Abstract
Background: In adults with sickle cell disease (SCD), an increased tricuspid regurgitant velocity (TRV) measured by Doppler echocardiography, an increased serum N-terminal pro–brain natriuretic peptide (NT-pro-BNP) level, and pulmonary hypertension (PH) diagnosed by right heart catheterization (RHC) are independent risk factors for mortality.
Methods: A multidisciplinary committee was formed by clinician-investigators experienced in the management of patients with PH and/or SCD. Clinically important questions were posed, related evidence was appraised, and questions were answered with evidence-based recommendations. Target audiences include all clinicians who take care of patients with SCD.
Results: Mortality risk stratification guides decision making. An increased risk for mortality is defined as a TRV equal to or greater than 2.5 m/second, an NT-pro-BNP level equal to or greater than 160 pg/ml, or RHC-confirmed PH. For patients identified as having increased mortality risk, we make a strong recommendation for hydroxyurea as first-line therapy and a weak recommendation for chronic transfusions as an alternative therapy. For all patients with SCD with elevated TRV alone or elevated NT-pro-BNP alone, and for patients with SCD with RHC-confirmed PH with elevated pulmonary artery wedge pressure and low pulmonary vascular resistance, we make a strong recommendation against PAH-specific therapy. However, for select patients with SCD with RHC-confirmed PH who have elevated pulmonary vascular resistance and normal pulmonary capillary wedge pressure, we make a weak recommendation for either prostacyclin agonist or endothelin receptor antagonist therapy and a strong recommendation against phosphodiesterase-5 inhibitor therapy.
Conclusions: Evidence-based recommendations for the management of patients with SCD with increased mortality risk are provided, but will require frequent reassessment and updating.
Keywords: sickle cell disease, mortality, pulmonary hypertension, dyspnea, hemolysis
Contents
Overview
Introduction
Methods
How to Use These Guidelines
Definition of PH in SCD
Diagnosis of PH in SCDEstimating Mortality Risk in SCD
Doppler Echocardiography
N-Terminal Pro–Brain Natriuretic Peptide
Rationale for Risk Stratification
Treatment of Patients with SCD Who Have Increased Mortality Risk
Hydroxyurea
Chronic Transfusion Therapy
Chronic Anticoagulant Therapy
Associated Conditions
Targeted PAH Therapy
Future Directions
Overview
Pulmonary hypertension (PH) and right heart failure are well-established risk factors for mortality in sickle cell disease (SCD). Observational studies have consistently shown that increased tricuspid regurgitant jet velocity (TRV) measured by Doppler echocardiography, an increased serum N-terminal pro–brain natriuretic peptide (NT-pro-BNP) level, and pulmonary hypertension measured by right heart catheterization are all independent risk factors for mortality in adults. To reduce the variability and to improve the quality of care that patients with SCD receive, we developed clinical practice guidelines to advise hematologists, pulmonologists, cardiologists, pediatricians, and internists about how to identify and manage patients with SCD who are at increased risk for mortality. We make the following recommendations:
-
1.
Risk stratification guides clinical decision making in SCD:
-
a.
Mortality risk can be accurately determined by noninvasive measurement of the TRV via Doppler echocardiography (see Table E1 in the online supplement). (Note: In children ≥ 8 yr old the TRV determines morbidity risk, rather than mortality risk, and provides a baseline for future comparisons.)
-
b.
Serum NT-pro-BNP measurement is a reasonable noninvasive alternative when Doppler echocardiography is either unavailable or cannot obtain adequate images (Table E2). (Note: Measurements may be misleading in patients with renal insufficiency.)
-
c.
Mortality risk can also be determined invasively by direct hemodynamic measurements via right heart catheterization (RHC).
-
a.
-
2.
An increased risk for mortality is defined as a TRV ≥ 2.5 m/second, an NT-pro-BNP level ≥ 160 pg/ml, or RHC-confirmed PH (a resting mean pulmonary arterial pressure ≥ 25 mm Hg). Hemodynamics in PH of SCD may be consistent with pre- or postcapillary PH or have features of both.
-
3.
For patients with SCD who have an increased risk for mortality, we recommend hydroxyurea (strong recommendation, moderate-quality evidence) (Table E3).
-
4.
For patients with SCD who have an increased risk for mortality and who either are not responsive to or not candidates for hydroxyurea, we suggest chronic transfusion therapy (weak recommendation, low-quality evidence) (Table E4).
-
5.
For patients with SCD who have RHC-confirmed PH, venous thromboembolism, and no additional risk factors for hemorrhage, we suggest indefinite anticoagulant therapy rather than a limited duration of therapy (weak recommendation, low-quality evidence) (Table E5).
-
6.
For all patients with SCD who have elevated TRV alone or elevated NT-pro-BNP alone, we recommend against targeted PAH therapy (strong recommendation, moderate-quality evidence). Targeted PAH therapy currently includes prostacyclin agonist, endothelin receptor antagonist, and phosphodiesterase-5 inhibitor therapy.
-
7.
For most patients with SCD who have RHC-confirmed PH, we recommend against targeted PAH therapy (strong recommendation, moderate-quality evidence).
-
8.
For select patients with SCD who have RHC-confirmed marked elevation of their pulmonary vascular resistance, normal pulmonary artery wedge pressure, and related symptoms, we suggest a trial of either a prostacyclin agonist or an endothelin receptor antagonist (weak recommendation, very low-quality evidence).
-
9.
For patients with SCD who have RHC-confirmed marked elevation of their pulmonary vascular resistance, normal pulmonary artery wedge pressure, and related symptoms we recommend against phosphodiesterase-5 inhibitor therapy as a first-line agent (strong recommendation, moderate-quality evidence).
Introduction
Pulmonary hypertension (PH) is common and associated with increased morbidity and mortality in sickle cell disease (SCD) (1, 2). In the past, many patients with SCD died during childhood or early adulthood before the development of PH. However, now that SCD-specific therapies exist, more patients are surviving long enough to develop PH. Right heart catheterization (RHC) studies indicate that the prevalence of PH in SCD is 6–11%, which is similar to that observed in systemic sclerosis (1–3).
An elevated tricuspid regurgitant jet velocity (TRV) detected by Doppler echocardiography is a well-known indicator of possible PH and predicts mortality in patients with SCD. Studies indicate that a TRV 2 standard deviations above the normal age-adjusted mean value occurs in approximately 30% of hemoglobin SS (HbSS) and 10–25% of hemoglobin SC (HbSC) adults. HbSS adults with even a modest elevation in TRV have decreased survival, estimated to be only 40% at 40 months (4–7). In addition, 10–20% of the pediatric SCD population have an elevated TRV, although the effects on survival are unclear (8, 9).
Despite the prevalence of PH and an elevated TRV in patients with SCD, as well as the association of each with mortality, a standardized approach to identifying and managing such patients does not exist. To reduce the variability and to improve the quality of care that patients with SCD receive, we developed clinical practice guidelines to advise hematologists, pulmonologists, cardiologists, pediatricians, and internists about how to identify and manage patients with SCD who are at increased risk for mortality. The discussion that follows describes the definition and diagnosis of PH in SCD, mortality risk assessment, and treatment. Epidemiology, pregnancy, and issues related to the pediatric population are reviewed in the online supplement.
Methods
The methods used to develop this guideline are summarized in Table 1 and described in the online supplement.
Table 1:
Overview of Methods Used for Guideline Development
| Yes | No | |
|---|---|---|
| Panel assembly | ||
| • Included experts for relevant clinical and nonclinical disciplines | X | |
| • Included individual who represents the views of patients and society at large | X | |
| • Included a methodologist with appropriate expertise (documented expertise in conducting systematic reviews to identify the evidence base and the development of evidence-based recommendations) | X | |
| Literature review | ||
| • Performed in collaboration with librarian | X | |
| • Searched multiple electronic databases | X | |
| • Reviewed reference lists of retrieved articles | X | |
| Evidence synthesis | ||
| • Applied prespecified inclusion and exclusion criteria | X | |
| • Evaluated included studies for sources of bias | X | |
| • Explicitly summarized benefits and harms | X | |
| • Used PRISMA1 to report systematic review | X | |
| • Used GRADE to describe quality of evidence | X | |
| Generation of recommendations | ||
| • Used GRADE to rate the strength of recommendations | X |
Definition of abbreviations: GRADE = Grading of Recommendations Assessment, Development, and Evaluation; PRISMA1 = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, version 1.
How to Use These Guidelines
These American Thoracic Society guidelines about the diagnosis, risk stratification, and management of SCD-PH are not intended to impose a standard of care. They provide the basis for rational decisions in the management of patients with SCD who have an increased risk for death, as defined by an elevated TRV, elevated serum N-terminal pro–brain natriuretic peptide (NT-pro-BNP) level, or RHC-confirmed PH. Clinicians, patients, third-party payers, institutional review committees, other stakeholders, or the courts should never view these recommendations as dictates. No guidelines and recommendations can take into account all of the often compelling unique individual clinical circumstances. Therefore, no one charged with evaluating clinicians’ actions should attempt to apply the recommendations from these guidelines by rote or in a blanket fashion. Statements about the underlying values and preferences as well as qualifying remarks accompanying each recommendation are its integral parts and serve to facilitate more accurate interpretation. They should never be omitted when quoting or translating recommendations from these guidelines.
Definition of PH in SCD
PH is defined as a resting mean pulmonary arterial pressure (mPAP) equal to or exceeding 25 mm Hg. Although 6–11% of patients with SCD have PH by this definition, the hemodynamics observed vary across patients. As a result, soon to be published guidelines for the diagnosis and treatment of PH have placed it within group 5 PH (10). In some patients, there is predominantly precapillary PH. In others, hemodynamics are more consistent with postcapillary PH. Some patients have hemodynamics with features of both.
Precapillary PH associated with SCD is defined similarly to other types of group 1 PAH: an mPAP of at least 25 mm Hg with a mean pulmonary artery wedge pressure (PAWP) or left ventricular end-diastolic pressure less than or equal to 15 mm Hg, plus increased pulmonary vascular resistance (PVR). However, what constitutes increased PVR is different in SCD-related PAH compared with other types of group 1 PAH because patients with SCD have an anemia-induced elevation of their cardiac output and reduction in their blood viscosity, which result in a lower baseline PVR than observed in nonanemic patients (11).
This is illustrated by the following observations. In idiopathic PAH, increased PVR is defined as at least 240 dyn⋅seconds⋅cm−5 (3 Wood units), which is 2 standard deviations above the PVR of 80–120 dyn⋅seconds⋅cm−5 (1 Wood unit) or less observed in healthy volunteers. However, three studies (2, 3, 12) that measured hemodynamic values in adults with SCD via RHC (Table 2) found that adults without PH had a mean cardiac output of 8–9 L/minute with a PVR of 72–74 (±25–38) dyn⋅seconds⋅cm−5. In the absence of a consensus definition of what constitutes an elevated PVR in SCD, most clinicians consider values that are 2–3 standard deviations above normal (i.e., approximately ≥160 dyn⋅s⋅cm−5 [2 Wood units]) as indicative of a high PVR.
Table 2:
Hemodynamic Profiles of Patients with Sickle Cell Disease with or without Pulmonary Hypertension: Three Cohorts*
| PH | No PH | P Value | |
|---|---|---|---|
| NIH cohort | n = 56 | n = 30 | |
| CVP, mm Hg | 10 ± 5 | 6 ± 3 | <0.001 |
| mPAP, mm Hg | 36 ± 9 | 19 ± 4 | <0.001 |
| PAWP, mm Hg | 16 ± 5 | 12 ± 3 | <0.001 |
| CO, L/min | 8 ± 3 | 9 ± 2 | 0.14 |
| CI, L/min/m2 | 5 ± 1 | 5 ± 1 | 0.063 |
| PVR, dyn⋅s⋅cm−5 | 229 ± 149 | 74 ± 38 | <0.001 |
| French cohort | n = 24 | n = 72 | |
| CVP, mm Hg | 10 ± 6 | 7 ± 2 | <0.001 |
| mPAP, mm Hg | 30 ± 6 | 19 ± 3 | <0.001 |
| PAWP, mm Hg | 16 ± 7 | 11 ± 3 | <0.001 |
| CO, L/min | 8.7 ± 1.9 | 8.4 ± 2.1 | 0.60 |
| PVR, dyn⋅s⋅cm−5 | 138 ± 58 | 72 ± 26 | <0.001 |
| Brazilian cohort† | n = 8 | n = 18 | |
| mPAP, mm Hg | 33.1 ± 8.9 | 18.7 ± 2.8 | <0.001 |
| PAWP, mm Hg | 16.0 ± 5.7 | 13.3 ± 2 | 0.07 |
| CI, L/min/m2 | 5 ± 1.36 | 4.9 ± 1.7 | 0.85 |
| PVR, dyn⋅s⋅cm−5 | 179 ± 120 | 64 ± 48 | 0.002 |
Definition of abbreviations: CI = cardiac index; CO = cardiac output; CVP = central venous pressure; mPAP = mean pulmonary artery pressure; NIH = National Institutes of Health; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance.
CVP data not provided for Brazilian cohort.
Postcapillary PH occurs in SCD when the left atrial pressure and, therefore, the pulmonary venous pressure (i.e., the mean PAWP or left ventricular end-diastolic pressure) is greater than 15 mm Hg (in the absence of mitral valve disease) without an increase in PVR (13).
Patients with SCD frequently have hemodynamics with features of both pre- and postcapillary PH. This is characterized by a mean PAP of 25 mm Hg or more, a PAWP greater than 15 mm Hg, and an increased PVR. These patients often have an elevated transpulmonary gradient or PA diastolic pressure − pulmonary artery wedge pressure difference (>12 mm Hg) reflective of reactive precapillary PH (12).
Diagnosis of PH in SCD
Noninvasive tests are frequently performed to determine whether or not PH might be present. However, such tests are not sufficiently accurate to replace direct hemodynamic measurements in patients with SCD. Therefore, the diagnosis of PH requires RHC.
Doppler echocardiography is one such noninvasive test (Figure 1). It evaluates the heart for abnormalities suggestive of PH, such as right atrial enlargement, right ventricular dilation or hypertrophy, tricuspid regurgitation, and/or a right-to-left septum shift. It also measures the TRV and uses this to estimate the pulmonary artery systolic pressure. Although an attractive option because it is noninvasive and readily available in most institutions, Doppler echocardiography is imperfect as a diagnostic test. This has been illustrated by a study of a population of patients with SCD with a prevalence of PH of 6%; the positive predictive value for PH was only 25% among patients with a TRV of at least 2.5 m/second, although this improved to 64% when a TRV greater than 2.9 m/second was used as the threshold instead. The positive predictive value of Doppler echocardiography was also improved by combining it with other tests. The combination of a TRV of at least 2.5 m/second and either an NT-pro-BNP level greater than 164 pg/ml or a 6-minute walk distance less than 333 m improved the positive predictive value to 62% (2). Because only positive results were reported, it is not possible to estimate how many results were negative. In another study, a TRV of at least 2.5 m/second identified PH with a sensitivity and specificity of 78 and 19%, respectively. The sensitivity decreased but the specificity increased to 67 and 81%, respectively, when a TRV of at least 2.88 m/second was used instead (14).
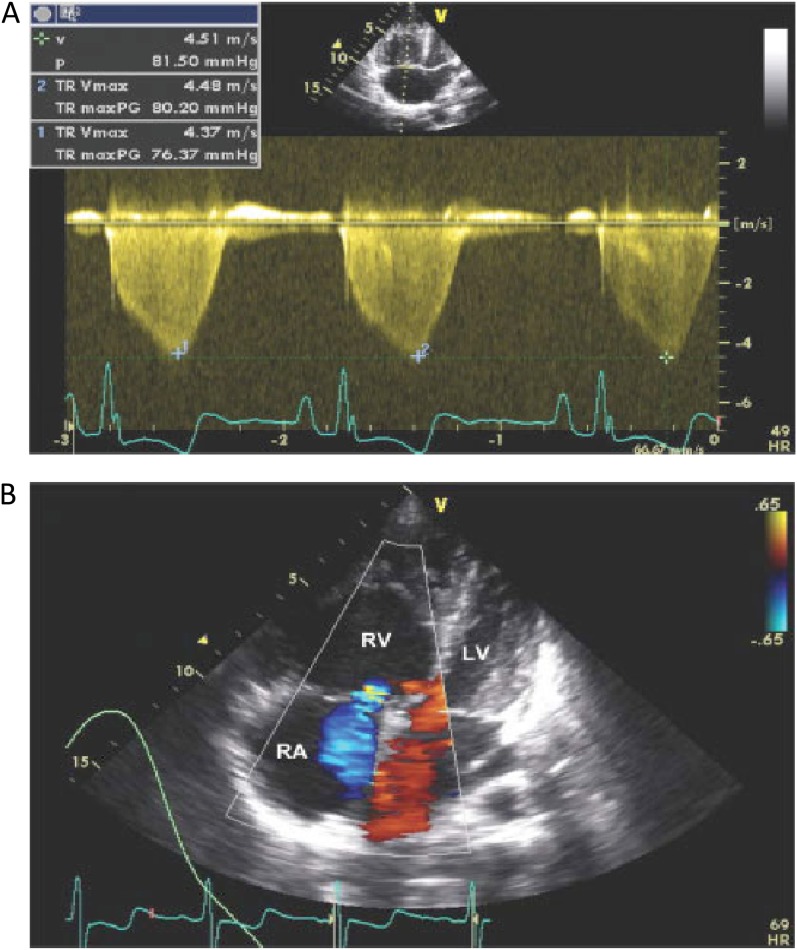
Figure 1.
Echocardiogram of a patient with pulmonary hypertension related to sickle cell disease, and determination of tricuspid regurgitant jet velocity. (A) As demonstrated in this echocardiogram of a patient with an estimated pulmonary artery systolic pressure of 44 mm Hg, in patients with pulmonary hypertension of sickle cell disease there is dilation of the right ventricular (RV) chamber so that it is larger than the left ventricle (LV) with shift of the interventricular septum into the LV. (B) Doppler flow of tricuspid regurgitation (demonstrated in blue) from the right ventricle (RV) into the right atrium (RA). The velocity of this flow is measured and allows for calculation of an estimated pulmonary artery systolic pressure. HR = heart rate; p = pressure; TR maxPG = peak gradient of tricuspid regurgitation; TR Vmax = tricuspid regurgitation peak velocity; v = velocity.
Serum NT-pro-BNP measurement is another noninvasive test that is imperfect diagnostically. It is a marker of right and left ventricular strain whose diagnostic characteristics have not been extensively studied. Most studies that evaluated the diagnostic characteristics of NT-pro-BNP were limited because they used an elevated TRV as the reference standard for PH, rather than direct hemodynamic measurements. As an example, a study of 230 patients with SCD found that a serum NT-pro-BNP level of at least 160 pg/ml detected PH with a sensitivity and specificity of 57 and 91%, respectively (15). Another study of 84 patients with HbS/β-thalassemia similarly reported that a serum NT-pro-BNP level of at least 153.6 pg/ml identified PH with a sensitivity and specificity of 85.7 and 94.6%, respectively (16). Limitations of these studies are that they did not use right heart catheterization to define PH.
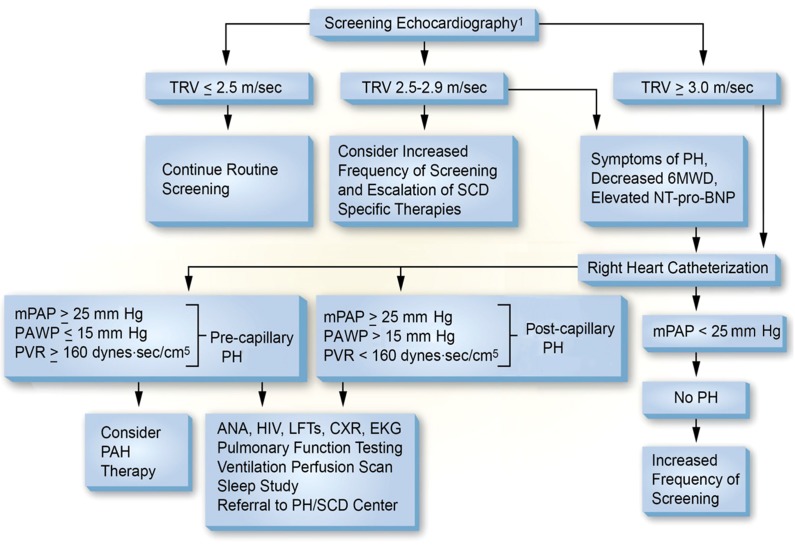
Additional details about the diagnostic performance of Doppler echocardiography and NT-pro-BNP are provided in the online supplement, as are the roles of the history, physical examination, chest imaging, and laboratory and pulmonary function testing in the evaluation of suspected PH. Table 3 provides sample questions that a provider can use to elicit a history of dyspnea, and Figure 2 provides a suggested diagnostic algorithm for PH of SCD.
Table 3:
Sample Questions for Evaluation of Dyspnea
| 1. Do you get short of breath at rest? |
| 2. Do you get short of breath with minimal exertion? (e.g., walking across a room, doing light housework, putting on your shoes, talking on the telephone) |
| 3. How far can you walk before stopping due to shortness of breath? |
| 4. How far can you walk on an incline before stopping? |
| 5. How many flights of stairs can you climb before stopping? |
| 6. For how long have you felt short of breath? |
| 7. Do you have chest pain with exertion? |
| 8. Do you have any trouble breathing while you sleep or lie flat in bed? |
| 9. Have you ever passed out? |
| 10. Have you ever felt you were about to pass out? |
| 11. Do you have any dizziness or light-headedness at rest or with exertion? |
| 12. Do you have swelling in your legs? |
| 13. Do you have swelling in your abdomen? |
Figure 2.
Proposed algorithm for evaluation of pulmonary hypertension related to sickle cell disease. 6MWD = 6-minute walk distance; ANA = anti-nuclear antibody; CXR = chest X-ray; EKG = electrocardiogram; LFTs = liver function tests; mPAP = mean pulmonary artery pressure; NT-pro-BNP = N-terminal pro–brain natriuretic peptide; PAWP = pulmonary artery wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; SCD = sickle cell disease; TRV = tricuspid regurgitant jet velocity. 1Note: The use of the term screening refers to mortality risk assessment. Echocardiography should be performed while patients are clinically stable. Patients with an mPAP between 20 and 25 mm Hg need further study as they may be at increased mortality risk. Note: PAH therapy is to be considered on the basis of a weak recommendation and very low-quality evidence.
Estimating Mortality Risk in SCD
Confirmed PH is an accepted risk factor for mortality regardless of whether the patient has SCD (12) or does not have SCD. It should be noted that PH is an independent risk factor for death in a diverse array of human diseases, from hereditable PH, to PH secondary to systemic sclerosis, HIV infection, portal hypertension, interstitial lung disease, and chronic obstructive pulmonary disease (17–24). The problem with using RHC for primary screening risk stratification is that the approach is not acceptable to many patients because it is invasive and expensive. Noninvasive alternatives include measurement of the estimated pulmonary artery systolic pressure (calculated from the TRV) via Doppler echocardiography and measurement of the serum NT-pro-BNP. These tests identify patients at higher risk of having PH by right heart catheterization and for increased mortality.
Doppler Echocardiography
We performed a systematic review to look for studies that evaluated how accurately Doppler echocardiography identifies patients with SCD with an increased mortality risk (Table E6). The review identified three studies in adults and one in children (Figure E1 and Table E1) (4, 9, 25, 26).
The first study in adults reported that the mortality risk among patients with a TRV of at least 2.5 m/second was 10.1 (95% confidence interval [CI], 2.2–47.0) times greater than that observed among patients with a TRV less than 2.5 m/second (4). A follow-up analysis demonstrated that the degree of TRV elevation correlated with mortality: among patients with a TRV of 2.5–2.9 m/second and patients with a TRV of at least 3.0 m/second, the risk ratio for mortality was 4.4 (95% CI, 1.6–12.2) and 10.6 (95% CI, 3.3–33.6), respectively (27). These findings were supported by two subsequent adult studies that similarly found a 5.1 (95% CI, 2.0–13.3) to 5.9 (95% CI, 1.7–20.8) times increased risk of mortality among patients with a TRV of at least 2.5 m/second (Table E1) (25, 26). The study in children had few events (i.e., deaths) and, therefore, could not detect a similar mortality risk among children with an elevated TRV (after only 22 mo of follow-up) (9). All of the studies had wide confidence intervals around the estimates, indicating uncertainty about the exact magnitude of the increased risk of death among patients with an increased TRV.
Assuming mortality rates of approximately 2, 10, and 20% for adults with a TRV less than 2.5 m/second, 2.5–2.9 m/second, and 3.0 m/second or more, respectively (27), and a 42% (95% CI, 15–61%) relative reduction in mortality due to hydroxyurea (Table E3), the expected absolute mortality reduction due to hydroxyurea therapy is 8 per 1,000 patients (95% CI, 3–12) among adults with a TRV less than 2.5 m/second, 42 per 1,000 patients (95% CI, 15–61) among adults with a TRV of 2.5–2.9 m/second, and 84 per 1,000 patients (95% CI, 30–122) among adults with a TRV of at least 3.0 m/second. These estimates should be interpreted cautiously given the uncertainty about the exact magnitude of the increased risk of death among patients with an increased TRV and the low confidence in the estimated reduction in mortality with hydroxyurea (Table E3). Similar calculations could be performed for the other benefits of hydroxyurea therapy (i.e., decreased hospitalizations, fewer vaso-occlusive crises, and fewer episodes of acute chest syndrome) that are described below.
The committee members routinely perform risk stratification on their patients with SCD by measuring the TRV via Doppler echocardiography. This reflects the committee’s opinion, that the potential for an at least 4% absolute reduction in mortality (and the other effects of therapy) among adults who are identified as having a TRV of at least 2.5 m/second and are then treated with hydroxyurea outweighs the costs and burdens of testing, the impact of erroneous test results (i.e., unnecessary therapy for false-positive results), and the risks of subsequent treatment (i.e., neutropenia requiring temporary discontinuation of therapy followed by dose reduction). The committee also recognizes that identification of an increased mortality risk affects the way a physician manages a patient in ways that cannot be measured; for example, the physician may more aggressively manage coexisting diseases and may also avoid some treatments of comorbid diseases that could be hazardous to the patient who is at higher mortality risk. An elevated TRV also identifies patients at increased risk for a number of conditions, such as venous thromboembolic disease and sleep-disordered breathing, with effective therapies. This is particularly the case for pulmonary thromboembolic disease. Patients with elevated TRV are at higher risk of having a high-probability ventilation–perfusion scan and chronic thromboembolic pulmonary hypertension (28), amenable to anticoagulation and consideration of pulmonary endarterectomy.
The TRV should be measured by Doppler echocardiography, using the same conditions as the studies described previously, because the TRV varies according to the clinical status of the patient (i.e., the TRV may be acutely and transiently increased during an acute illness such as a vaso-occlusive crisis [VOC] or acute chest syndrome [ACS]) (29). Therefore, Doppler echocardiography should be performed when the patient is in a stable clinical state, defined as more than 4 weeks after hospitalization for ACS and more than 2 weeks after hospitalization for a VOC or a blood transfusion that is part of a chronic transfusion program.
The optimal frequency of Doppler echocardiography is unknown. Longitudinal studies of adults with SCD suggest that 13% of those with a normal echocardiogram develop an elevated TRV after approximately 3 years of follow-up (5). On the basis of these studies, echocardiography every 1 to 3 years seems reasonable.
In children, we perform a Doppler echocardiogram to obtain a baseline for subsequent comparisons and to identify children who have an increased morbidity risk (the Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease [PUSH] trial demonstrated that children and adolescents with an elevated TRV and severe hemolytic anemia were at highest risk for a decreased 6-minute walk distance after 2 years of follow-up [9]).
N-Terminal Pro–Brain Natriuretic Peptide
We performed similar systematic reviews of the literature to identify evidence relevant to the use of NT-pro-BNP levels to assess mortality risk in patients with SCD (Table E7). Two relevant studies were identified (15, 30) (Figure E2 and Table E2), one of which included two separate cohorts of consecutive adults with SCD. In one cohort, the degree of NT-pro-BNP elevation correlated with mortality: among patients with an NT-pro-BNP level not exceeding 30 pg/ml, 31–159 pg/ml, and at least 160 pg/ml, respectively, mortality was 6, 7, and 26%. The mortality risk among adults with an NT-pro-BNP level of at least 160 pg/ml was 5.1 (95% CI, 2.1–12.5) times greater than that observed among patients with an NT-pro-BNP level less than 160 pg/ml. In the other cohort, the degree of NT-pro-BNP elevation also correlated with mortality: among patients with an NT-pro-BNP level not exceeding 30 pg/ml, 31–159 pg/ml, and at least 160 pg/ml, respectively, mortality was 6, 17, and 49%. The risk of mortality was 2.9 (95% CI, 1.2–6.6) times greater among patients with an NT-pro-BNP level of at least 160 pg/ml compared with those with lower levels of NT-pro-BNP (15). These findings were confirmed by a study of 330 adults recruited as part of the Cooperative Study of Sickle Cell Disease, in which an NT-pro-BNP level of at least 160 pg/ml was associated with a relative risk of death of 6.24 (95% CI, 2.9–13.3) compared with those with lower levels (30).
Assuming mortality rates of 6, 7, and 26% for adults with an NT-pro-BNP level not exceeding 30 pg/ml, 31–159 pg/ml, and at least 160 pg/ml, respectively, and a 40% relative reduction in mortality due to hydroxyurea (Table E3), the expected absolute mortality reduction due to hydroxyurea is 24 per 1,000 patients among adults with an NT-pro-BNP level not greater than 30 pg/ml, 28 per 1,000 patients among adults with an NT-pro-BNP level of 31–159 pg/ml, and 104 per 1,000 patients among adults with an NT-pro-BNP level of at least 160 pg/ml. These estimates should be interpreted cautiously given the uncertainty about the magnitude of the increased risk of death among patients with an increased NT-pro-BNP level and the low confidence in the estimated reduction in mortality with hydroxyurea. Similar calculations could be performed for the other benefits of hydroxyurea therapy (i.e., decreased hospitalizations, fewer vaso-occlusive crises, and fewer episodes of acute chest syndrome) that are described below.
Serum NT-pro-BNP is generally measured in adults with SCD when Doppler echocardiography is either unavailable or unable to obtain adequate images. In the committee’s opinion, the anticipated 10.4% (or more) absolute reduction in mortality (and the other effects of therapy) among adults who are identified as having an NT-pro-BNP level of at least 160 pg/ml and are then treated with hydroxyurea outweighs the costs and burdens of testing, the impact of erroneous test results (i.e., unnecessary therapy for false-positive results), and the risks of subsequent treatment (i.e., neutropenia requiring temporary discontinuation of therapy followed by dose reduction). The committee also recognizes that identification of an increased mortality risk affects the way a physician manages a patient in ways that cannot be measured; for example, the physician may more aggressively manage coexisting diseases and may also avoid some treatments of comorbid diseases that could be hazardous to the patient who is at higher mortality risk.
An important exception to our recommendation is patients with renal disease. Renal dysfunction is independently associated with high NT-pro-BNP levels (15, 30) and, thus, testing is more likely to provide false-positive results in patients with renal dysfunction than in patients with normal renal function. In such patients, it is possible that the undesirable consequences of false-positive results (i.e., unnecessary therapy and the risks associated with it) might exceed the benefits of NT-pro-BNP testing. Until the impact of renal dysfunction on NT-pro-BNP levels is further studied, we suggest that NT-pro-BNP testing not be used in patients with renal dysfunction.
Rationale for Risk Stratification
Identifying patients at increased risk for mortality is important because it guides decision making. Specifically, it identifies patients who may benefit from SCD-specific therapies that have been shown to improve outcomes in patients with various complications of SCD, as illustrated previously with hydroxyurea. Risk stratification also allows patients to anticipate their future health care needs.
Conclusions from the Committee:
Risk stratification guides decision making.
Mortality risk can be determined by noninvasive measurement of the TRV via Doppler echocardiography.
Serum NT-pro-BNP measurement is a reasonable noninvasive alternative if Doppler echocardiography is either unavailable or cannot obtain adequate images. (Note: Measurements may be misleading in patients with renal insufficiency.)
Mortality risk can also be determined invasively by direct hemodynamic measurements via right heart catheterization.
Treatment of Patients with SCD Who Have Increased Mortality Risk
Patients with SCD who have been determined to have an increased mortality risk (i.e., TRV ≥ 2.5 m/s, NT-pro-BNP ≥ 160 pg/ml, or RHC-confirmed PH) may benefit from referral to an experienced PH center (preferably with expertise in SCD as well) so that they can be comanaged by clinicians with experience in both PH and SCD.
Hydroxyurea
Mild SCD genotypes (such as HbSC) have a lower prevalence of PH and mortality than severe SCD genotypes (such as HbSS). This suggests that decreasing sickle cell formation and hemolysis (31–33) may improve PH and mortality in patients with SCD. Hydroxyurea (HU) is an agent that has been shown to reduce both sickle cell formation and hemolysis.
We performed a systematic review of the literature to identify studies pertinent to the decision about whether patients with SCD with an increased risk for mortality (i.e., TRV ≥ 2.5 m/s, NT-pro-BNP ≥ 160 pg/ml, or RHC-confirmed PH) should be treated with HU. Our search and selection criteria are shown in Table E8. The search criteria were intentionally broad because we anticipated that there would be minimal direct evidence and, therefore, we wanted to capture relevant indirect evidence. The review identified three relevant randomized trials (34–36) and two case series (37, 38) (Figure E3).
The largest trial (the Multicenter Study of Hydroxyurea in Sickle Cell Anemia [MSH] trial) randomly assigned 299 adults with SCD (HbSS genotype) and three or more VOC per year to receive HU or placebo (35). Patients who received HU were less likely to have an episode of ACS, had a lower annual rate of VOC, had a longer duration until their first and second crises, and required fewer transfusions.
With respect to mortality, there was no difference in mortality during the trial (2 of 152 [1.3%] vs. 5 of 147 [3.4%]). However, during a subsequent extension study, there was a significant reduction in mortality at 9 years among patients who received HU for at least 1 year compared with those who either did not receive HU or received HU for less than 1 year (21 vs. 36.3%; relative risk [RR], 0.58; 95% CI, 0.39–0.85). Similarly, at 17 years, there was a significant decrease in mortality when patients who received HU for at least 5 years were compared with those who either did not receive HU or who received HU for less than 5 years (30.4 vs. 51.1%; RR, 0.60; 95% CI, 0.44–0.81). At both 9 and 17 years, there was no significant difference in mortality when patients who received HU for any duration were compared with patients who never received HU, suggesting that prolonged therapy may be necessary to achieve a clinical benefit (39, 40).
Patients who received HU during the MSH trial also had improvement in some measures of quality of life (i.e., social function and general health perception) (37). Hydroxyurea therapy was not associated with an increased incidence of birth defects (38), infection (40), stroke (40), or neoplasia (40). Bone marrow suppression (i.e., decreased counts in one or more cell lines) occurred in most patients, but resolved within 2 weeks of temporary discontinuation of therapy (40). Therapy was later resumed at a lower dose.
Another trial enrolled 25 children and young adults with SCD (HbSS genotype) and a history of at least three VOC over the previous year, stroke, or ACS (36). The patients were randomly assigned to receive HU or placebo for 6 months and then crossed over to the other arm of the trial; those who received HU were significantly less likely to require hospitalization. Adverse effects were not reported, although there was a significant decrease in the neutrophil count following the initiation of HU therapy (from a mean of 12.47 × 109/L to a mean of 8.90 × 109/L).
Finally, a trial that randomly assigned 193 very young (<18 mo) children with SCD (HbSS genotype) to receive either HU or placebo found that HU decreased hospitalizations, episodes of VOC, and episodes of ACS (34). Mild to moderate neutropenia (i.e., an absolute neutrophil count of 500–1,249/mm3) was significantly more common among the HU group (46.9 vs. 18.6%; RR, 2.53; 95% CI, 1.58–4.03) and required long-term dose reductions in nine patients. Severe neutropenia (i.e., absolute neutrophil count < 500/mm3) was rare and not complicated by infection. There were no other adverse effects reported.
On the basis of these studies, it is widely accepted that HU is indicated for patients with SCD who have the HbSS genotype and least three VOC per year or at least one episode of ACS (41). Our consensus is that the studies are also applicable to patients with an increased risk for mortality (i.e., TRV ≥ 2.5 m/s, NT-pro-BNP ≥ 160 pg/ml, or RHC-confirmed PH). This reflects our recognition that VOC, ACS, PH, elevated TRV, and elevated NT-pro-BNP are all established independent risk factors for death among patients with SCD.
Furthermore, there is now a well-characterized interaction between acute episodes of ACS and death from PH and cor pulmonale in patients with sickle cell disease (2). ACS and VOC increase pulmonary pressures acutely and can precipitate right-sided heart failure; patients with ACS and right heart failure are at increased risk of death both during and after that hospitalization (2, 29). A history of heart failure was an independent risk factor for mechanical ventilation in patients with ACS in the National Acute Chest Syndrome Study Group (odds ratio, 0.67; 95% CI, 2.1–22.3; P = 0.002) (42) and both VOC and ACS increase the risk for mortality in patients with SCD with PH (12). Both these study results and the experience of the committee members strongly suggest that intermittent episodes of acute chest syndrome clearly worsen outcomes of patients with established PH and right heart dysfunction, and this can be prevented by HU therapy.
The committee recognizes that the use of such indirect evidence to inform judgments is controversial, with some individuals contending that the indirectness is so minor that it should have no impact on our confidence in the estimated effects and others arguing that the indirectness is so profound that the evidence should not be used. The final judgment of the committee was to compromise; that is, to apply the evidence in our decision making, but to lower the quality of evidence to reflect the indirectness of the population. The case series support this extrapolation of evidence, finding that HU treatment of patients with SCD with elevated TRV produces a decline in TRV (31, 32). This occurred most often in children and young adults with a mild TRV elevation in whom HU increased the fetal hemoglobin to more than 20%.
We recommend HU therapy for HbSS patients who have an increased risk for mortality (i.e., TRV ≥ 2.5 m/s, NT-pro-BNP ≥ 160 pg/ml, or RHC-confirmed PH) because the numerous desirable consequences of HU therapy (12.3% decrease in 9-yr mortality, 23% reduction in hospitalizations, 15.4% fewer episodes of ACS, and fewer episodes of VOC) substantially outweigh the undesirable consequences (bone marrow suppression, cost, and burden) (Table E3). The bone marrow suppression is generally mild, resolves when the HU is held, and is managed by dose reduction.
The recommendation is strong because the committee is certain that the desirable effects of HU outweigh the undesirable effects in the population for which the recommendation is intended. Our certainty derives from both the greater patient-importance of the desirable consequences than the undesirable consequences, as well as our moderate confidence in the estimated effects. The quality of evidence is moderate because our critical outcomes were estimated from randomized trials downgraded for indirectness of the population (Table E3).
Recommendation: For patients with SCD who have an increased risk for mortality, we recommend hydroxyurea therapy (strong recommendation, moderate-quality evidence).
Remarks: We define an increased risk for mortality as a TRV of ≥2.5 m/second, an NT-pro-BNP level of ≥160 pg/ml, or RHC-confirmed PH.
Values and preferences: This recommendation attaches a relatively high value to the potential benefits from therapy and a lower value to the risks and burdens of therapy.
Chronic Transfusion Therapy
Chronic scheduled transfusion of packed red blood cells is effective in reducing most complications of SCD by decreasing both the burden of sickle erythrocytes and the hemolytic rate (33). The HbS level can be reduced to less than 50% within weeks by simple transfusions or within hours by erythrocytapheresis, as opposed to the months usually required for the gradual titration of HU to reach optimal fetal hemoglobin levels. A goal hemoglobin concentration of 10–12 g/dl is often attainable via chronic transfusion therapy.
We performed a systematic review of the literature to identify studies pertinent to the decision about whether patients who have SCD and either RHC-confirmed PH or an increased risk for mortality (i.e., TRV ≥ 2.5 m/s or NT-pro-BNP ≥ 160 pg/ml) should be managed with chronic transfusion therapy. Our search and selection criteria are listed in Table E9. The search criteria were intentionally broad because we anticipated that there would be minimal direct evidence and, therefore, we wanted to capture relevant indirect evidence. The review identified two relevant randomized trials, the Stroke Prevention in Sickle Cell Anemia (STOP)-1 and -2 trials (43–45); and a case series (46, 47) (Figure E4). Long-term follow-up reports of the STOP-1 trial were also identified (45, 48).
Both randomized trials enrolled children with SCD and a transcranial Doppler velocity greater than 200 cm/second in the middle cerebral or internal carotid artery, findings that place them at increased risk for a stroke. The trials compared mortality and stroke rates among children who received either chronic transfusion therapy or standard care. Chronic transfusion therapy significantly reduced the stroke rate (1 vs. 12%; odds ratio, 0.10; 95% CI, 0.02–0.58), but had no effect on mortality (48). Long-term follow-up of patients from the STOP-1 trial suggested that chronic transfusions may improve children’s growth (49) and reduced the frequency of ACS and VOC (50) (Table E4).
Chronic transfusion therapy is warranted in children with SCD with an increased risk for stroke, according to these trials. Our consensus is that these results are also applicable to patients with SCD with an increased risk for mortality (i.e., RHC-confirmed PH, TRV ≥ 2.5 m/s, or NT-pro-BNP ≥ 160 pg/ml). This is based on a rationale similar to that provided previously for HU. Specifically, we recognize that stroke risk, VOC, ACS, PH, elevated TRV, and elevated NT-pro-BNP are associated with increased risk of mortality. Chronic transfusion therapy reduces vaso-occlusive and acute chest syndrome events as well as hemolysis; thus, it is likely that chronic transfusion therapy will benefit patients with any of these complications of SCD. A similar argument for preventing intercurrent ACS and VOC events in the patient with PH and right heart failure is considered. This extrapolation of evidence is supported by a case series of patients with SCD with PH that reported that maintaining HbS at 30–50% via chronic red cell transfusion improved the TRV (47). One caveat to keep in mind is that chronic transfusions may reduce the response to HU.
Risks of chronic transfusion therapy include febrile reactions, allergic reactions, hemolytic reactions, and volume overload. Such adverse effects collectively occur in 15% of patients. Red cell alloimmunization is another consequence of chronic transfusion therapy, occurring in 11% of patients. Alloimmunization can be reduced by matching for the red cell antigens D, C, E, and Kell. Iron accumulates in all patients who receive chronic transfusions, with the magnitude of accumulation correlating with the volume transfused. In a series of 45 patients with SCD who received chronic transfusions for SCD, most patients exhibited a serum ferritin level greater than 1,000 ng/ml by the time they had been transfused with 100 ml/kg, and all patients exhibited a serum ferritin level greater than 1,000 ng/ml by the time they had been transfused with 200 ml/kg. Among patients who received a cumulative transfusion volume greater than 250 ml/kg, 44% reached a serum ferritin level greater than 3,000 ng/ml, whereas the remaining 56% reached a plateau at a serum ferritin level less than 3,000 ng/ml (51). Iron overload may be minimized by the aggressive use of iron chelators (deferasirox, deferoxamine) or by replacing simple transfusion therapy with erythrocytapheresis.
We suggest chronic transfusion therapy for patients with SCD who have an increased risk for mortality (i.e., RHC-confirmed PH, TRV ≥ 2.5 m/s, or NT-pro-BNP ≥ 160 pg/ml), and who are not responsive to or are not candidates for HU therapy. This reflects our judgment that the desirable consequences of chronic transfusion therapy (11% fewer strokes, an 18.1% decrease in episodes of ACS, and an 8.3% decrease in episodes of VOC) outweigh the undesirable consequences (cost, burden, 11% incidence of alloimmunization, and 15% frequency of minor adverse effects, including febrile reactions, allergic reactions, hemolytic reactions, and volume overload) (Table E4). The recommendation is weak, reflecting the committee’s recognition that the desirable and undesirable effects are finely balanced, and that the estimated effects are based on low-quality evidence (two randomized trials downgraded for indirectness of the population and imprecision) (Table E4).
Recommendation: For patients with SCD who have an increased risk for mortality and who are either are not responsive to hydroxyurea or not candidates for hydroxyurea, we suggest chronic transfusion therapy (weak recommendation, low-quality evidence).
Remarks: We define an increased risk for mortality as a TRV of ≥2.5 m/second, an NT-pro-BNP level of ≥160 pg/ml, or RHC-confirmed PH.
Chronic Anticoagulant Therapy
SCD is associated with an increased risk of both large and small vessel pulmonary embolism (52–58), as well as an increased risk for intracerebral hemorrhage. Short-term anticoagulant therapy is widely accepted for most patients with acute venous thromboembolism (VTE), but the opposing tendencies toward both VTE and hemorrhage make decisions about long-term anticoagulation challenging. These decisions are even more complicated in patients with SCD with PH because such patients are less likely to tolerate recurrent VTE.
We performed a systematic review of the literature to identify studies that compared indefinite anticoagulant therapy with a shorter duration of anticoagulant therapy in patients with SCD-related PH and VTE. Our review did not identify any relevant studies, so we broadened our selection criteria to identify studies that performed the same comparison in patients with SCD (with or without PH) and VTE. Again, our review did not identify any relevant studies. Our search and selection criteria are listed in Tables E10 and E11.
Given the paucity of evidence regarding the benefits versus risks of indefinite anticoagulant therapy in patients with SCD and VTE, we sought evidence from heterogeneous populations of patients with VTE. We did not perform our own systematic review; rather, we updated a well-done published systematic review (59). Four randomized trials were identified that compared long-term anticoagulant therapy (i.e., uninterrupted therapy until the end of the trial) with a limited duration of therapy (60–63). A meta-analysis found that patients who received indefinite anticoagulant therapy had fewer recurrent VTE events (3.5 vs. 17.3%; RR, 0.16; 95% CI, 0.06–0.41) and a trend toward lower mortality (2.8 vs. 5.0%; RR, 0.56; 95% CI, 0.31–1.01). This was partially offset by an increase in major bleeding (3.3 vs. 0.9%; RR, 3.14; 95% CI, 1.27–7.77). After the cessation of anticoagulant therapy, the risk of recurrent VTE appears to increase, regardless of treatment duration (59).
We suggest indefinite anticoagulant therapy for patients with RHC-confirmed PH plus VTE, and no additional risk factors for bleeding. This reflects the committee’s judgment that the upsides of indefinite anticoagulation (13.8% less recurrent VTE and, possibly, lower mortality) outweigh the downsides (2.4% increased bleeding risk, cost, and burden of monitoring) in most patients with SCD-related PH and VTE. The recommendation is weak because it is based on low-quality evidence (randomized trials downgraded for indirectness of the population and imprecision) and we recognize that some patients and providers may not find the risk of major bleeding to be an acceptable trade-off for reducing the likelihood of recurrent VTE and, possibly, mortality (i.e., the potential benefits and risks are finely balanced).
Recommendation: For patients with SCD who have RHC-confirmed PH, venous thromboembolism, and no additional risk factors for bleeding, we suggest indefinite anticoagulant therapy rather than a limited duration of therapy (weak recommendation, low-quality evidence).
Values and preferences: This recommendation attaches a relatively high value to the prevention of recurrent venous thromboembolic events and a lower value to the risks and burdens of anticoagulant therapy.
Associated Conditions
Standard care for patients with SCD-related PH is similar to that for patients with other forms of PH. Hypoxemia is treated with supplemental oxygen to maintain an arterial oxyhemoglobin saturation of at least 90% at rest, with exertion, and during sleep (13). Proteinuria and microalbuminuria are common in SCD-related PH (26) and may improve with angiotensin-converting enzyme inhibitors (64). Suspected obstructive sleep apnea is evaluated via polysomnography and treated accordingly. In a small case series of children with SCD, treatment of obstructive sleep apnea, nocturnal hypoxemia, and asthma reduced the elevated TRV, suggesting that mortality risk can be modified by early intervention (65). Diuretics are used to treat right ventricular volume overload (13), but this must be done carefully to minimize the risk of volume depletion–induced erythrocyte sickling. The risk of this occurring may be reduced by HU or chronic transfusions with concomitant treatment for iron overload.
Targeted PAH Therapy
Targeted PAH therapy refers currently to treatment with prostacyclin agonists (e.g., epoprostenol, treprostinil, iloprost), endothelin receptor antagonists (e.g., bosentan, macitentan, ambrisentan), soluble guanylate cyclase stimulators (e.g., riociguat), or phosphodiesterase-5 inhibitors (e.g., sildenafil, vardenafil, tadalafil). To identify evidence related to targeted PAH therapy in patients with SCD-related PH, a systematic review was performed, using the search and selection criteria shown in Table E12). Three randomized trials and four case series were identified (Figure E5).
Two of the trials compared treatment with bosentan to placebo in patients with SCD with RHC-defined precapillary PH (the ASSET-1 trial) or postcapillary PH with a PVR of at least 100 dyn · seconds⋅cm−5 (the ASSET-2 trial) (66). After the randomization of only 14 subjects in ASSET-1 and 12 patients in ASSET-2, the trials were prematurely terminated because of sponsor's withdrawal of support for the study. Although few patients were enrolled, there were no apparent toxicity issues. The third trial, Walk-PHaSST (Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy) (67), compared the safety and efficacy of sildenafil with that of placebo in patients with SCD with a TRV of at least 2.7 m/second. After 74 (of a targeted 132) subjects were enrolled, the study was prematurely discontinued because of an increase in serious adverse events in the sildenafil group, primarily hospitalization for pain.
These trials were judged by the committee to be insufficient to determine whether or not patients with SCD with an RHC-confirmed marked elevation of the PVR with normal PAWP should receive targeted PAH therapy, for two reasons. First, the trials collectively included only 14 patients with an elevated PVR and normal PAWP by RHC (fewer than half of whom actually received targeted PAH therapy); the remaining patients had either postcapillary PH alone or elevated TRV alone. Second, as a result of the small sample size, there were few events and the estimated effects were imprecise.
Given the serious limitations of the randomized trials, the committee decided that its judgments regarding whether targeted PAH therapy is indicated in patients with SCD with an elevated PVR and normal PAWP should be informed by indirect evidence from group 1 PAH populations and our own clinical experience. In group 1 patients with PAH, it has been well established by meta-analyses of randomized trials that targeted PAH therapy consistently improves exercise capacity, functional status, symptoms, cardiopulmonary hemodynamics, and outcome (68–70). This indirect evidence is supported by four case series in which patients with SCD with RHC-confirmed precapillary PH received targeted PAH therapy with bosentan, sildenafil, and/or epoprostenol. Targeted PAH therapy was associated with improvement in exercise capacity, with the 6-minute walk distance increasing 41 to 144 m beyond baseline (71–74). There were also improvements in the mean PAP, PVR, and cardiac index, although these parameters were measured in only a few patients (74). The magnitude of the benefits was greatest among symptomatic patients. The most common adverse effects were headache (15%) and peripheral edema (21%); transaminase elevation was reported in 14% of patients during endothelin receptor antagonist therapy (71–74).
Although sildenafil has been shown to produce clinical and hemodynamic improvement compared with placebo in a small number of non-SCD patients with PVH due to diastolic dysfunction, this needs to be studied in a larger population before this becomes widely accepted (75). Targeted PAH therapy has never been shown to benefit patients with elevated TRV alone or elevated NT-pro-BNP alone.
After taking the evidence and the committee’s collective clinical experience into consideration, we recommend against targeted PAH therapy for all patients with SCD who have elevated TRV alone, or an elevated NT-pro-BNP level alone, and for most patients with SCD and RHC-confirmed PH, because targeted PAH therapy has not been shown to be beneficial in such patients. Therefore, the risks, costs, and burdens may exceed the benefits. The recommendation is strong, to emphasize the importance of avoiding costly and potentially harmful therapies in the absence of evidence of a benefit.
Recommendation: For all patients with SCD who have elevated TRV alone, or elevated NT-pro-BNP alone, and for most patients with SCD who have RHC-confirmed PH, we recommend against targeted PAH therapy (strong recommendation, moderate-quality evidence).
Remarks: Targeted PAH therapy refers to prostacyclin agonist, endothelin receptor antagonist, soluble guanylate cyclase stimulator, and phosphodiesterase-5 inhibitor therapy.
Values and preferences: This recommendation places a greater value on avoiding expensive and potentially harmful agents in patients who may not benefit and a lower value on avoiding the complications of invasive hemodynamic testing by using noninvasive results for decision making.
In carefully selected symptomatic patients with RHC-confirmed hemodynamics demonstrating elevated PVR and normal PAWP, however, we suggest targeted PAH therapy with either a prostacyclin agonist or an endothelin receptor antagonist. This reflects our judgment that the benefits of such therapies (i.e., improved exercise capacity and hemodynamic measures) likely outweigh the cost, burdens, and risks (i.e., headache and peripheral edema) in most patients. The recommendation is weak because the low quality of evidence (randomized trials downgraded for indirectness of the population and imprecision of the estimated effects, as well as case series) allows little confidence in the estimated effects of targeted PAH therapy and, therefore, in the balance of benefits versus harms in this population.
Recommendation: For select patients with SCD who have an RHC-confirmed marked elevation of their pulmonary vascular resistance, normal pulmonary artery wedge pressure, and related symptoms, we suggest a trial with either a prostacyclin agonist or an endothelin receptor antagonist (weak recommendation, low-quality evidence).
Remarks: This group of patients with SCD is characterized by a mean pulmonary arterial pressure (mPAP) of ≥25 mm Hg with a pulmonary artery wedge pressure < 15 mm Hg, plus increased pulmonary vascular resistance (PVR) of ≥160 dyn · seconds⋅cm−5 (2 Wood units). Patients with SCD who have confirmed PH are now categorized as being within WHO group 5, instead of WHO group 1. As a result, use of targeted PAH therapy in such patients is considered off-label.
Values and preferences: This recommendation places a greater value on improving symptoms and hemodynamic measures and a lower value on the costs, burdens, and risks of therapy.
In contrast, we recommend against phosphodiestase-5 inhibitor therapy in light of the harmful effects of sildenafil in the Walk-PHaSST trial described previously. The recommendation is strong to emphasize the importance of avoiding harmful therapies, especially when alternative agents are available. As the soluble guanylate cyclase stimulators have never been studied in SCD, we cannot comment on their use in this patient group, but have similar concerns related to the observations with phosphodiesterase-5 inhibitor therapy.
Recommendation: For patients with SCD who have an RHC-confirmed marked elevation of their pulmonary vascular resistance, a normal pulmonary artery wedge pressure, and related symptoms, we recommend against phosphodiesterase-5 inhibitor therapy as a first-line treatment (strong recommendation, moderate-quality evidence).
Remarks: The recommendation is based on the observation that phosphodiesterase-5 inhibitor therapy may increase the risk of hospitalization for vaso-occlusive crisis.
Values and preferences: This recommendation places a higher value on avoiding serious complications and a lower value on symptomatic and hemodynamic improvement.
Future Directions
These guidelines reflect the complexity and caveats of pulmonary hypertension in sickle cell disease and are reflective of the current state of the literature as of 2014. We anticipate that changes to this document will need to be made in the future as our understanding of this disease grows. In the future, the management of patients with SCD with an increased risk for mortality and PH is certain to include collaboration across the disciplines of adult and pediatric pulmonary medicine, cardiology, and hematology. Controlled clinical trials for the treatment of such patients are needed, particularly trials that evaluate combination therapy targeting the hemoglobinopathy and pulmonary vasculopathy of these patients. Continued research is expected to lead to earlier disease detection and enhanced treatment strategies to improve the prognosis and quality of life for these patients.
Acknowledgments
This guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Pulmonary Circulation.
Writing Committee
Elizabeth S. Klings, M.D. (Chair)*
Roberto F. Machado, M.D. (Co-Chair)*
Robyn J. Barst, M.D.
Claudia R. Morris, M.D.
Kamal K. Mubarak, M.D.
Victor R. Gordeuk, M.D.
Gregory J. Kato, M.D.
Kenneth I. Ataga, M.D.
J. Simon Gibbs, M.D.
Oswaldo Castro, M.D.
Erika B. Rosenzweig, M.D.
Namita Sood, M.D.
Lewis Hsu, M.D., Ph.D.
Kevin C. Wilson, M.D.
Marilyn J. Telen, M.D.
Laura M. DeCastro, M.D.
Lakshmanan Krishnamurti, M.D.
Martin H. Steinberg, M.D.
David B. Badesch, M.D.
Mark T. Gladwin, M.D. (Co-Chair)
*Drs. Klings and Machado are co–first authors of this document.
Committee members were as follows (in alphabetical order):
Committee Chair
Elizabeth S. Klings, M.D.
Committee Co-Chairs
Mark T. Gladwin, M.D.
Roberto F. Machado, M.D.
Pulmonary—Adult
David B. Badesch, M.D.
Kamal K. Mubarak, M.D.
Namita Sood, M.D.
Pulmonary—Pediatric
Robert Strunk, M.D.
Pulmonary and Evidence-Based Approaches
Kevin C. Wilson, M.D.
Cardiology—Adult
Hunter C. Champion, M.D.
J. Simon Gibbs, M.D.
Cardiology—Pediatric
Robyn J. Barst, M.D.
Erika B. Rosenzweig, M.D.
Hematology—Adult
Kenneth Ataga, M.D.
Oswaldo Castro, M.D.
Laura M. DeCastro, M.D.
Victor R. Gordeuk, M.D.
Martin H. Steinberg, M.D.
Marilyn J. Telen, M.D.
Hematology—Pediatric
Lewis Hsu, M.D., Ph.D.
Gregory J. Kato, M.D.
Lakshmanan Krishnamurti, M.D.
Elliott Vichinsky, M.D.
Emergency Medicine—Pediatric
Claudia R. Morris, M.D.
Acknowledgments
Acknowledgment
The members of the committee dedicate this document to the memory of Dr. Robyn J. Barst, who was an integral part of our group until her passing in April 2013. Dr. Barst was a passionate advocate for the importance of pulmonary hypertension as a devastating complication for patients with sickle cell disease. She brought her wealth of experience as an international leader in the care of patients with pulmonary hypertension to her committee, and all of us learned a tremendous amount from working with her. The committee acknowledges the American Thoracic Society (ATS) for support of this project. The committee acknowledges Mr. Lance Lucas, Ms. Jessica Wisk, Ms. Miriam Rodriguez, and the ATS staff for administrative assistance with conference calls and face-to-face meetings; and the clinicians and patients with sickle cell disease who have participated in the studies that have made this document possible.
Footnotes
E.S.K. receives support from NIH grant R21HL107993. M.T.G. receives research support from NIH grants R01HL098032, R01HL096973, RC1DK085852, and 1P01HL103455-01, the Institute for Transfusion Medicine, and the Hemophilia Center of Western Pennsylvania. R.F.M. receives support from NIH grants K23HL098454 and R01HL111656-01.
This Official Clinical Practice Guideline of the American Thoracic Society was approved by the ATS Board of Directors, November 2013. These Guidelines were also endorsed by the American College of Chest Physicians, October 2013, and by the Pulmonary Hypertension Association, November 2013
This statement has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Listen to accompanying podcast discussion at www.atsjournals.org
Author Disclosures: R.J.B. was a consultant to Actelion ($50,000–99,999), Gilead ($1,000–9,999), Ikaria ($10,000–49,999), Novartis ($10,000–49,999), and Pfizer ($100,000–249,999); she was a speaker for Actelion ($1,000–9,999). K.K.M. was a consultant to Actelion ($1,000–9,999) and Gilead ($1,000–9,999) and served on advisory committees of Actelion ($1,000–9,999) and Gilead ($1,000–9,999); he was a speaker for Actelion ($1,000–9,999) and Gilead ($1,000–9,999), and received research support from Actelion ($25,000–49,999), Fibrogen ($25,000–49,999), Gilead ($25,000–49,999), Novartis ($25,000–49,999), and Pfizer ($25,000–49,999). G.J.K. received research support from Ikaria ($100,000–249,999). K.I.A. received research support from BioMarin ($25,000–49,999), HemaQuest ($100,000–249,999), Eli Lilly ($25,000–49,999), and TRF Pharma ($50,000–99,999). J.S.G. served on advisory committees of Actelion ($1,000–4,999), Bayer Schering ($1,000–4,999), GlaxoSmithKline ($1,000–4,999), Pfizer ($1,000–4,999), and United Therapeutics ($1,000–4,999); he was a speaker for Actelion ($1,000–4,999), GlaxoSmithKline ($1,000–4,999), Lilly ($1,000–4,999), Pfizer ($1,000–4,999), and Schering ($1,000–4,999); he received research support from BioMarin ($10,000–49,999) and Lung Rx ($1,000–9,999). O.C. was a consultant to Actelion ($1–4,999), Icagen ($1–4,999), and Cellerant ($1–4,999). E.B.R. was on advisory committees of Actelion ($5,000–24,999) and United Therapeutics ($5,000–24,999), and a speaker for Actelion ($5,000–24,999), Gilead ($5,000–24,999), and United Therapeutics ($5,000–24,999); she received research support from Actelion ($50,000–99,999), Gilead ($50,000–99,999), Lilly ICOS ($25,000–49,999), and United Therapeutics ($50,000–99,999). L.H. was a consultant to BioMarin ($1–4,999) and Eli Lilly ($1–4,999), and received research support from BioMarin ($50,000–99,999), Ikaria/INO Therapeutics ($50,000–99,999), and GlycoMimetics ($25,000–49,999). K.C.W.\x{2019}s spouse previously held stocks or options of Moody Lynn and Co. and State Street Bank ($50,000–99,999). M.J.T. was a consultant to GlycoMimetics ($25,000–49,999) and received research support from Dilafor ($25,000–49,999). L.M.D. received research support from Anthera Pharmaceuticals ($1,000–4,999) and GlycoMimetics ($1,000–4,999). L.K. received research support from INO Therapeutics/Ikaria ($50,000–99,999). M.H.S. was a consultant to Ikaria ($1–4,999) and served on an advisory committee of TRF Pharma ($1–4,999). D.B.B. was a consultant to Actelion ($25,000–49,999), Arena ($1–4,999), Bayer ($5,000–24,999), Gilead ($5,000–24,999), Ikaria ($1–4,999), Lung Rx ($1–4,999), Pfizer ($5,000–24,999), and United Therapeutics ($1–4,999); he was on an advisory committee of MondoGen ($1–4,999), and received research support from Actelion ($25,000–49,999), Bayer ($100,000–249,999), Gilead ($100,000–249,999), Ikaria ($1–4,999), Novartis ($50,000–99,999), and United Therapeutics ($100,000–249,999). M.T.G. received research support from Bayer (USA) ($10,000–49,999) and Gilead ($100,000–249,999); he received royalties from a patent for the use of nitrite salts for cardiovascular indications ($1,000–4,999). E.S.K., R.F.M., C.R.M., V.R.G., and N.S. reported no relevant commercial interests.
References
- 1.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca GH, Souza R, Salemi VC, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterization in sickle cell disease. Eur Respir J. 2011 doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 4.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 5.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–115. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 6.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 7.Klings ES, Anton BD, Rosenman D, Princeton S, Odhiambo A, Li G, Bernard SA, Steinberg MH, Farber HW. Pulmonary arterial hypertension and left-sided heart disease in sickle cell disease: clinical characteristics and association with soluble adhesion molecule expression. Am J Hematol. 2008;83:547–553. doi: 10.1002/ajh.21187. [DOI] [PubMed] [Google Scholar]
- 8.Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary hypertension in sickle cell disease: relevance to children. Pediatr Hematol Oncol. 2007;24:159–170. doi: 10.1080/08880010601185892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordeuk V, Minniti CP, Nouraie M, Campbell AD, Rana S, Luchtman-Jones L, Sable C, Dham N, Ensing G, Prchal JT, et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow-up in children and adolescents with sickle cell anemia Haematologica 20119633–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension J Amer Coll Cardiol 20136225 SupplD34–D41 [DOI] [PubMed] [Google Scholar]
- 11.Gladwin MT, Machado RF. Pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:1646–1647. doi: 10.1056/NEJMc1109130. [DOI] [PubMed] [Google Scholar]
- 12.Mehari A, Alam S, Tian X, Cuttica MJ, Barnett CF, Miles G, Xu D, Seamon C, Adams-Graves P, Castro OL, et al. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187:840–847. doi: 10.1164/rccm.201207-1222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. Circulation. 2009 doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald M, Fagan K, Herbert DE, Al Ali M, Mugal M, Haynes J., Jr Misclassification of pulmonary hypertension in adults with sickle hemoglobinopathies using Doppler echocardiography. South Med J. 2012;105:300–305. doi: 10.1097/SMJ.0b013e318256b55b. [DOI] [PubMed] [Google Scholar]
- 15.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X, et al. N-terminal pro–brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 16.Voskaridou E, Tsetsos G, Tsoutsias A, Spyropoulou E, Christoulas D, Terpos E. Pulmonary hypertension in patients with sickle cell/β thalassemia: incidence and correlation with serum N-terminal pro–brain natriuretic peptide concentrations. Haematologica. 2007;92:738–743. doi: 10.3324/haematol.11136. [DOI] [PubMed] [Google Scholar]
- 17.Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- 18.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. JAMA. 2008;299:324–331. doi: 10.1001/jama.299.3.324. [DOI] [PubMed] [Google Scholar]
- 19.Burger CD. Pulmonary hypertension in COPD: a review and consideration of the role of arterial vasodilators. COPD. 2009;6:137–144. doi: 10.1080/15412550902754252. [DOI] [PubMed] [Google Scholar]
- 20.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 21.Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, Jais X, Yaici A, Humbert M, Simonneau G, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637–643. doi: 10.1164/rccm.200804-613OC. [DOI] [PubMed] [Google Scholar]
- 22.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 23.Hassoun PM. Pulmonary arterial hypertension complicating connective tissue diseases. Semin Respir Crit Care Med. 2009;30:429–439. doi: 10.1055/s-0029-1233312. [DOI] [PubMed] [Google Scholar]
- 24.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132:998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 25.Sachdev V, Kato GJ, Gibbs JS, Barst RJ, Machado RF, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and UK Circulation 20111241452–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 27.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 28.Anthi A, Machado RF, Jison ML, Taveira-DaSilva AM, Rubin LJ, Hunter L, Hunter CJ, Coles W, Nichols J, Avila NA, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado RF, Kyle MA, Martyr S, Barnett C, Macarthur P, Sachdev V, Ernst I, Hunter LA, Coles WA, Nichols JP, et al. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease Br J Haematol 2007136319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado RF, Hildesheim M, Mendelsohn L, Remaley AT, Kato GJ, Gladwin MT. NT-pro brain natriuretic peptide levels and the risk of death in the Cooperative Study of Sickle Cell Disease. Br J Haematol. 2011;154:512–520. doi: 10.1111/j.1365-2141.2011.08777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Longitudinal follow up of elevated pulmonary artery pressures in children with sickle cell disease. Br J Haematol. 2009;144:736–741. doi: 10.1111/j.1365-2141.2008.07501.x. [DOI] [PubMed] [Google Scholar]
- 32.Olnes M, Chi A, Haney C, May R, Minniti C, Taylor J, Kato GJ. Improvement in hemolysis and pulmonary arterial systolic pressure in adult patients with sickle cell disease during treatment with hydroxyurea. Am J Hematol. 2009;84:530–532. doi: 10.1002/ajh.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37:1424–1426. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 34.Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 36.Ferster A, Vermylen C, Cornu G, Buyse M, Corazza F, Devalck C, Fondu P, Toppet M, Sariban E. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–1964. [PubMed] [Google Scholar]
- 37.Ballas SK, Barton FB, Waclawiw MA, Swerdlow P, Eckman JR, Pegelow CH, Koshy M, Barton BA, Bonds DR. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual Life Outcomes. 2006;4:59. doi: 10.1186/1477-7525-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballas SK, McCarthy WF, Guo N, DeCastro L, Bellevue R, Barton BA, Waclawiw MA. Exposure to hydroxyurea and pregnancy outcomes in patients with sickle cell anemia. J Natl Med Assoc. 2009;101:1046–1051. doi: 10.1016/s0027-9684(15)31072-5. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg MH, McCarthy WF, Castro O, Ballas SK, Armstrong FD, Smith W, Ataga K, Swerdlow P, Kutlar A, DeCastro L, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5 year follow-up. Am J Hematol. 2010;85:403–408. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, et al. National Institutes of Health consensus development conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008;148:932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 42.Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, et al. National Acute Chest Syndrome Study Group. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 43.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 44.Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 45.Lee MT, Piomelli S, Granger S, Miller ST, Harkness S, Brambilla DJ, Adams RJ. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins FS, Orringer EP. Pulmonary hypertension and cor pulmonale in the sickle hemoglobinopathies. Am J Med. 1982;73:814–821. doi: 10.1016/0002-9343(82)90763-x. [DOI] [PubMed] [Google Scholar]
- 47.Claster S, Hammer M, Hagar W, Ataga K, Orringer E, Vichinsky E. Treatment of pulmonary hypertension in sickle cell disease with transfusion [abstract] Blood. 1999;94:420A. [Google Scholar]
- 48.Riddington C, Wang W.Blood transfusion for preventing stroke in people with sickle cell disease Cochrane Database Syst Rev 2002. 1:CD003146. [DOI] [PubMed] [Google Scholar]
- 49.Wang WC, Morales KH, Scher CD, Styles L, Olivieri N, Adams R, Brambilla D. Effect of long-term transfusion on growth in children with sickle cell anemia: results of the STOP trial. J Pediatr. 2005;147:244–247. doi: 10.1016/j.jpeds.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 50.Miller ST, Wright E, Abboud M, Berman B, Files B, Scher CD, Styles L, Adams RJ. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr. 2001;139:785–789. doi: 10.1067/mpd.2001.119593. [DOI] [PubMed] [Google Scholar]
- 51.Files B, Brambilla D, Kutlar A, Miller S, Vichinsky E, Wang W, Granger S, Adams RJ. Longitudinal changes in ferritin during chronic transfusion: a report from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) J Pediatr Hematol Oncol. 2002;24:284–290. doi: 10.1097/00043426-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE.Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease Am J Med 2006119897.e7–e11 [DOI] [PubMed] [Google Scholar]
- 53.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, Sohier C, Hinderliter A, Parise LV, Orringer EP. Coagulation activation and inflammation in sickle cell disease–associated pulmonary hypertension. Haematologica. 2008;93:20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 54.van Beers EJ, Spronk HM, Ten Cate H, Duits AJ, Brandjes DP, van Esser JW, Biemond BJ, Schnog JB. No association of the hypercoagulable state with sickle cell disease related pulmonary hypertension. Haematologica. 2008;93:e42–e44. doi: 10.3324/haematol.12632. [DOI] [PubMed] [Google Scholar]
- 55.Minter KR, Gladwin MT. Pulmonary complications of sickle cell anemia: a need for increased recognition, treatment, and research. Am J Respir Crit Care Med. 2001;164:2016–2019. doi: 10.1164/ajrccm.164.11.2104101. [DOI] [PubMed] [Google Scholar]
- 56.Thomas AN, Pattison C, Serjeant GR. Causes of death in sickle-cell disease in Jamaica. Br Med J (Clin Res Ed) 1982;285:633–635. doi: 10.1136/bmj.285.6342.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisbona R, Derbekyan V, Novales-Diaz JA. Scintigraphic evidence of pulmonary vascular occlusion in sickle cell disease. J Nucl Med. 1997;38:1151–1153. [PubMed] [Google Scholar]
- 58.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Cooperative Study of Sickle Cell Disease. Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- 59.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest 2008133454S–545S.[Published erratum appears in Chest 134:892.] [DOI] [PubMed] [Google Scholar]
- 60.Schulman S, Granqvist S, Holmstrom M, Carlsson A, Lindmarker P, Nicol P, Eklund SG, Nordlander S, Larfars G, Leijd B, et al. Duration of Anticoagulation Trial Study Group. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. N Engl J Med. 1997;336:393–398. doi: 10.1056/NEJM199702063360601. [DOI] [PubMed] [Google Scholar]
- 61.Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–907. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 62.Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, Pengo V, Ghirarduzzi A, Pattacini C, Testa S, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780–1789. doi: 10.1056/NEJMoa054444. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, Cushman M, Moll S, Kessler CM, Elliott CG, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425–1434. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 64.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326:910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- 65.Ambrusko SJ, Gunawardena S, Sakara A, Windsor B, Lanford L, Michelson P, Krishnamurti L. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47:907–913. doi: 10.1002/pbc.20791. [DOI] [PubMed] [Google Scholar]
- 66.Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, Naeije R, Sood N, Swerdlow PS, Hildesheim M, et al. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149:426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011 doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Chen J, Gao Y, Deng B, Liu K.Endothelin receptor antagonists for pulmonary arterial hypertension Cochrane Database Syst Rev[serial on the Internet]. 2009[accessed January 2014];3:CD004434Available from: http://summaries.cochrane.org/ [DOI] [PubMed] [Google Scholar]
- 69.Paramothayan NS, Lasserson TJ, Wells AU, Walters EH.Prostacyclin for pulmonary hypertension in adults Cochrane Database Syst Rev[serial on the Internet]. 2005[accessed January 2014];2:CD002994Available from: http://summaries.cochrane.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanthapillai P, Lasserson T, Walters E.Sildenafil for pulmonary hypertension Cochrane Database Syst Rev[serial on the Internet]. 2004[accessed January 2014];4:CD003562Available from: http://summaries.cochrane.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, Hunter L, Coles W, Nichols J, Hunter C, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derchi G, Forni GL, Formisano F, Cappellini MD, Galanello R, D'Ascola G, Bina P, Magnano C, Lamagna M. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:452–458. [PubMed] [Google Scholar]
- 73.Morris CR. New strategies for the treatment of pulmonary hypertension in sickle cell disease: the rationale for arginine therapy. Treat Respir Med. 2006;5:31–45. doi: 10.2165/00151829-200605010-00003. [DOI] [PubMed] [Google Scholar]
- 74.Minniti CP, Machado RF, Coles WA, Sachdev V, Gladwin MT, Kato GJ. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009;147:737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]