresponse or unsure responses to some survey questions may also indicate a need for further education. Seventh, because our survey data are self-reported, they are subject to social desirability and recall bias and may not truly reflect actual screening practices. Despite these limitations, the current study represents an important contribution to the understanding of cervical cancer screening practices in the USAPIJ because few comprehensive assessments of screening practices have been conducted in those jurisdictions. Findings from this comprehensive assessment will be helpful for informing program planning and training.
Conclusion
Although cervical cancer screening is a priority in clinical practice, costs associated with screening pose barriers to the provision of proper care throughout the USAPIJ. Providers, particularly in non-NBCCEDP-funded jurisdictions, report use of and interest in alternative screening technologies. Two of these jurisdictions have already adapted their own screening guidelines, which are more resource appropriate and have the potential to screen each woman at least twice in her lifetime, including those living on remote islands. These guidelines may further be refined to better reflect 2013 WHO guidelines. Additionally, several USAPIJs are interested in conducting feasibility testing of WHO algorithms utilizing HPV primary screening, followed by VIA and cryotherapy of precancerous lesions. However, providers and health departments and ministries of health interested in adopting these new technologies may need to set policies and standards for screening, diagnosis, and referral, and women in the community will need to be comfortable with different testing methods [10, 33, 46, 47, 52–54]. Quality assurance and program monitoring to ensure that all eligible women are reached and that women with abnormal test results are followed in a timely manner will be key to reducing the cervical cancer burden and ensuring effective use of resources. Efforts are currently under way to develop a joint committee opinion through the American Society for Colposcopy and Cervical Pathology and ACOG on alternative screening technologies for U.S. territories and Pacific Island Jurisdictions [55]. Further exploration of using evidence-based, lower-cost, and more sustainable screening technologies is warranted for all jurisdictions, as is development of guidelines that can be achieved in the USAPIJ, given the region’s geographic isolation, limited resources, history, and cultural diversity.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank the Cancer Council of the Pacific Islands, health care providers and public health programs in the U.S. Affiliated Pacific Island Jurisdictions for their participation and assistance in our assessment of cervical cancer screening practices in the U.S. Affiliated Pacific Island Jurisdictions. We also thank Dr. Tom Richards for producing a regional map for us. This work was supported through a Centers for Disease Control and Prevention cooperative agreement, U58 DP000779 Federated States of Micronesia National Comprehensive Cancer Control Program. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Furthermore, the use of any product names, trade names, images, or commercial sources is for identification only and does not imply endorsement or government sanction by the U.S. Department of Health and Human Services.
Author Contributions
Conception/Design: Lee Buenconsejo-Lum, Mona Saraiya
Provision of study material or patients: Katherine B. Roland, Lee Buenconsejo-Lum
Collection and/or assembly of data: Analía Romina Stormo, Lee Buenconsejo-Lum
Data analysis and interpretation: Julie S. Townsend, Analía Romina Stormo, Katherine B. Roland, Lee Buenconsejo-Lum
Manuscript writing: Julie S. Townsend, Analía Romina Stormo, Katherine B. Roland, Lee Buenconsejo-Lum, Susan White, Mona Saraiya
Final approval of manuscript: Julie S. Townsend, Analía Romina Stormo, Katherine B. Roland, Lee Buenconsejo-Lum, Susan White, Mona Saraiya
Disclosures
Lee Buenconsejo-Lum: Centers for Disease Control and Prevention Cooperative Agreements (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Hoppenot C, Stampler K, Dunton C. Cervical cancer screening in high- and low-resource countries: Implications and new developments. Obstet Gynecol Surv. 2012;67:658–667. doi: 10.1097/OGX.0b013e3182732375. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Council of the Pacific Islands. U.S. Affiliated Pacific Island Nations: Pacific regional comprehensive cancer control plan 2007-2012. 2007. Available at http://cancercontrolplanet.cancer.gov/state_plans/Pacific_Regional_Cancer_Control_Plan.pdf Accessed August 2, 2013.
- 4.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force Recommendation statement. Ann Intern Med. 2012;156:880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee on Practice Bulletins—Gynecology ACOG practice bulletin number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120:1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 7.ACOG Committee on Practice Bulletins--Gynecology ACOG practice bulletin no. 109: Cervical cytology screening. Obstet Gynecol. 2009;114:1409–1420. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 8.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: Meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO guidance note: Comprehensive cervical cancer prevention and control: A healthier future for girls and women. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 11.The Federated States of Micronesia National Department of Health and Social Affairs. Workshop to develop national breast and cervical cancer client management guidelines for prevention, detection, treatment and care in the Federated States of Micronesia. August 29–September 2, 2008; Pohnpei, Federated States of Micronesia. Available at: http://pacificcancer.org/Cancer/CaResources/CCC/ Accessed January 30, 2014.
- 12.Republic of the Marshall Islands Ministry of Health. National screening standards for cervical cancer. Majuro, Republic of the Marshall Islands: Ministry of Health, 2010.
- 13.Sherris J, Wittet S, Kleine A, et al. Evidence-based, alternative cervical cancer screening approaches in low-resource settings. Int Perspect Sex Reprod Health. 2009;35:147–154. doi: 10.1363/ifpp.35.147.09. [DOI] [PubMed] [Google Scholar]
- 14.Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: A cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 15.Luciani S, Gonzales M, Munoz S, et al. Effectiveness of cryotherapy treatment for cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2008;101:172–177. doi: 10.1016/j.ijgo.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Shastri S, Mittra I, Mishra G et al. Effect of visual inspection with acetic acid (VIA) screening by primary health workers on cervical cancer mortality: A cluster randomized controlled trial in Mumbai, India. Paper presented at: Annual Meeting of the American Society of Clinical Oncology; May 31-June 4, 2013; Chicago, IL. [Google Scholar]
- 17.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 18.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 19.Palafox NA, Tsark J. Cancer in the US Associated Pacific Islands (UASPI): History and participatory development. Pac Health Dialog. 2004;11:8–13. [PubMed] [Google Scholar]
- 20.Gunawardane K, Demei Y. Cancer Council of the Pacific Islands: Speaking with one voice. Pac Health Dialog. 2004;11:14–16. [PubMed] [Google Scholar]
- 21.Berkowitz Z, Saraiya M, Sawaya GF. Cervical cancer screening intervals, 2006 to 2009: Moving beyond annual testing. JAMA Intern Med. 2013;173:922–924. doi: 10.1001/jamainternmed.2013.368. [DOI] [PubMed] [Google Scholar]
- 22.Roland KB, Benard VB, Soman A, et al. Cervical cancer screening among young adult women in the United States. Cancer Epidemiol Biomarkers Prev. 2013;22:580–588. doi: 10.1158/1055-9965.EPI-12-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saraiya M, Berkowitz Z, Yabroff KR, et al. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: What screening intervals are physicians recommending? Arch Intern Med. 2010;170:977–985. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- 24.Kroon E, Reddy R, Gunawardane K, et al. Cancer in the Republic of the Marshall Islands. Pac Health Dialog. 2004;11:70–77. [PubMed] [Google Scholar]
- 25.Ichiho HM, Wong V, Hedson J, et al. Cancer in Pohnpei state, Federated States of Micronesia. Pac Health Dialog. 2004;11:44–49. [PubMed] [Google Scholar]
- 26.Ichiho HM, Gladu R, Keybond K, et al. Cancer in Chuuk state, Federated States of Micronesia. Pac Health Dialog. 2004;11:30–36. [PubMed] [Google Scholar]
- 27.Taoka S, Hancock T, Ngaden V, et al. Cancer in Yap state, Federated States of Micronesia. Pac Health Dialog. 2004;11:50–56. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System: Prevalence and trends data. Available at http://apps.nccd.cdc.gov/brfss/ Accessed August 14, 2013.
- 29.Katz AR, Palafox NA, Johnson DB, et al. Cancer epidemiology in the freely associated U.S. Pacific Island jurisdictions: Challenges and methodologic issues. Pac Health Dialog. 2004;11:84–87. [PubMed] [Google Scholar]
- 30.Tsu VD, Pollack AE. Preventing cervical cancer in low-resource settings: How far have we come and what does the future hold? Int J Gynaecol Obstet. 2005;89(suppl 2):S55–S59. doi: 10.1016/j.ijgo.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Benard VB, Saraiya MS, Soman A, et al. Cancer screening practices among physicians in the National Breast and Cervical Cancer Early Detection Program. J Womens Health (Larchmt) 2011;20:1479–1484. doi: 10.1089/jwh.2010.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrero R, Ferreccio C, Salmerón J, et al. New approaches to cervical cancer screening in Latin America and the Caribbean. Vaccine. 2008;26(suppl 11):L49–L58. doi: 10.1016/j.vaccine.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Gravitt PE, Belinson JL, Salmeron J, et al. Looking ahead: A case for human papillomavirus testing of self-sampled vaginal specimens as a cervical cancer screening strategy. Int J Cancer. 2011;129:517–527. doi: 10.1002/ijc.25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitlock EP, Vesco KK, Eder M, et al. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687–697. doi: 10.7326/0003-4819-155-10-201111150-00376. [DOI] [PubMed] [Google Scholar]
- 35.Cox JT, Castle PE, Behrens CM, et al. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: Results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208:184.e1–184.e11. doi: 10.1016/j.ajog.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 37.Ogilvie GS, Krajden M, van Niekerk DJ, et al. Primary cervical cancer screening with HPV testing compared with liquid-based cytology: Results of round 1 of a randomised controlled trial — the HPV FOCAL Study. Br J Cancer. 2012;107:1917–1924. doi: 10.1038/bjc.2012.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 39.Cuzick J, Bergeron C, von Knebel Doeberitz M, et al. New technologies and procedures for cervical cancer screening. Vaccine. 2012;30(suppl 5):F107–F116. doi: 10.1016/j.vaccine.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 40.Almonte M, Murillo R, Sánchez GI, et al. New paradigms and challenges in cervical cancer prevention and control in Latin America [in Spanish] Salud Publica Mex. 2010;52:544–559. doi: 10.1590/s0036-36342010000600010. [DOI] [PubMed] [Google Scholar]
- 41.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: A cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 42.Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): A community-based randomised controlled trial. Lancet. 2011;378:1868–1873. doi: 10.1016/S0140-6736(11)61522-5. [DOI] [PubMed] [Google Scholar]
- 43.Ogilvie GS, Mitchell S, Sekikubo M, et al. Results of a community-based cervical cancer screening pilot project using human papillomavirus self-sampling in Kampala, Uganda. Int J Gynaecol Obstet. 2013;122:118–123. doi: 10.1016/j.ijgo.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Ogilvie G, Krajden M, Maginley J, et al. Feasibility of self-collection of specimens for human papillomavirus testing in hard-to-reach women. CMAJ. 2007;177:480–483. doi: 10.1503/cmaj.070013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell S, Ogilvie G, Steinberg M, et al. Assessing women’s willingness to collect their own cervical samples for HPV testing as part of the ASPIRE cervical cancer screening project in Uganda. Int J Gynaecol Obstet. 2011;114:111–115. doi: 10.1016/j.ijgo.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Busingye P, Nakimuli A, Nabunya E, et al. Acceptability of cervical cancer screening via visual inspection with acetic acid or Lugol’s iodine at Mulago Hospital, Uganda. Int J Gynaecol Obstet. 2012;119:262–265. doi: 10.1016/j.ijgo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(suppl 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Waller J, McCaffery K, Forrest S, et al. Acceptability of unsupervised HPV self-sampling using written instructions. J Med Screen. 2006;13:208–213. doi: 10.1177/096914130601300409. [DOI] [PubMed] [Google Scholar]
- 49.Wong VS, Kawamoto CT. Understanding cervical cancer prevention and screening in Chuukese women in Hawaii. Hawaii Med J. 2010;69(suppl 3):13–16. [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L, Colby E, Iongi-Filiaga A, et al. American Samoan women’s health: Experiences and attitudes toward breast and cervical cancer screening. Hawaii Med J. 2010;69(suppl 3):17–20. [PMC free article] [PubMed] [Google Scholar]
- 51.Rosario AM. Meeting Chamorro women’s health care needs: Examining the cultural impact of mamahlao on gynaecological screening. Pac Health Dialog. 2010;16:81–90. [PubMed] [Google Scholar]
- 52.United Nations Population Fund . Comprehensive cervical cancer prevention and control: Programme guidance for countries. New York, NY: United Nations Population Fund; 2011. [Google Scholar]
- 53.Stormo AR, Altamirano VC, Pérez-Castells M, et al. Bolivian health providers’ attitudes toward alternative technologies for cervical cancer prevention: A focus on visual inspection with acetic acid and cryotherapy. J Womens Health (Larchmt) 2012;21:801–808. doi: 10.1089/jwh.2012.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Audet CM, Silva Matos C, Blevins M, et al. Acceptability of cervical cancer screening in rural Mozambique. Health Educ Res. 2012;27:544–551. doi: 10.1093/her/cys008. [DOI] [PubMed] [Google Scholar]
- 55.Expert panel on cervical cancer screening in the U.S. territories and Pacific Island Jurisdictions. September 9–10, 2013; Washington, D.C. Available at: http://www.ent-s-t.com/Cervical_Cancer_Screening/. Accessed January 31, 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.