Abstract
Background
Urinary tract involvement in patients with peritoneal surface disease treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) often requires complex urologic resections and reconstruction to achieve optimal cytoreduction. The impact of these combined procedures on surgical outcomes is not well defined.
Methods
A prospective database of CRS/HIPEC procedures was analyzed retrospectively. Type of malignancy, performance status, resection status, hospital and intensive care unit stay, morbidity, mortality, and overall survival were reviewed.
Results
A total of 864 patients underwent 933 CRS/HI-PEC procedures, while 64 % (550) had preoperative ureteral stent placement. A total of 7.3 % had an additional urologic procedure without an increase in 30-day (p = 0.4) or 90-day (p = 1.0) mortality. Urologic procedures correlated with increased length of operating time (p < 0.001), blood loss (p < 0.001), and length of hospitalization (p = 0.003), yet were not associated with increased overall 30-day major morbidity (grade III/IV, p = 0.14). In multivariate analysis, independent predictors of additional urologic procedures were prior surgical score (p < 0.001), number of resected organs (p = 0.001), and low anterior resection (p = 0.03). Long-term survival was not statistically different between patients with and without urologic resection for low-grade appendiceal primary lesions (p = 0.23), high-grade appendiceal primary lesions (p = 0.40), or colorectal primary lesions (p = 0.14).
Conclusions
Urinary tract involvement in patients with peritoneal surface disease does not increase overall surgical morbidity. Patients with urologic procedures demonstrate survival patterns with meaningful prolongation of life. Urologic involvement should not be considered a contraindication for CRS/HIPEC in patients with resectable peritoneal surface disease.
Cytoreductive surgery (CRS) followed by intraperitoneal hyperthermic chemotherapy (HIPEC) has resulted in improved survival outcomes for selected cohorts of patients with peritoneal surface disease (PSD).1-5 Urinary tract involvement is not uncommon, especially when prior surgical explorations have exposed the retroperitoneal structures. Therefore, complex urologic resection and reconstruction may be required to achieve optimal cytoreduction. Few reports exist on the impact of resected urinary tract involvement on outcomes of patients undergoing CRS/HIPEC.6,7 Whether urinary tract involvement functions as an indicator of more aggressive biologic behavior with subsequent impact on long-term survival is also unknown.
The primary aim of this study was to identify variables predicting urologic resections during CRS/HIPEC and determine their incidence. The secondary aim was to define the impact of urinary tract procedures both in surgical outcomes and long-term survival of patients with PSD.
METHODS
This is a retrospective analysis of a prospectively maintained database of 933 CRS/HIPEC procedures. An Institutional Review Board approval was obtained for this study. Data relevant to our analysis included demographics, age, race, gender, Eastern Cooperative Oncology Group (ECOG) performance status, R status of resection, type of malignancy, type of urologic intervention, nutritional status, comorbidities, morbidity, mortality and survival. Eligibility criteria for CRS/HIPEC were histologic or cytologic diagnosis of peritoneal carcinomatosis, complete recovery from prior systemic chemotherapy or radiation treatments, resectable or resected primary lesion, debulkable peritoneal disease, and no extra-abdominal disease. The presence of peripheral liver metastases, if easily resectable, was not a contraindication. Urinary tract disease with involvement of major pelvic vascular structures was considered unresectable.
Preoperative cystoscopy, retrograde pyelograms, and externalized ureteral stenting was performed for cases felt to have a higher likelihood of ureteral involvement based either on the volume of disease on preoperative imaging or the extent of previous surgery. If evidence of ureteral obstruction was seen on retrograde pyelography, an internal stent was placed. Externalized stents were removed postoperatively. Urinary tract organs were resected when macroscopically invaded by tumor. Intraoperative injuries to the ureter and bladder were repaired with absorbable suture, and when not possible to repair primarily, reconstructed using standard techniques. All ureteral repairs underwent placement of JJ stents before the completion of the surgery.
In addition to a complete history and physical, all patients had tumor markers and CT of the chest, abdomen and pelvis before CRS/HIPEC procedures. Previous surgical score was assigned for each patient.8 Ureteral stents were placed on a case by case basis at the discretion of the surgeon on the basis of volume of disease, prior surgical exploration, and preoperative imaging. The CRS/HIPEC procedure was conducted as previously described by our group.9 Surgical morbidity and mortality were recorded according to Clavien and Dindo classification system.10 R0 and R1 resections were grouped together as complete cytoreductions. Cytoreductions with residual macroscopic disease were characterized as R2 and subdivided on the basis of the size of residual disease (R2a ≤5 mm, R2b ≤2 cm, R2c >2 cm).
Descriptive statistics, including frequencies and proportions for categorical data, and means and standard deviations for continuous outcomes were calculated for all study measures. To assess for differences between study groups for categorical measures, Fisher’s exact tests were used, and Wilcoxon rank-sum tests were used for continuous variables. Multiple logistic regression models were used to determine predictors of a urologic procedure. Overall survival (OS) was estimated using the Kaplan-Meier method and compared with the log-rank test. All analyses were performed by SAS 9.3 (SAS, Cary, NC). A p value of 0.05 or less was considered statistically significant.
RESULTS
A total of 864 patients underwent 933 CRS/HIPEC procedures; 550 (63.7 %) of which underwent preoperative ureteral stent placement. The specific characteristics are included in Table 1.
TABLE 1.
Patient and tumor characteristics
| Characteristic | All patients (n = 864) |
|---|---|
| Age (years), mean ± SD | 53 ± 12.4 |
| Gender, n (%) | |
| Male | 400 (46.3) |
| Female | 464 (53.7) |
| Body mass index (kg/m2), mean ± SD (n = 710) | 27.9 ± 6.4 |
| ECOG performance status, n (%) (n = 856) | |
| 0 | 373 (43.6) |
| 1 | 345 (40.3) |
| 2–4 | 138 (16.1) |
| Malnutrition, n (%)a | 210 (24.3) |
| Preoperative chemotherapy, n (%) (n = 771) | 258 (33.5) |
| Prior surgical score, n (%) (n = 835) | |
| 0 | 152 (18.2) |
| 1 | 314 (37.6) |
| 2–3 | 369 (44.2) |
| Ureteral stents, n (%) (n = 860) | 550 (64.0) |
| Site of primary disease, n (%) | |
| Appendix | 404 (46.8) |
| Colon | 199 (23.0) |
| Ovary | 59 (6.8) |
| Mesothelioma | 56 (6.5) |
| Other/unknown | 146 (16.9) |
| More than 1 CRS/HIPEC, n (%) | 64 (7.4) |
| Resection status, n (%) (n = 838) | |
| R0/1 | 383 (45.7) |
| R2 | 455 (54.3) |
| Length of procedure (h), mean ± SD, (n = 601) | 8.4 ± 3.2 |
| Total no. of organs resected, mean ± SD | 3.1 (1.8) |
| Rectal resection, n (%) | 70 (8.1) |
| Ureteral resection, n (%) | 7 (0.81) |
| Estimated blood loss (mL), mean ± SD (n = 665) | 769 ± 726 |
| No. of days in ICU, mean ± SD (n = 649) | 3.5 (9.3) |
| No. of days in hospital, mean ± SD (n = 856) | 14.6 (17.2) |
SD standard deviation, ECOG Eastern Cooperative Oncology Group, CRS/HIPEC cytoreductive surgery–hyperthermic intraperitoneal chemotherapy, ICU intensive care unit
Malnutrition was defined as prealbumin <18 mg/dL, albumin <3 g/dL, or presence of cachexia
In univariate analysis preoperative ureteral stent placement was more frequent in patients with either higher volume or unfavorable location of disease as indicated by the total numbers of resected organs (p < 0.001), increased incidence of low anterior resection (p < 0.001), and R2 resection (p < 0.001). Ureteral stent placement was more common in patients with malnutrition (p < 0.001) and was associated with increased operative blood loss (p < 0.001), increased length of surgery (p < 0.001), as well as increased length of both hospitalization and intensive care unit stay (p < 0.001) (Table 2).
TABLE 2.
Comparison of patients undergoing CRS/HIPEC with and without ureteral stents
| Characteristic | No stents (n = 310) | Stents (n = 550) | p Value |
|---|---|---|---|
| Age (years), mean ± SD | 52.4 ± 13.1 | 53.4 ± 12.0 | 0.23 |
| Gender, n (%) | 0.62 | ||
| Male | 148 (47.7) | 252 (45.8) | |
| Female | 162 (52.3) | 298 (54.2) | |
| Body mass index (kg/m2), mean ± SD (n = 706) | 28.2 ± 6.0 | 27.7 ± 6.6 | 0.22 |
| ECOG performance status, n (%) (n = 831) | 0.13 | ||
| 0 | 148 (49.5) | 224 (42.1) | |
| 1 | 117 (39.1) | 227 (42.7) | |
| 2–4 | 43 (14.4) | 93 (17.5) | |
| Malnutrition, n (%)a | 44 (14.2) | 165 (30) | <0.001 |
| Preoperative chemotherapy, n (%) (n = 767) | 76 (28.8) | 181 (36.0) | 0.05 |
| Prior surgical score, n (%) (n = 831) | 0.1 | ||
| 0 | 127 (42.5) | 186 (35.0) | |
| 1 | 52 (17.4) | 100 (18.8) | |
| 2–3 | 120 (40.1) | 246 (46.2) | |
| Site of primary disease, n (%) | <0.001 | ||
| Appendix | 127 (41.0) | 276 (50.2) | |
| Colon | 62 (20.0) | 137 (24.9) | |
| Ovary | 29 (9.4) | 29 (5.3) | |
| Mesothelioma | 15 (4.8) | 41 (7.5) | |
| Other/unknown | 77 (24.8) | 67 (12.2) | |
| More than 1 CRS/HIPEC, n (%) | 26 (8.4) | 38 (6.9) | 0.42 |
| Resection status, n (%) (n = 835) | <0.001 | ||
| R0/1 | 181 (59.9) | 200 (37.5) | |
| R2 | 121 (40.1) | 333 (62.5) | |
| Length of procedure (h), mean ± SD (n = 599) | 7.1 ± 2.6 | 9.1 ± 3.2 | <0.001 |
| Total no. of organs resected, mean ± SD | 2.7 ± 1.6 | 3.3 ± 1.8 | <0.001 |
| Rectal resection, n (%) | 12 (3.9) | 58 (10.5) | <0.001 |
| Resection of uterus, n (%) | 24 (7.7) | 52 (9.5) | 0.45 |
| Estimated blood loss (mL), mean ± SD (n = 633) | 554 ± 614 | 875 ± 754 | <0.001 |
| No. of days in ICU, mean ± SD (n = 646) | 2.3 ± 7.1 | 4.1 ± 10.3 | <0.001 |
| No. of days in hospital, mean ± SD (n = 852) | 12.1 ± 12.9 | 16.0 ± 19.1 | <0.001 |
CRS/HIPEC cytoreductive surgery–hyperthermic intraperitoneal chemotherapy, SD standard deviation, ECOG Eastern Cooperative Oncology Group, ICU intensive care unit
Malnutrition was defined as prealbumin <18 mg/dL, albumin <3 g/dL, or presence of cachexia
Urologic complications are listed in Table 3. Significant predictors of these complications were female sex (p = 0.04) and malnutrition (p = 0.05). Ureteral injury occurred in 0.81 % (7) of procedures. In 5 (71.4 %) of these cases, the injury was recognized and addressed intraoperatively. Ureteral stents were present in every case where a ureteral injury was recognized at the time of injury. Of the two unrecognized injuries, one patient was stented preoperatively and the other one was not. Urinary fistulas occurred in 9 (1.0 %) of all patients after CRS/HIPEC, the majority of which (7 of 9, 77.8 %) followed cases where a urologic procedure was performed. Two vesicovaginal fistulas were repaired transvaginally. One ureteral leak was treated with a ureteral stent, Foley catheter, and nephrostomy drainage, while another patient with a ureteral leak refused therapy and was lost to follow-up. One bladder leak resolved with catheter drainage and the other required reoperation for closure. A vesicocutaneous fistula resolved with catheter drainage. One enterovesical fistula resolved spontaneously before localization and a colovesical fistula required a diverting ileostomy.
TABLE 3.
Urologic complications and procedures in 864 patients undergoing CRS/HIPEC
| All patients (n = 864) | |
|---|---|
| Complications | |
| Ureteral injury | 7 |
| Cystotomy | 13 |
| Urinary tract fistula | 9 |
| Procedures | |
| Nephrectomy | 8 |
| Partial nephrectomy | 1 |
| Ureteral resection with | |
| Ureteroureterostomy | 1 |
| Ureteral reimplantation | 3 |
| Boari flap | 1 |
| Renal autotransplant | 1 |
| Ureterolysis | 2 |
| Ureterorrhaphies | 5 |
| Cystorrhaphies | 12 |
| Partial cystectomy | 27 |
| Radical cystectomy with urinary diversion | 1 |
| Excision of seminal vesicle | 2 |
| Partial prostatectomya | 1 |
CRS/HIPEC cytoreductive surgery–hyperthermic intraperitoneal chemotherapy
Resection of posterior wall of prostate because of involvement
A total of 65 urologic procedures, whether anticipated or not, were performed in 63 (7.3 %) patients (Table 3). These were generally performed to achieve a complete cytoreduction or address an intraoperative injury that occurred while attempting complete cytoreduction. In univariate analysis, factors associated with an increased risk of requiring an additional urologic procedure were those indicating increased volume of disease, unfavorable location or prior surgical exploration such as prior surgical score (p = 0.01), ureteral stent placement (p = 0.01), low anterior resection (p < 0.001) and number of resected organs (p < 0.001). Urologic procedures were associated with increased length of operating time (p < 0.001), blood loss (p < 0.001), hospital stay (p = 0.003) along with a marginally significantly increased length of intensive care unit hospitalization (p = 0.05) (Table 4).
TABLE 4.
Comparison of patients with and without an additional urologic procedure as a component of CRS/HIPEC
| Characteristic | No urologic procedure (n = 801) | Urologic procedure (n = 63) | Univariate p value | Multivariate p value |
|---|---|---|---|---|
| Age (years), mean ± SD | 53.2 ± 12.4 | 51.4 ± 12.8 | 0.36 | |
| Gender, n (%) | 0.04 | |||
| Male | 379 (47.3) | 21 (33.3) | ||
| Female | 422 (52.7) | 42 (66.7) | ||
| Body mass index (kg/m2), mean ± SD (n = 710) | 27.9 ± 6.4) | 27.7 ± 6.6 | 0.59 | |
| ECOG performance status, n (%) (n = 856) | 0.85 | |||
| 0 | 347 (43.7) | 26 (41.9) | ||
| 1 | 318 (40.1) | 27 (43.5) | ||
| 2–4 | 129 (16.2) | 9 (14.5) | ||
| Malnutrition, n (%)a | 191 (23.8) | 19 (30.2) | 0.29 | |
| Preoperative chemotherapy, n (%) (n = 771) | 234 (32.9) | 24 (40.0) | 0.26 | |
| Prior surgical score, n (%) (n = 835) | 0.01 | <0.001 | ||
| 0 | 146 (18.9) | 6 (9.7) | ||
| 1 | 297 (38.4) | 17 (27.4) | ||
| 2–3 | 330 (42.7) | 39 (62.9) | ||
| Ureteral stents, n (%) (n = 860) | 501 (62.8) | 49 (79.0) | 0.01 | |
| Site of primary disease, n (%) | 0.27 | |||
| Appendix | 379 (47.3) | 25 (39.7) | ||
| Colon | 179 (22.3) | 20 (31.7) | ||
| Ovary | 56 (7.0) | 3 (4.8) | ||
| Mesothelioma | 54 (6.7) | 2 (3.2) | ||
| Other/unknown | 133 (16.6) | 13 (20.6) | ||
| More than 1 CRS/HIPEC, n (%) | 57 (7.1) | 7 (11.1) | 0.22 | |
| Resection status, n (%) (n = 838) | 0.90 | |||
| R0/1 | 355 (45.8) | 28 (44.4) | ||
| R2 | 420 (54.2) | 35 (55.6) | ||
| Length of procedure (h), mean ± SD (n = 601) | 8.3 ± 3.1 | 10.4 ± 3.6 | <0.001 | |
| Total no. of organs resected, mean ± SD | 3 ± 1.7 | 3.9 ± 1.7 | <0.001 | 0.001 |
| Rectal resection, n (%) | 57 (7.1) | 13 (20.6) | <0.001 | 0.03 |
| Resection of uterus, n (%) | 69 (8.6) | 8 (12.7) | ||
| Estimated blood loss (mL), mean ± SD (n = 665) | 742 ± 706 | 1,127 ± 874 | <0.001 | |
| No. of days in ICU, mean ± SD (n = 649) | 3.4 ± 9.0 | 4.3 ± 12.8 | 0.05 | |
| No. of days in hospital, mean ± SD (n = 856) | 14.3 ± 16.8 | 18.7 ± 21.1 | 0.003 |
CRS/HIPEC cytoreductive surgery–hyperthermic intraperitoneal chemotherapy, SD standard deviation, ECOG Eastern Cooperative Oncology Group, ICU intensive care unit
Malnutrition was defined as prealbumin <18 mg/dL, albumin <3 g/dL, or presence of cachexia
In multivariate analysis number of resected organs (p = 0.001), prior surgical score (p < 0.001), and low anterior resection (p = 0.03) remained significant in predicting the need for an additional urologic procedure in patients with PSD (Table 4).
Incidental Findings
A total of 2.9 % (17) of patients who were stented underwent internal stenting at the time of initial cystoscopy as a result of evidence of obstruction on retrograde pyelography. One patient had an unsuspected ureteral stone that was managed ureteroscopically before CRS/HIPEC. Only one out of 550 patients had findings at cystoscopy that led to early case termination as it was determined that the patient was unresectable.
Morbidity, Mortality, and Long-term Survival
For patients who needed a urologic procedure compared with those who did not, there was no difference in 30-day (27.0 vs. 19.3 %, p = 0.14) and 90-day (9.5 vs. 6.0 %, p = 0.27) major (grade III/IV) morbidity or 30-day (0 vs. 2.6 %, p = 0.39) and 90-day (1.6 vs. 1.9 %, p = 1.00) mortality. The proportions of complete and incomplete resections observed in these two groups were similar. More specifically patients with and without urologic procedure underwent 44.4 versus 45.8 % (p = 0.90) complete macroscopic cytoreductions.
Survival was similar between patients who had urologic procedures as a component of CRS/HIPEC compared with patients who did not have a urologic procedure for PSD originating from high-grade appendiceal primary lesions (median OS 17.4 vs. 18.5 months, p = 0.40) or colorectal primary lesions (median OS 23.0 vs. 15.8 months, p = 0.14). The difference observed in low-grade appendiceal primary lesions (median OS 43.6 vs. 106.9 months, p = 0.23), was not statistically significant possibly as a result of power attenuation (Fig. 1). When evaluating patients who had a urologic procedure, survival was similar between those with a prior surgical score of 0 or 1 and those with a prior surgical score of 2 or greater for all primary lesions (median OS 19.2 vs. 26.6 months, p = 0.40) as well as for the cohort with low-grade appendiceal primary lesions (median OS 19.4 vs. 35.8 months, p = 0.46).
FIG. 1.
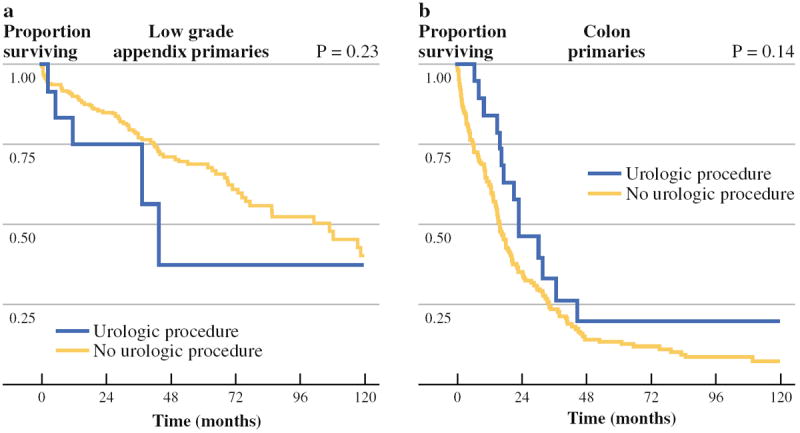
Survival comparison between patients with a urologic procedure as a component of their CRS/HIPEC and those without for PSD originating from a low-grade appendiceal primary disease and b colonic primary disease
DISCUSSION
Patients planned for CRS/HIPEC have externalized ureteral stents placed before surgery on a case by case basis. We routinely request stent placement in patients with increased volume of disease (notably PCI >18), prior surgical explorations, and preoperative imaging indicating urinary tract involvement. As a result of the above practice preferences, the patients who were stented preoperatively were also the patients who had a higher number of resected organs and were more likely not to achieve a complete cytoreduction. For the same reasons CRS/HIPEC procedures in stented patients were associated with increased operative blood loss, length of surgery, and hospitalization.
Despite the selection bias in stent placement, the incidence of ureteral injury in the stented population was 1.1 %, while 83.3 % of these injuries were recognized intraoperatively and addressed at that time. With an experienced team, ureteral stent placement adds less than 20 min to the length of the operation and can frequently be performed during anesthetic preparation time. Given the complexity of involvement and disease distribution in patients with PSD we feel that the relatively low incidence of ureteral injury likely reflects the benefits of ureteral stenting. In fact, our data indicate the rate of ureteral injury after CRS/HIPEC is similar to the reported incidence after anterior resection for rectal cancer.11
The role of routine cystoscopy as a separate procedure in the management of patients with PSD is minimal, considering that when cystoscopic findings are present, they rarely change the operative management or are sufficiently addressed during ureteral stent placement.
The incidence of urinary tract involvement requiring an additional urologic resection and reconstruction was 7.3 %, similar with the reported literature.7 Despite concerns of direct exposure of the urologic reconstruction to chemoperfusion, overall urologic complications were acceptably low, and when they did occur, those complications typically responded to conservative treatment without the need for additional surgical intervention.
There are very few reported studies in literature, addressing the urologic interventions in CRS/HIPEC. However, our results are in agreement with a recently published cohort of 598 patients, which indicated no differences in major morbidity or mortality between patients with and without urologic resection or reconstruction during CRS/HIPEC.7 Data were not available on patients who were considered appropriate candidates for CRS/HIPEC but did not receive HIPEC because of our inability to obtain a sufficient cytoreduction. Because of this selection bias, it is possible that some patients with urologic involvement will not benefit from CRS/HIPEC.
When patients were stratified by primary lesion, there were no statistically significant differences in survival on the basis of the presence or absence of urologic resection, possibly indicating that involvement of the urinary tract by peritoneal disease is not always a function of more aggressive biologic behavior. The survival difference observed in low-grade appendiceal patients was not statistically significant, but was clinically important. For patients with low-grade appendiceal primary lesions and urinary tract involvement, the smaller survival benefit might reflect a greater volume of disease at the onset as patients with urologic resections required more visceral resections to achieve similar resection status (Table 4). Even though survival in this cohort did not match that of patients without involvement, CRS/HIPEC still provided meaningful prolongation of life with a median survival of 44 months. Although urologic involvement was more commonly encountered in patients with higher prior surgical scores, survival was similar between patients regardless of the extent of prior resections. For patients with urologic involvement and no prior resection, we cannot exclude the possibility of a more aggressive tumor biology as a result of power attenuation in that particular subgroup analysis. Despite the possibility of more aggressive tumor biology in patients with urologic involvement and no prior surgical resection, CRS/HIPEC should still be attempted if complete cytoreduction is feasible because of the meaningful prolongation of life associated with R0/R1 resections.
In our experience, involvement of the urinary tract is more frequently encountered in patients having had prior exposure of the retroperitoneum during a hysterectomy or a low anterior resection, where the ureters are routinely exposed for identification. In these cases it is not the urinary tract involvement that makes a complete cytoreduction virtually impossible but rather the iatrogenic contamination of the pelvic vascular structures with tumor implants. Patients with PSD not amenable to complete cytoreduction face a dramatic decrease in their overall survival. Therefore, patients who present with synchronous PSD from rectal cancer or other primary lesions with secondary involvement of the uterus and ovaries would be better treated by referral to a center with CRS/HIPEC capabilities and not by staged resection.12
In conclusion, surgical resection and reconstruction of urologic involvement by PSD, despite the milieu of disseminated cancer, hyperthermia, chemotherapeutic agent, and wide variety of urologic extirpative and reconstructive procedures required, is feasible and does not increase the morbidity or the mortality of CRS/HIPEC procedures. Patients with PSD from colon and appendiceal primary lesions and urinary tract involvement, experience a clinically meaningful survival in cases of complete cytoreduction. Resectable urinary tract involvement in patients with PSD should not be considered a contraindication for CRS/ HIPEC.
Footnotes
Presented at the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction, 2012 Winter Meeting, March 3, 2012, New Orleans, LA; and the Southeastern Section of the American Urological Association, 76th Annual Meeting, March 22–25, 2012, Amelia Island, FL.
DISCLOSURE The authors declare no conflict of interest.
References
- 1.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 2.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–8. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 3.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 4.Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065–71. doi: 10.1097/SLA.0b013e31827e9289. [DOI] [PubMed] [Google Scholar]
- 5.Levine EA, Stewart JH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–53. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Smeenk RM, Bex A, Verwaal VJ, Horenblas S, Zoetmulder FA. Pseudomyxoma peritonei and the urinary tract: involvement and treatment related complications. J Surg Oncol. 2006;93:20–3. doi: 10.1002/jso.20427. [DOI] [PubMed] [Google Scholar]
- 7.Honore C, Souadka A, Goere D, Dumont F, Deschamps F, Elias D. HIPEC for peritoneal carcinomatosis: does an associated urologic procedure increase morbidity? Ann Surg Oncol. 2012;19:104–9. doi: 10.1245/s10434-011-1820-2. [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–31. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 9.Shen P, Stewart JH, Levine EA. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancy: overview and rationale. Curr Probl Cancer. 2009;33:125–41. doi: 10.1016/j.currproblcancer.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260–8. doi: 10.1097/01.sla.0000133185.23514.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Votanopoulos KI, Swett K, Blackham AU, Ihemelandu C, Shen P, Stewart JH, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann Surg Oncol. 2013;20:1088–92. doi: 10.1245/s10434-012-2787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


