Abstract
Beta-catenin is a multifunctional protein with critical roles in cell-cell adhesion, Wnt-signaling and the centrosome cycle. Whereas the roles of β-catenin in cell-cell adhesion and Wnt-signaling have been studied extensively, the mechanism(s) involving β-catenin in centrosome functions are poorly understood. β-Catenin localizes to centrosomes and promotes mitotic progression. NIMA-related protein kinase 2 (Nek2), which stimulates centrosome separation, binds to and phosphorylates β-catenin. β-Catenin interacting proteins involved in Wnt signaling such as APC, Axin and GSK3β, are also localized at centrosomes and play roles in promoting mitotic progression. Additionally, proteins associated with cell-cell adhesion sites, such as dynein, regulate mitotic spindle positioning. These roles of proteins at the cell cortex and Wnt signaling that involve β-catenin indicate a cross-talk between different sub-cellular sites in the cell at mitosis, and that different pools of β-catenin may co-ordinate centrosome functions and cell cycle progression.
What is the major role of centrosomes throughout the cell cycle?
In mammalian cells, the centrosome is the major organizer of the microtubule cytoskeleton [1]. A centrosome consists of two cylindrical microtubule-based structures termed centrioles, which recruit a matrix of associated pericentriolar material. During interphase, the centrosome coordinates an astral array of microtubules that participates in fundamental cellular functions including intracellular trafficking, cell motility, cell adhesion and cell polarity [2]. During mitosis, a pair of centrosomes coordinates the formation of the spindle, which separates the chromosomes to the daughter cells.
At the beginning of the cell cycle (G1) cells have one centrosome. Duplication of the centrioles in preparation for mitosis occurs only once per cell cycle at a specific cell cycle stage and site in the cell [3]. As a result of this duplication, the two centrioles contrast in age and maturity each with different functions. For example, the older of the two centrioles, termed the mother centriole, can initiate the polymerization of a ciliary axoneme at its distal end to form the primary cilium, a nexus for growth factor signaling pathways [4]. The centrosome also coordinates the G1/S transition, entry into mitosis, anaphase onset, cytokinesis and mitotic spindle formation [1]. The mitotic spindle is the macromolecular machine that segregates the chromosomes to two daughter cells during mitosis. The major structural elements of the spindle are microtubule polymers, whose intrinsic polarity and dynamic properties are critical for bipolar spindle organization and function [5,6]. Abnormalities in centrosome duplication and spindle organization lead to a variety of diseases, most notably cancer.
β-Catenin is also a component of the centrosome [7,8], in addition to a role in mediating cell-cell adhesion and Wnt signaling [9]. We hypothesize that β-catenin accumulates during mitosis via interactions with kinases and other regulatory proteins and utilizes the centrosome as a platform to coordinate the formation of the bipolar spindle during mitosis.
What are β-catenin functions in cell-cell adhesion and Wnt signaling?
β-Catenin is a multifunctional protein that plays an essential role in tissue organization, gene expression during development [9], and bipolar spindle formation[8]. β-Catenin localizes to cell-cell contacts [9], the nucleus, cytosol [10] and at centrosomes [7,8]. β-Catenin plays a significant role in the structural and functional organization of the Adherens junction and cell-cell adhesion, by linking the cytoplasmic domain of Type I cadherin cell-cell adhesion proteins at the cell membrane to the actin binding protein α-catenin [11]. The catenin-cadherin complex is stabilized by serine/threonine phosphorylation of β-catenin [12] or E-cadherin [13]. In contrast, tyrosine phosphorylation of β-catenin disrupts the complex, causing a reduction or loss of cell-cell adhesion and increased levels of cytoplasmic β-catenin [14].
Regulation of the cytoplasmic pool of β-catenin is the central switch in controlling the transcriptional activity of β-catenin in the Wnt signaling pathway [10]. The canonical Wnt pathway plays a key role in development and tissue homeostasis, and unregulated Wnt signaling leads to cancer progression [10]. In the absence of Wnt, cytosolic levels of β-catenin are kept low by a destruction complex, which consists of the scaffold proteins adenomatous polyposis coli (APC) and Axin which bind β-catenin, and glycogen synthase kinase 3beta (GSK3β). GSK3β and the priming kinase casein kinase 1 (CK1) phosphorylate the N-terminus of β-catenin at residues S33/S37/T41 and S45, respectively [15,16], leading to the binding of the E3 ligase β-TrCP which marks β-catenin for ubiquitination and degradation by the proteasome [15,16]. Conversely, in the presence of Wnt which binds the transmembrane receptor Frizzled (frz), phosphorylation of β-catenin by the destruction complex is inhibited, causing the accumulation of β-catenin in the cytoplasm. This stable pool of β-catenin is translocated to the nucleus and interacts with T cell factor/lymphoid enhanced factor (Tcf/Lef) to stimulate transcription of specific target genes [10,17–19] (Figure 1).
Figure 1.
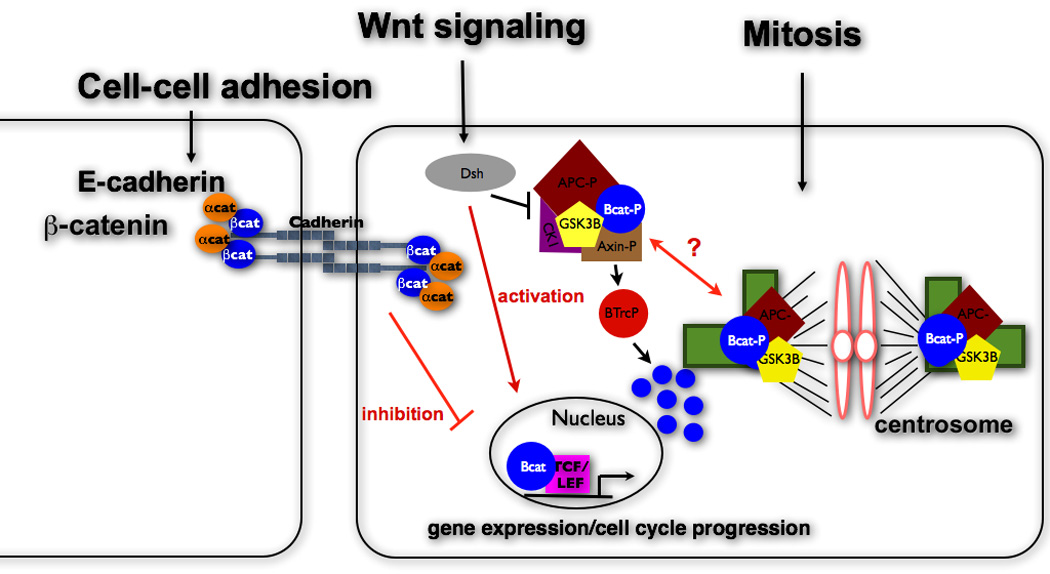
β-Catenin is a multifunctional protein that localizes at cell-cell contacts, the nucleus, cytosol and centrosomes. Cortical proteins and Wnt signaling components have been implicated in regulating cell cycle progression. Additionally, β-catenin destruction pathway proteins (APC, Axin, GSK3β) are also located at the centrosome and are involved in similar functions as β-catenin suggesting a cross-talk between these 3 distinct cellular pools of proteins.
What role does β-catenin play in regulating centrosome function?
The initial evidence that β-catenin is involved in centrosome regulation came from experiments in which β-catenin over-expression led to centrosome disorganization and loss of cortical anchoring of microtubules [20]. Normally, β-catenin levels in the cytoplasm and nucleus increase in S phase, reach a maximum level at late G2/M and then abruptly decrease as cells re-enter G1 after mitosis [21]. Manipulating β-catenin levels, particularly in G2, affects centrosome maturation and function, indicating roles for β-catenin in the centrosome [21].
During G2, centrosomes mature and accumulate high amounts of γ-tubulin and other centrosome proteins that are required for increased microtubule nucleation and spindle assembly [22]. Depletion of β-catenin inhibits microtubule nucleation from centrosomes [23], and β-catenin-null neuronal progenitor cells do not have γ-tubulin-containing centrosomes or a microtubule network [24]. However, these β-catenin-null neuronal progenitor were only analyzed for the presence of γ-tubulin, and it is not clear whether rudimentary centrosomes containing centrioles and other centrosome proteins remained. In addition, stabilizing mutations in the CK1/GSK3β phosphorylation sites in the N-terminus of β-catenin, which occur in cancer, increase the mobile fraction of γ-tubulin at centrosomes [25]. These results support a role for β-catenin in recruiting at least γ-tubulin, and potentially other proteins to centrosomes that result in centrosome maturation and full functionality.
β-Catenin is also involved in the establishment of a bipolar mitotic spindle [8]. During interphase, β-catenin colocalizes with the centriolar linker proteins Rootletin and C-Nap1 [8]. This interaction regulates β-catenin localization at centrosomes during interphase [7]. During mitosis β-catenin relocalizes from the centriolar linker to mitotic spindle poles and eventually to the mid-body. Reduction of β-catenin levels by RNAi leads to inhibition of centrosome separation and an increased number of cells with monopolar spindles [8], which consist of centrosomes in very close proximity and surrounded by chromosomes.
Does phospho-β-catenin also play a role at centrosomes?
The N-terminal domain of β-catenin contains phosphorylation sites for two serine/threonine kinases, GSK3β and CKI [15,16]. Phosphorylation by these kinases results in the subsequent ubiquitination and targeting of β-catenin for degradation by the proteasome [15,16]. Interestingly, immunofluorescence with an antibody that recognizes β-catenin phosphorylated at those sites (S33/S37/T41) indicates that phospho-β-catenin accumulates at the centrosome [23] and that during cell division phospho-β-catenin is preferentially segregated into one of the daughter cells [26]. Phospho-β-catenin localizes mainly to the mother centrosome during interphase and is recruited to the daughter centrosome in mitosis [23]. A phospho-mimetic β-catenin mutant can rescue disruption of microtubule organization and inhibition of microtubule assembly caused by depletion of β-catenin, although some of the cells over-expressing phospho-mimetic β-catenin have multiple centrosomes [23]. The effects of β-catenin depletion cannot by rescued by a phospho-mutant of β-catenin (S/T->A; also termed a ‘stabilized’ form of β-catenin) [23], which results in increased centrosome splitting [7], and extra non-microtubule nucleating structures that include γ-tubulin and centrin, but not polo-like kinase 4 (Plk4), SAS-6 or pericentrin [25].
Decreased centrosomal levels of S33/S34/T41 phosphorylated β-catenin are associated with mitotic spindle defects and centrosomal and microtubule defects [24]. Neuronal progenitor cells in mice expressing only phospho-mutant (S/T->A) β-catenin have defects in microtubules and polarity, similar to β-catenin loss-of-function neuronal progenitor cells [24]. Interestingly, Axin2 binds to CNap1 and promotes phosphorylation of Ser33/S37/T41 residues of centrosomal β-catenin [27]; Axin1 is involved in microtubule nucleation by forming a complex with γ-tubulin at the centrosome [28].
Taken together, these results imply a role for phospho-β-catenin at centrosomes in spindle and microtubule dynamics. However, there is no direct biochemical evidence to show how phosphorylated β-catenin is regulated at the centrosome, or how it promotes centrosome cohesion. Some recent studies have implied that GSK3β phosphorylates the S33/S34/T41 sites on β-catenin at centrosomes, which results in β-catenin stabilization at centrosomes [24,27] rather than targeting β-catenin for degradation. However, all of these data are based on immunofluorescence studies with an antibody that recognizes β-catenin phosphorylated at those sites (S33/S37/T41), without complementary biochemical analyses. Furthermore, broad claims have been made based on inferences from what is known about the β-catenin degradation pathway. Important questions that should be addressed biochemically are: how does inhibition of GSK3β affect S33/S34/T41 phosphorylated β-catenin levels and interactions at centrosomes; since CK1 priming is normally required for GSK3β phosphorylation of β-catenin, is there an increase in S45 phosphorylated β-catenin staining at the centrosome at the onset of mitosis; is GSK3β the (only) kinase that phosphorylates these residues on β-catenin at centrosomes? Additional biochemical and genetic studies are needed to elucidate the downstream signaling pathway involving β-catenin at the centrosome, and address how β-catenin promotes mitotic progression and proper bipolar spindle formation.
Which proteins are the key β-catenin interactors at the centrosome?
Biochemical experiments demonstrate that β-catenin is a binding partner and substrate of Nek2 [7]. Nek2 is a serine/threonine protein kinase that phosphorylates the centrosome linker proteins Rootletin and C-Nap1 resulting in centrosome separation and bipolar spindle formation [29]. Nek2 activity is cell cycle regulated and peaks at the G2/M boundary [29], coincident with β-catenin localization to centrosomes [7]. Whereas Nek2 phosphorylation of Rootletin and C-Nap removes them from centrosomes, the level of β-catenin at centrosomes increases [7,30,31]. An inactive form of Nek2 affects centrosome disjunction, spindle formation and chromosome segregation [32]. Significantly, cytoplasmic levels of Nek2 and β-catenin are increased in invasive ductal carcinoma in situ suggesting that abnormal expression of Nek2 and β-catenin might be involved in tumor proliferation [33].
Although β-catenin has been identified as a component of the inter-centrosomal linker [7] and a role for β-catenin during mitosis has been established, how β-catenin stability and function are regulated during mitosis remains undetermined. Furthermore, the role of Nek2 in regulating β-catenin stability during mitosis has yet to be characterized. Nek2 kinase activity is regulated by PLK1, a central mitotic kinase that regulates centrosome maturation including the recruitment of γ-tubulin and other centrosome components at G2/M [22,34]. At later stages in mitosis, PLK1 also regulates the interaction between dynein-dynactin and astral microtubules at the cell cortex and hence proper mitotic spindle positioning. The cortical localization of dynein is negatively regulated by spindle pole proximity resulting in spindle oscillations that center the spindle within the cell [35,36]. Most of β-catenin is also localized at the cortex where it mediates cell-cell adhesion in a complex with E-cadherin [11]. β-Catenin may be involved in cortical anchoring of interphase microtubules in a complex with dynein [7] and mitotic spindle positioning, since reduced levels of β-catenin in neuronal progenitor cells causes defects in the orientation of the mitotic spindle and increased asymmetric cell divisions [24].
These somewhat disparate observations on β-catenin functions raise the interesting question whether the pathways regulating spindle formation and orientation not only share PLK1 as a common regulator but also β-catenin as a common effector (Figure 2). Further work is needed to answer this question.
Figure 2.
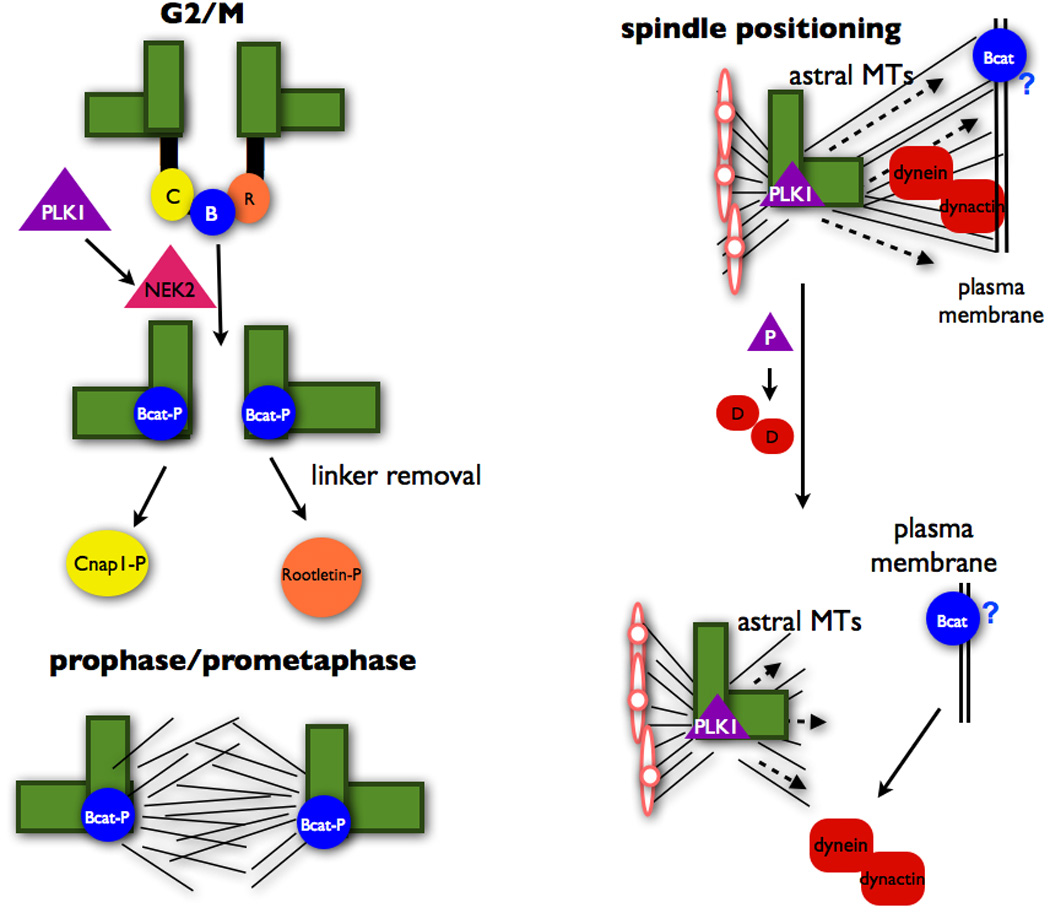
PLK1 is upstream of Nek2 in promoting removal of the centriolar linker at the G2/M transition. PLK1 also negative regulates the dynein-dynactin complex at the cell cortex in order to ensure proper spindle positioning. These processes promote mitotic spindle formation and centrosome separation during cell division.
Do Wnt pathway regulators have additional roles in mediating centrosome function and mitotic progression?
β-Catenin interactors and effectors in the Wnt signaling pathway have been linked to the regulation of the centrosome and mitosis. For example, activation of the Wnt signaling pathway in C. elegans ensures the timing and orientation of spindle rotation during developmentally regulated cell divisions [24,37,38]. Recent in vitro experiments have shown directly that a spatially restricted Wnt signal orients the plane of mitotic division and directs asymmetric inheritance of centrosomes [39]. Additionally, Wnt signals lead to abnormal mitotic progression and missegreation of chromosomes [40,41]. Lastly, Chibby (Cby), a highly conserved antagonist of the Wnt/β-catenin pathway, is localized at the distal end of the mother centriole and is essential for assembly of the primary cilium[42].
APC, a scaffold protein in the β-catenin destruction complex, localizes to the mother centriole [43]. Loss and/or truncation of APC causes mitotic spindle defects that trigger aneuploidy upon somatic inactivation of other known chromosomal instability genes including spindle and cell cycle checkpoint genes, DNA repair, telomere maintenance [44]. APC is targeted to multiple subcellular sites, and there is recent evidence linking novel protein interactions and functions of APC in the nucleus, at centrosomes and in mitochondria [45]. Axin2, which binds to APC and regulates β-catenin levels, is localized to the centrosome and regulates centrosome cohesion [27,46]. While these results are intriguing, additional studies are needed to understand whether these proteins control centrosome cycle by locally regulating β-catenin interactions at the centrosome, or whether they have functions independent of those of β-catenin. Finally, it is well known that hyperactivation of the Wnt pathway mediated by stabilization of β-catenin is a common cause of cancer, and that centrosome and spindle defects lead to genomic instability and tumorigenesis [47].
The discovery that β-catenin localizes to centrosomes, that it is regulated by the centrosomal kinase Nek2, and that decreased β-catenin levels cause defects in microtubule nucleation and spindle formation indicates a third function for β-catenin at centrosomes in addition to its roles in cell-cell adhesion and transcription. Therefore, mutations in β-catenin that are commonly observed in cancer may contribute in novel and previously unexpected ways to cancer progression [25]. This brings into question how much cross-talk is occurring between these three β-catenin pools and the global role β-catenin plays in the cell. As mentioned earlier there is evidence of proteins at the cortex that mediate cell cycle progression [35,36]. Additionally, contact inhibition has been shown to block cell cycle progression [48]. Though there is no evidence of a direct role for cortical β-catenin in mitotic regulation, this is an area that deserves further study.
Conclusions and Outlook
In addition to its well characterized roles in cell-cell adhesion and Wnt signaling, β-catenin is also localized to the centrosome and promotes cell cycle progression. A pool of β-catenin phosphorylated at the S33/S37/T41 residues has also been observed at centrosomes and appears to be involved in spindle and microtubule dynamics. β-Catenin interacts with known centrosomal proteins and mitotic regulators, including CNAP1, Rootletin and Nek2. There is also evidence of Wnt signaling proteins that interact with β-catenin being involved in cell cycle regulation. Future studies of β-catenin at the centrosome need to address how it promotes centrosome cohesion. What is β-catenin’s function to ensure proper bipolar spindle formation? Does it need to be phosphorylated at the S33/S37/T41 residues in order to carry out its function? Which kinase is phosphorylating those sites and are there additional centrosomal β-catenin interactors required for its stabilization and regulation? Additionally, the study of centrosomal β-catenin should broaden to experiments of the destruction pathway and other Wnt signaling proteins that are present at the centrosome and address the questions, are these proteins interacting with β-catenin at the centrosome and are those interactions regulating mitotic spindle dynamics?
Acknowledgements
BM is an HHMI Gilliam Fellow. Work from the Nelson laboratory was supported by the NIH (RO1GM35527).
Abbreviations
- APC
Adenomatous polyposis coli
- GSK3β
glycogen synthase kinase 3beta
- CKI
casein kinase I
- PLK-1
polo-like kinase 1
- Tcf/Lef
T cell factor /lymphoid enhanced factor
References
- 1.Schatten H. The mammalian centrosome and its functional significance. Histochemistry and cell biology. 2008;129:667–686. doi: 10.1007/s00418-008-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azimzadeh J, Bornens M. Structure and duplication of the centrosome. J Cell Sci. 2007;120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- 3.Wong C, Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nature cell biology. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Current biology : CB. 2009;19:1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolniak SM. The regulation of mitotic spindle function. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1988;66:490–514. doi: 10.1139/o88-061. [DOI] [PubMed] [Google Scholar]
- 6.Busson S, Dujardin D, Moreau A, Dompierre J, De Mey JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Current biology : CB. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- 7.Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH, Jr., O'Toole ET, et al. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes & development. 2008;22:91–105. doi: 10.1101/gad.1596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan DD, Meigs TE, Kelly P, Casey PJ. Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. The Journal of biological chemistry. 2004;279:10829–10832. doi: 10.1074/jbc.C400035200. [DOI] [PubMed] [Google Scholar]
- 9.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1847. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 11.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angers S. Proteomic analyses of protein complexes in the Wnt pathway. Methods Mol Biol. 2008;468:223–230. doi: 10.1007/978-1-59745-249-6_17. [DOI] [PubMed] [Google Scholar]
- 13.Major MB, Roberts BS, Berndt JD, Marine S, Anastas J, et al. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Sci Signal. 2008:1:ra12. doi: 10.1126/scisignal.2000037. [DOI] [PubMed] [Google Scholar]
- 14.Fixler D, Garcia J, Zalevsky Z, Weiss A, Deutsch M. Speckle random coding for 2D super resolving fluorescent microscopic imaging. Micron. 2007;38:121–128. doi: 10.1016/j.micron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Li Y, Semenov M, Han C, Baeg GH, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 16.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 17.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 18.Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 19.Arias AM, Brown AM, Brennan K. Wnt signalling: pathway or network? Curr Opin Genet Dev. 1999;9:447–454. doi: 10.1016/s0959-437x(99)80068-9. [DOI] [PubMed] [Google Scholar]
- 20.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nature cell biology. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 21.Olmeda D, Castel S, Vilaro S, Cano A. Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Molecular biology of the cell. 2003;14:2844–2860. doi: 10.1091/mbc.E03-01-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, Chen Q, Zhang X, Zhang B, Zhuo X, et al. PCM1 Recruits Plk1 to Pericentriolar Matrix to Promote Primary Cilia Disassembly before Mitotic Entry. Journal of cell science. 2013 doi: 10.1242/jcs.114918. [DOI] [PubMed] [Google Scholar]
- 23.Huang P, Senga T, Hamaguchi M. A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 2007;26:4357–4371. doi: 10.1038/sj.onc.1210217. [DOI] [PubMed] [Google Scholar]
- 24.Chilov D, Sinjushina N, Rita H, Taketo MM, Makela TP, et al. Phosphorylated beta-catenin localizes to centrosomes of neuronal progenitors and is required for cell polarity and neurogenesis in developing midbrain. Developmental biology. 2011;357:259–268. doi: 10.1016/j.ydbio.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Bahmanyar S, Guiney EL, Hatch EM, Nelson WJ, Barth AI. Formation of extra centrosomal structures is dependent on beta-catenin. Journal of cell science. 2010;123:3125–3135. doi: 10.1242/jcs.064782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuentealba LC, Eivers E, Geissert D, Taelman V, De Robertis EM. Asymmetric mitosis: Unequal segregation of proteins destined for degradation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7732–7737. doi: 10.1073/pnas.0803027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjihannas MV, Bruckner M, Behrens J. Conductin/axin2 and Wnt signalling regulates centrosome cohesion. EMBO reports. 2010;11:317–324. doi: 10.1038/embor.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fumoto K, Kadono M, Izumi N, Kikuchi A. Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO reports. 2009;10:606–613. doi: 10.1038/embor.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184–6194. doi: 10.1038/sj.onc.1205711. [DOI] [PubMed] [Google Scholar]
- 30.Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. The Journal of cell biology. 2005;171:27–33. doi: 10.1083/jcb.200504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, et al. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. The Journal of cell biology. 1998;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell. 2003;14:2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Li W, Lv S, Wang Y, Liu Z, et al. Abnormal expression of Nek2 and beta-catenin in breast carcinoma: clinicopathological correlations. Histopathology. 2011;59:631–642. doi: 10.1111/j.1365-2559.2011.03941.x. [DOI] [PubMed] [Google Scholar]
- 34.Mardin BR, Agircan FG, Lange C, Schiebel E. Plk1 controls the Nek2A-PP1gamma antagonism in centrosome disjunction. Current biology : CB. 2011;21:1145–1151. doi: 10.1016/j.cub.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 35.Kiyomitsu T, Cheeseman IM. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nature cell biology. 2012;14:311–317. doi: 10.1038/ncb2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins ES, Balchand SK, Faraci JL, Wadsworth P, Lee WL. Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase. Molecular biology of the cell. 2012;23:3380–3390. doi: 10.1091/mbc.E12-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walston T, Tuskey C, Edgar L, Hawkins N, Ellis G, et al. Multiple Wnt signaling pathways converge to orient the mitotic spindle in early C. elegans embryos. Developmental cell. 2004;7:831–841. doi: 10.1016/j.devcel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Ishidate T, Sharma R, Soto MC, Conte D, Jr., et al. Wnt and CDK-1 regulate cortical release of WRM-1/beta-catenin to control cell division orientation in early Caenorhabditis elegans embryos. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E918–E927. doi: 10.1073/pnas.1300769110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki K, Aoki M, Sugai M, Harada N, Miyoshi H, et al. Chromosomal instability by beta-catenin/TCF transcription in APC or beta-catenin mutant cells. Oncogene. 2007;26:3511–3520. doi: 10.1038/sj.onc.1210141. [DOI] [PubMed] [Google Scholar]
- 41.Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, et al. Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10747–10752. doi: 10.1073/pnas.0604206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steere N, Chae V, Burke M, Li FQ, Takemaru K, et al. A Wnt/beta-catenin pathway antagonist Chibby binds Cenexin at the distal end of mother centrioles and functions in primary cilia formation. PloS one. 2012;7:e41077. doi: 10.1371/journal.pone.0041077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louie RK, Bahmanyar S, Siemers KA, Votin V, Chang P, et al. Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. Journal of cell science. 2004;117:1117–1128. doi: 10.1242/jcs.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberici P, Fodde R. The role of the APC tumor suppressor in chromosomal instability. Genome dynamics. 2006;1:149–170. doi: 10.1159/000092506. [DOI] [PubMed] [Google Scholar]
- 45.Lui C, Mills K, Brocardo MG, Sharma M, Henderson BR. APC as a mobile scaffold: regulation and function at the nucleus, centrosomes, and mitochondria. IUBMB life. 2012;64:209–214. doi: 10.1002/iub.599. [DOI] [PubMed] [Google Scholar]
- 46.Alexandrova EM, Sokol SY. Xenopus axin-related protein: a link between its centrosomal localization and function in the Wnt/beta-catenin pathway. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:261–270. doi: 10.1002/dvdy.22125. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Fang X, Wei Z, York JP, Zhang P. Loss of spindle assembly checkpoint-mediated inhibition of Cdc20 promotes tumorigenesis in mice. The Journal of cell biology. 2009;185:983–994. doi: 10.1083/jcb.200904020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes & development. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]


