Abstract
Background.
Standard first-line chemotherapy for elderly non-small cell lung cancer (NSCLC) patients has been monotherapy with vinorelbine or gemcitabine. Docetaxel has also been considered as an alternative option for the elderly population in Japan. We have previously demonstrated the high efficacy of carboplatin plus weekly paclitaxel for elderly NSCLC patients. Consequently, we conducted a randomized phase II study to select the proper regimen for a future phase III trial.
Methods.
Eligible patients were aged 70 years or older with newly diagnosed advanced NSCLC. Patients were randomly assigned either to a combination of carboplatin (area under the curve: 6 mg/mL per minute) with weekly paclitaxel (70 mg/m2) (CP regimen) or to single-agent docetaxel (60 mg/m2). The primary endpoint of this study was objective response rate. Secondary endpoints were progression-free survival, overall survival, and toxicity profile.
Results.
Among 83 eligible patients (41 to CP, 42 to docetaxel), the objective response rates were 54% (95% confidence interval: 39%–69%) and 24% (95% confidence interval: 11%–37%) and median progression-free survival was 6.6 months and 3.5 months in the CP arm and the docetaxel arm, respectively. Severe neutropenia, febrile neutropenia, and nausea were significantly frequent in the docetaxel arm, whereas toxicities in the CP arm were generally moderate. One treatment-related death was observed in the docetaxel arm.
Conclusion.
The CP regimen achieved higher activity with less toxicity than single-agent docetaxel. Considering the results of this phase II trial and the IFCT-0501 trial, we have selected the CP regimen for a future phase III trial in elderly patients with advanced NSCLC.
Author Summary
Discussion
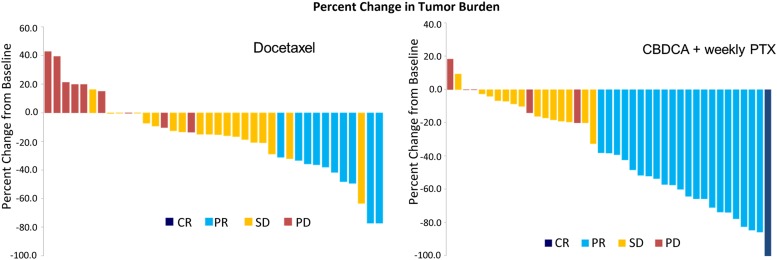
The objective response rate (ORR) of carboplatin (area under the plasma curve: 6 mg/mL per minute) with weekly paclitaxel (70 mg/m2) (CP regimen) met the primary endpoint of this study, achieving a higher response rate than single-agent docetaxel in this population of elderly patients with non-small cell lung cancer (NSCLC) (Fig. 1). In addition, the CP regimen achieved longer progression-free survival with less toxicity excluding moderate anemia and thrombocytopenia in comparison with docetaxel. Consequently, we have selected the CP regimen as a candidate for a future phase III trial.
Figure 1.
Waterfall plots of the docetaxel arm and the CP arm in this study.
Abbreviations: CBDCA, carboplatin; CP, carboplatin with weekly paclitaxel; CR, complete response, PD, progressive disease; PR, partial response; PTX, paclitaxel; SD, stable disease.
Although monotherapy with third-generation agents has been regarded as the preferred treatment option for elderly patients with NSCLC [1–6], Quoix et al. recently reported the results of IFCT-0501, a phase III study comparing a similar CP regimen (carboplatin [area under the plasma curve: 6 mg/mL per minute] plus weekly paclitaxel at 90 mg/m2) with monotherapy with either vinorelbine or gemcitabine in an elderly population [7]. IFCT-0501 demonstrated significant superiority to the CP regimen in terms of the efficacy (ORR and overall survival); however, severe toxicity in the CP arm, including a treatment-related death (TRD) rate of 4.4%, was of concern. The dose of paclitaxel in the current study was 70 mg/m2, and this could explain the lower toxicity of CP. No TRDs have been observed in the CP arm of this study or in our previous study using the same regimen.
Regarding the efficacy of CP, the ORR and progression-free survival in this study (54% and 6.6 months) are consistent with results achieved with the same regimen in our previous study (55% and 6.0 months) [8]. Because the evaluation of response in this study was performed by centralized review blinded as to the treatment, we believe the results were not biased. Furthermore, the ORR of the docetaxel arm in this study (24%) was quite consistent with previous results achieved with docetaxel in Japanese phase III trials with elderly NSCLC patients (23% in WJTOG9904 and 25% in JCOG0802) [6, 9]. Importantly, the rate of febrile neutropenia, an independent and poor prognostic factor in elderly NSCLC patients receiving chemotherapy, has been consistently high (>10%) in the docetaxel arm in the current study and in previous Japanese studies. In addition, one TRD was observed in the docetaxel arm in this study. All of these observations suggest that monotherapy with docetaxel might be more toxic than CP for elderly patients.
Supplementary Material
Footnotes
Access the full results at: Inoue-13-411.theoncologist.com
UMIN-CTR Identifier: UMIN000002616
Sponsor(s): Self-funding
Principal Investigator: Makoto Maemondo
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.