Abstract
Background.
Temsirolimus, an inhibitor of mammalian target of rapamycin (mTOR) complex 1, is approved for the treatment of metastatic renal cell carcinoma (RCC). Bryostatin-1 inhibits protein kinase C, a downstream effector of mTOR complex 2. We observed antitumor effects with the combination of temsirolimus and bryostatin-1 in RCC cell lines.
Methods.
Four cohorts of patients received weekly bryostatin-1 (20 μg/m2) with temsirolimus (10, 15, 25, or 37.5 mg) in 28-day cycles.
Results.
Thirty patients received a total of 138 cycles across four dose levels. Twenty-five patients had RCC (17 clear cell, 7 papillary, and 1 unclassified). Two sarcoma patients with prior cytotoxic therapy experienced dose-limiting toxicity at 15 mg of temsirolimus (grade 3 neutropenia and grade 3 hypophosphatemia). Subsequently, patients with prior cytotoxic therapy were excluded. Two additional dose-limiting toxicities were noted with 37.5 mg of temsirolimus (grade 3 neutropenia and grade 3 creatinine elevation). Consequently, the maximum tolerated dose was defined as temsirolimus at 25 mg and bryostatin-1 at 20 μg/m2 every 28 days. Of the 25 RCC patients, 3 patients had partial responses that lasted for 14 months, 28 months, and ≥80 months, respectively. Partial responses were seen in both clear cell and papillary histology.
Conclusion.
This combination of 37.5 mg of temsirolimus with 20 μg/m2 of bryostatin-1 was reasonably safe and well tolerated. Durable responses were observed in 3 of 25 patients with RCC.
Author Summary
Discussion
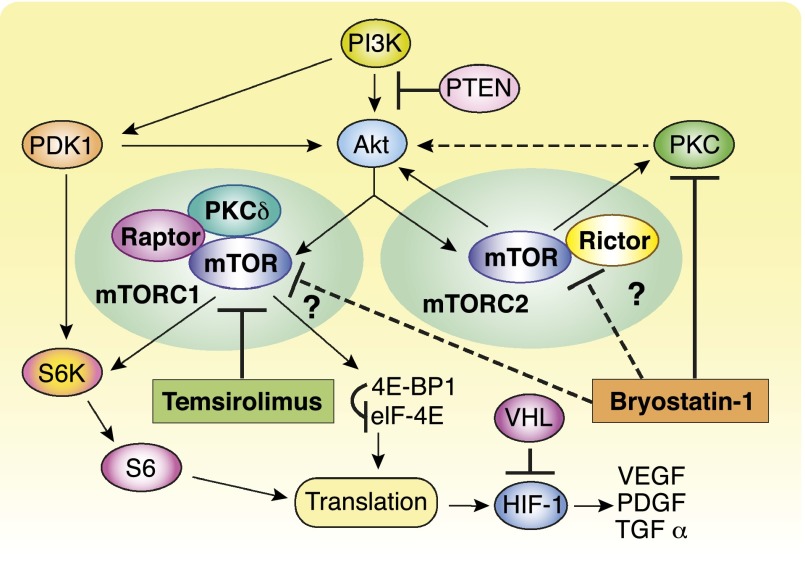
Temsirolimus is a selective inhibitor of mTOR kinase that was approved in the U.S. for advanced renal cell carcinoma (RCC). One suggested mechanism of resistance to temsirolimus is its inability to block mTOR complex 2 and, in turn, phosphorylation and activation of downstream AKT (Fig. 1). We observed that three patients who had received bryostatin-1 approximately 2 months prior to temsirolimus demonstrated durable partial responses. This finding prompted further preclinical studies in which bryostatin-1 and temsirolimus demonstrated at least additive antitumor effects in RCC cell lines. These observations suggested that the combination of temsirolimus with a protein kinase C (PKC) inhibitor such as bryostatin-1 may produce greater antitumor activity in RCC through more complete inhibition of mTOR signaling by inhibiting both p70 S6 kinase (mTOR complex 1) and mTOR complex 2.
Figure 1.
Proposed mechanism of action of temsirolimus plus bryostatin-1.
Abbreviations: mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; PKC, protein kinase C.
We report the results of a phase I clinical trial of temsirolimus and bryostatin-1. Four successive cohorts of patients received weekly bryostatin-1 at a fixed dose of 20 μg/m2, which was previously established as a safe single-agent dose on this schedule, together with weekly temsirolimus at 10, 15, 25, or 37.5 mg in 28-day cycles. At the time of analysis, all 30 patients had discontinued treatment because of disease progression (22 patients), treatment toxicity (5 patients), concomitant illness (2 patients), or withdrawal of consent (1 patient). The most common adverse events of any grade noted were fatigue (83%) and anemia (77%). The most commonly reported grade 3 or 4 toxicities related to therapy were thrombosis (10%) and neutropenia (10%). Two dose-limiting toxicities were noted with temsirolimus at 37.5 mg: grade 3 neutropenia in a non-RCC patient with prior radiation and grade 3 creatinine elevation in an RCC patient. Consequently, the maximum tolerated dose was defined as temsirolimus at 25 mg and bryostatin-1 at 20 μg/m2 every 28 days.
Of the 25 evaluable RCC patients who had received a median of one prior systemic therapy, 3 (12%) had partial responses (PRs) and 13 (52%) had stable disease, as defined by RECIST. Median progression-free survival was 4.3 months. Two patients had prolonged duration of PR until disease progression—one with papillary type II RCC for 14 months and one with clear cell RCC for 28 months. A third clear cell patient who had a PR discontinued treatment after 12 cycles because she required prednisone for interstitial lung disease, perhaps due to temsirolimus-induced pneumonitis. She remained in PR as of the last follow-up, for a total of 80 months.
Our study suggests that the combination of bryostatin-1 and temsirolimus was reasonably well tolerated for multiple cycles at phase II doses of each agent on a weekly schedule. Unfortunately, development of bryostatin-1 has been discontinued and the agent is no longer available for clinical study. The development of potent PKC inhibitors, potentially with isoform specificity, may facilitate further study of the interaction between mTOR and PKC inhibition.
Supplementary Material
Acknowledgment
The authors gratefully acknowledge the support of the late Dr. Igor Espinoza-Delgado at the Cancer Therapy Evaluation Program at the Investigational Drug Branch of the National Cancer Institute.
Footnotes
Access the full results at: Tan_Plimack-14-20.theoncologist.com
ClinicalTrials.gov Identifier: NCT00112476
Sponsor(s): National Cancer Institute
Principal Investigator: Gary R. Hudes
IRB Approved: Yes
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.