Abstract
Background.
Bosutinib is an oral, selective Src/Abl tyrosine kinase inhibitor with activity in breast cancer (BC). We evaluated bosutinib plus exemestane as second-line therapy in previously treated hormone receptor-positive (HR+) locally advanced or metastatic BC.
Methods.
This was a phase II study with patients enrolled in a single-arm safety lead-in phase. Patients receiving bosutinib at 400 mg or 300 mg/day (based on toxicity) plus exemestane at 25 mg/day were monitored for adverse events (AEs) and dose-limiting toxicities for 28 days, and initial efficacy was assessed. After the lead-in and dose-determination phase, randomized evaluation of combination therapy versus exemestane was planned.
Results.
Thirty-nine of 42 patients (93%) experienced treatment-related AEs including diarrhea in 28 (67%) and hepatotoxicity in 11 (26%); overall serious treatment-related AEs were recorded in 4 (10%). No liver toxicity met Hy’s law criteria. Dose-limiting toxicities occurred in 5 of 13 patients receiving 400 mg (38%) and 3 of 26 patients receiving 300 mg (12%) of bosutinib; all resolved on treatment discontinuation. One patient (300 mg/day) achieved confirmed partial response; three (400 mg/day, n = 2; 300 mg/day, n = 1) maintained stable disease for >24 weeks; a best response of progressive disease occurred in 15 of 42 patients (36%). Median progression-free survival was 12.3 weeks (80% confidence interval: 11.0–15.6).
Conclusion.
The risk-benefit profile of bosutinib at 300 mg/day plus exemestane resulted in early study termination before the randomized portion. Alternative bosutinib regimens merit investigation in BC.
Author Summary
Discussion
This phase II study was designed to evaluate the safety and efficacy of exemestane with or without bosutinib in previously treated postmenopausal women with HR+/HER2− locally advanced or metastatic breast cancer (BC) (Table 1). In the safety lead-in phase (part 1), 39 of 42 patients (93%) experienced treatment-related adverse events (AEs), and 8 of 39 evaluable patients (21%) experienced dose-limiting toxicities (DLTs), with a higher incidence of DLTs in the group given bosutinib at 400 mg/day plus exemestane (5 of 13, 38%) than in the group given the recommended phase II dose of bosutinib at 300 mg/day plus exemestane (3 of 26, 12%). No case met Hy’s law criteria for drug-induced hepatic injury [1]. One patient in the bosutinib 300 mg/day group achieved a confirmed partial response. The median progression-free survival (PFS) was 12.3 weeks (80% confidence interval [CI]: 8.1–23.7) for bosutinib 400 mg/day plus exemestane and 12.3 weeks (80% CI: 11.0–15.6) for bosutinib 300 mg/day plus exemestane. For bosutinib 300 mg/day plus exemestane, the 80% CI upper boundary for PFS was below the benchmark of 16 weeks for the median PFS for exemestane alone.
Table 1.
Treatment summary (safety population)
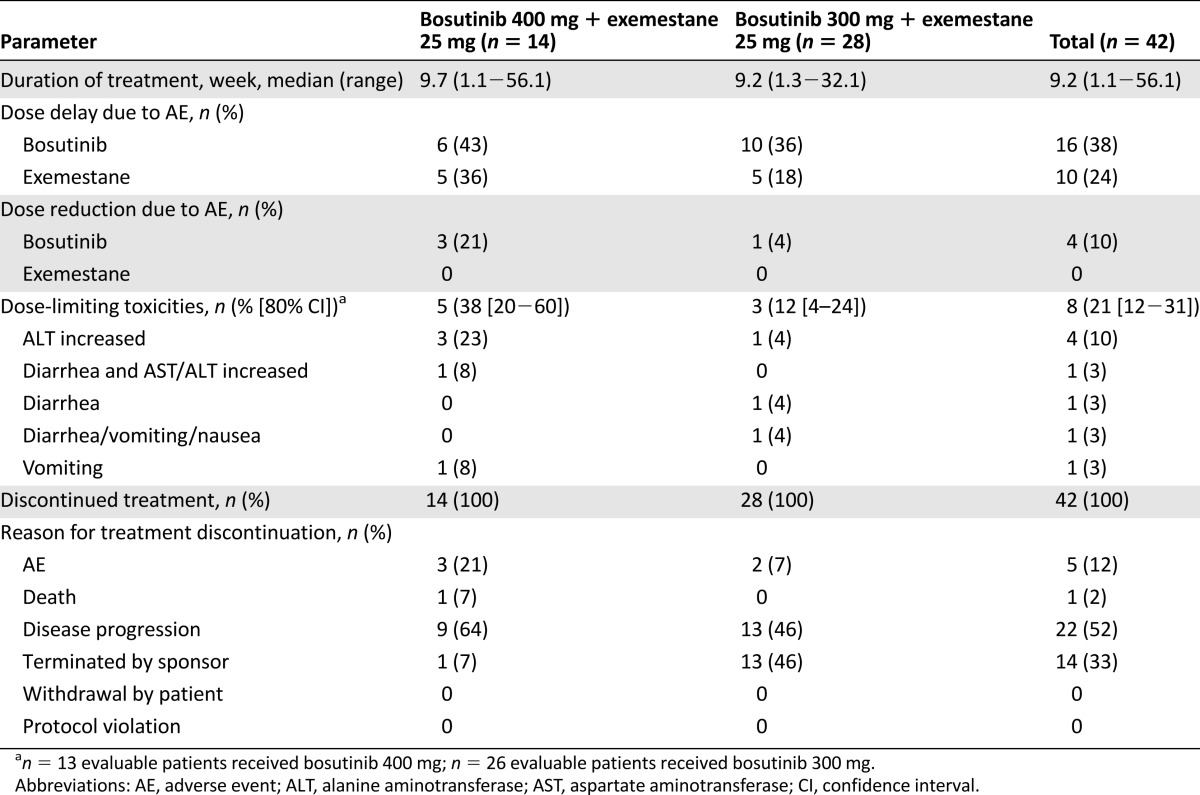
Bosutinib at 300 mg/day plus exemestane had a generally acceptable safety profile based on the DLT rate (12%); however, the rate of treatment-related hepatotoxicity (25%) prohibited further evaluation of this combination, given the lower-than-expected antitumor activity. Consequently, the study was terminated early, before initiation of the randomized portion of the study (part 2). Early termination was supported by the results of another phase II trial with the same design of bosutinib at 400 mg/day plus letrozole at 25 mg/day as first-line endocrine therapy in locally advanced or metastatic HR+/HER2− BC patients (ClinicalTrials.gov identifier NCT00880009) [2]. Bosutinib plus letrozole was associated with DLTs in 4 of 15 evaluable patients (27%). Six of 16 patients experienced treatment-related hepatotoxicity. In that study, one patient with hepatotoxicity met Hy’s law criteria for drug-induced liver injury, which also led to early study termination.
Bosutinib has demonstrated antitumor activity in BC cells in vitro and in mammary tumors in vivo [3–5]. The efficacy of single-agent bosutinib at 400 mg/day was demonstrated in a phase II trial in pretreated BC patients, with clinical benefit observed in 27% of patients (the four patients whose tumors responded had HR+ tumors) and a PFS rate of 40% [6]. The most common AEs were gastrointestinal (diarrhea, nausea, vomiting), and grade 3/4 alanine aminotransferase or aspartate aminotransferase elevations also occurred; these AEs were manageable and not life-threatening [6]. A phase I study of bosutinib in advanced solid tumors indicated a similar safety profile [7].
In conclusion, the initial findings from the present study indicate an unfavorable risk-benefit profile of the combination of bosutinib and exemestane in pretreated postmenopausal women with HR+/HER2− BC and resulted in early termination of this study. However, the efficacy of single-agent bosutinib underscores the potential clinical benefits of Src/Abl inhibition in BC [6] and suggests that studies of other combination regimens with bosutinib may be warranted.
Supplementary Material
Footnotes
Access the full results at: Moy-14-22.theoncologist.com
ClinicalTrials.gov Identifier: NCT00793546
Sponsor(s): Wyeth Research (acquired by Pfizer in October 2009)
Principal Investigator: Beverly Moy
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


