Abstract
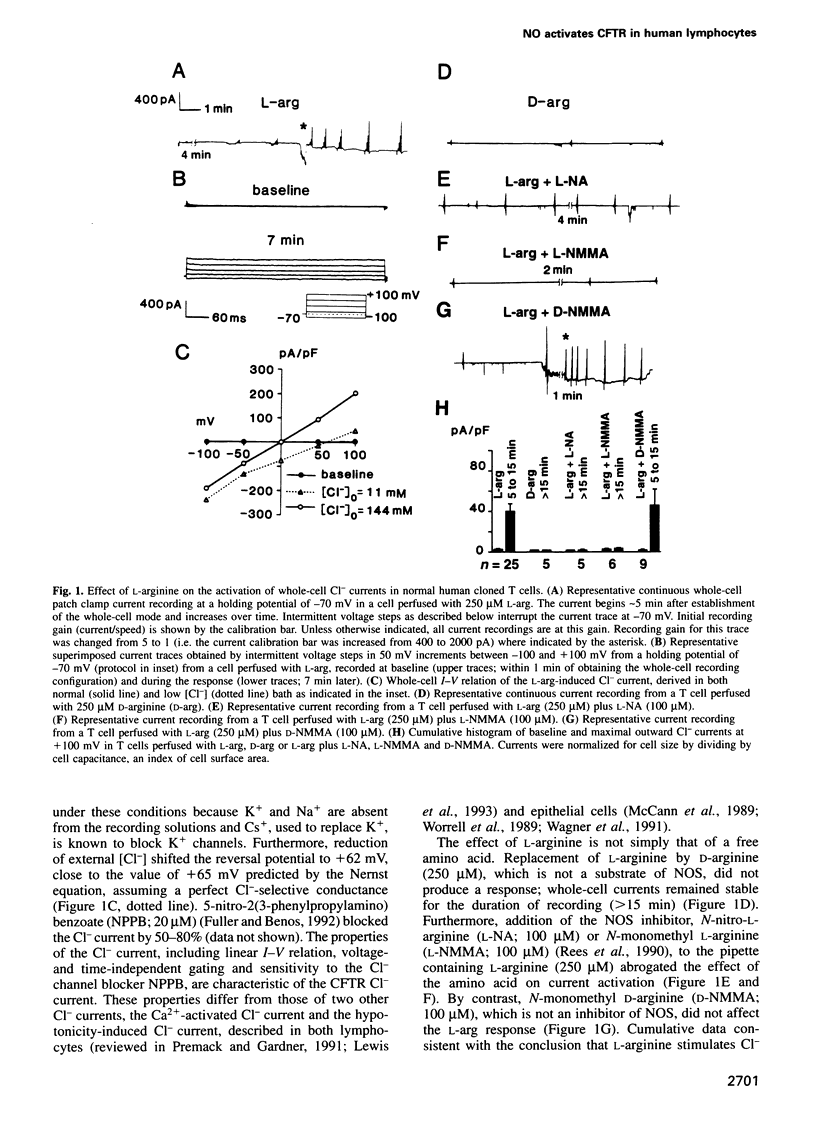
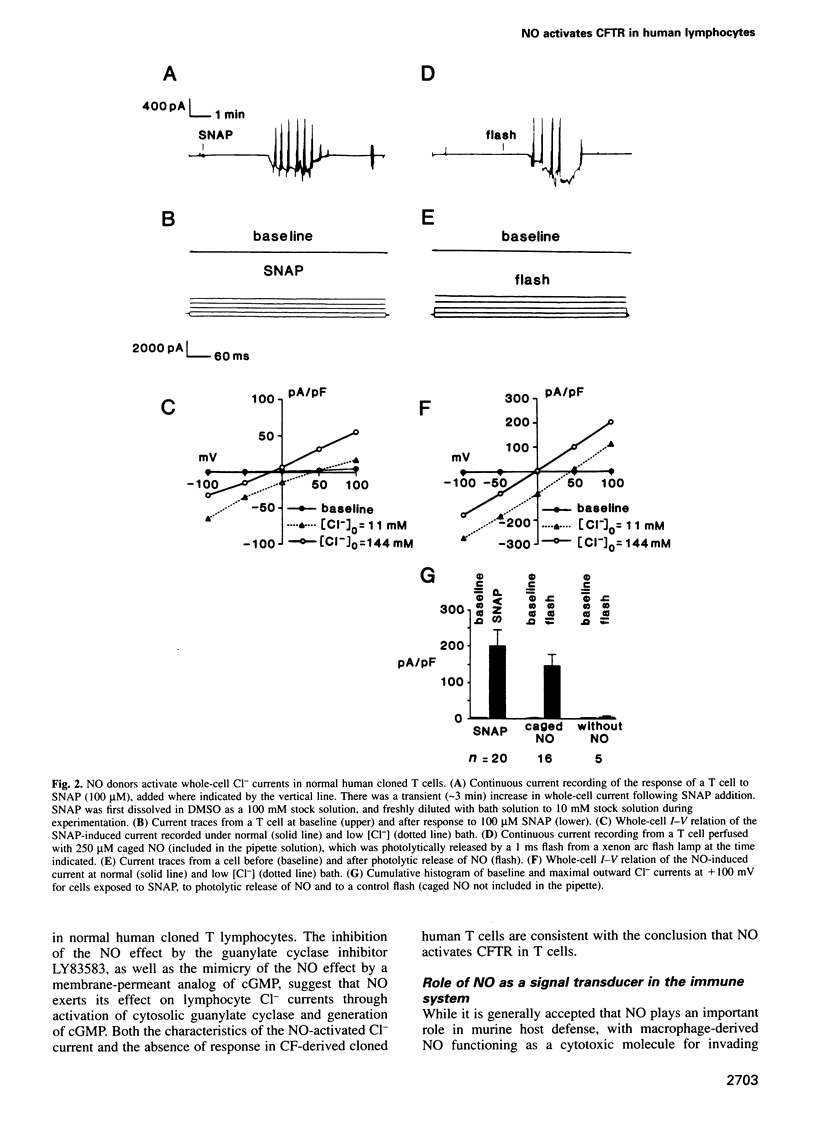
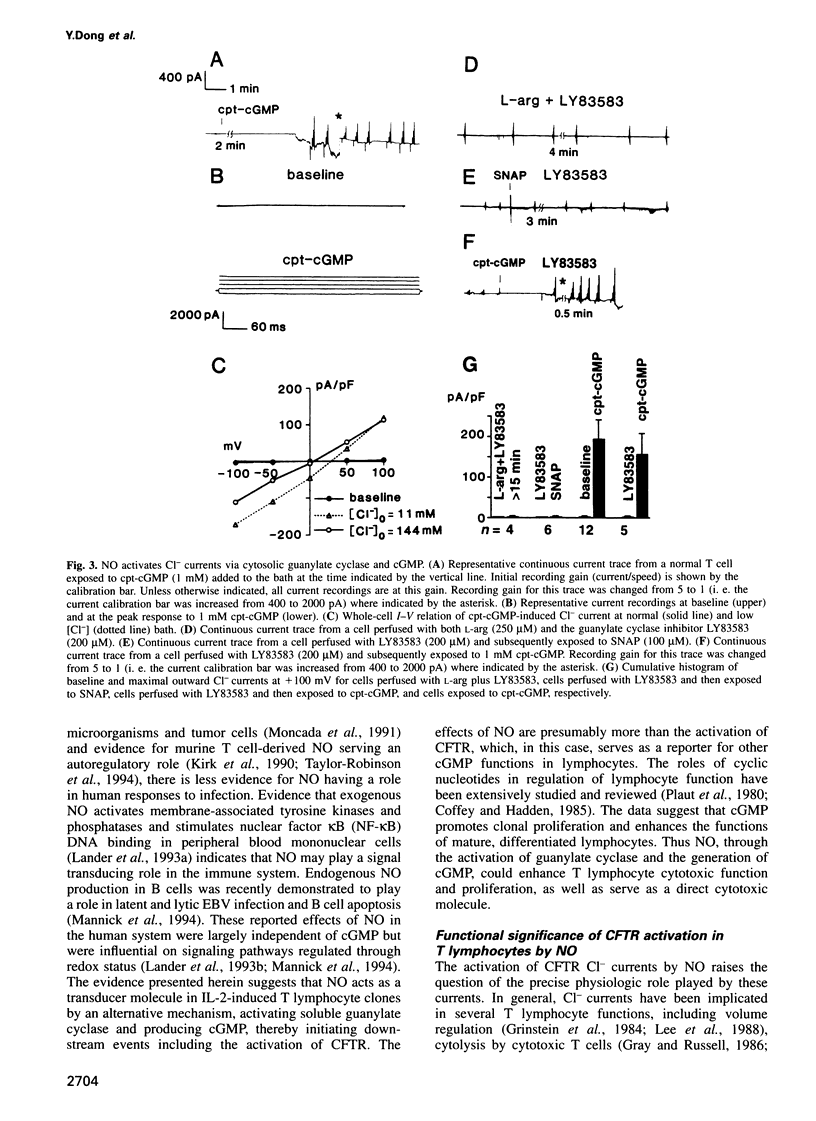
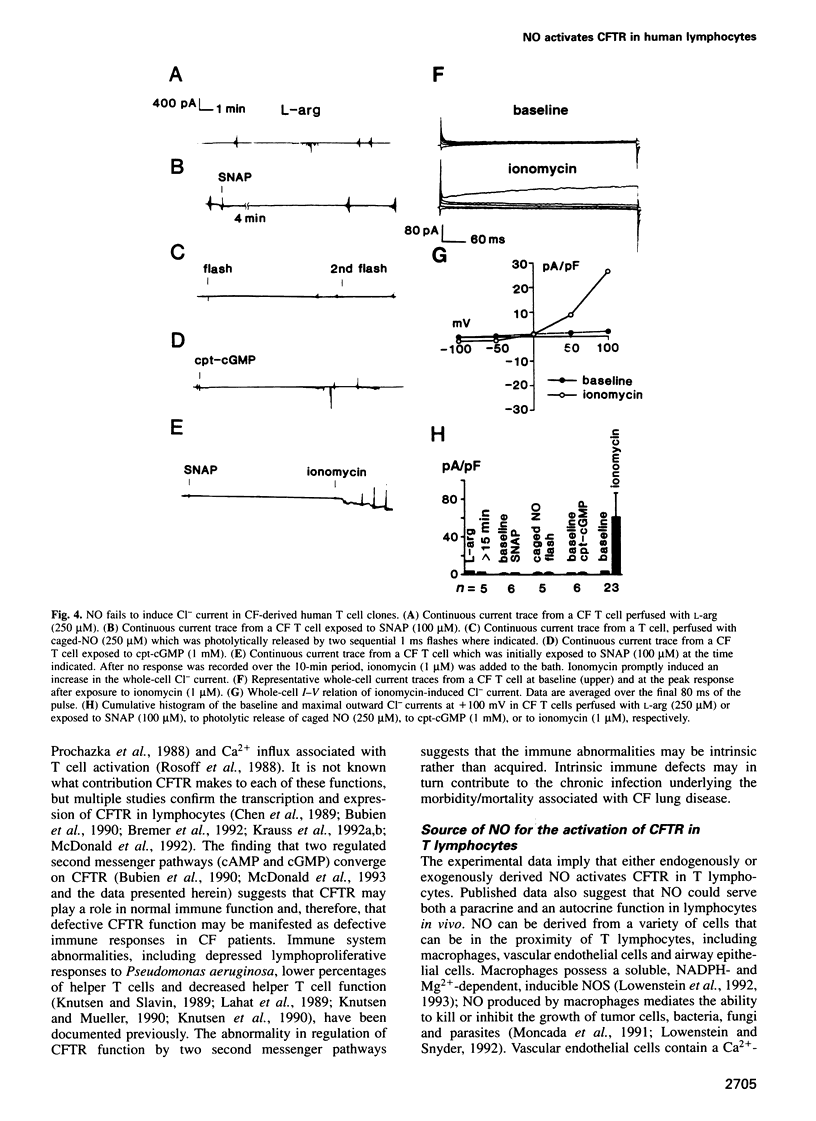
Nitric oxide, which is produced by cytokine-activated mononuclear cells, is thought to play an important role in inflammation and immunity. While the function of nitric oxide as a direct cytotoxic effector molecule is well established, its function as a transducer molecule in immune cells is not. By use of whole-cell patch clamp recordings, we show that nitric oxide activates cystic fibrosis transmembrane conductance regulator CI- currents in normal human cloned T cells by a cGMP-dependent mechanism. This pathway is defective in cystic fibrosis-derived human cloned T cells. These findings not only delineate a novel transduction mechanism for nitric oxide but also support the hypothesis that an intrinsic immune defect may exist in cystic fibrosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer S., Hoof T., Wilke M., Busche R., Scholte B., Riordan J. R., Maass G., Tümmler B. Quantitative expression patterns of multidrug-resistance P-glycoprotein (MDR1) and differentially spliced cystic-fibrosis transmembrane-conductance regulator mRNA transcripts in human epithelia. Eur J Biochem. 1992 May 15;206(1):137–149. doi: 10.1111/j.1432-1033.1992.tb16911.x. [DOI] [PubMed] [Google Scholar]
- Bubien J. K., Kirk K. L., Rado T. A., Frizzell R. A. Cell cycle dependence of chloride permeability in normal and cystic fibrosis lymphocytes. Science. 1990 Jun 15;248(4961):1416–1419. doi: 10.1126/science.2162561. [DOI] [PubMed] [Google Scholar]
- Böhme E., Grossmann G., Herz J., Mülsch A., Spies C., Schultz G. Regulation of cyclic GMP formation by soluble guanylate cyclase: stimulation by NO-containing compounds. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:259–266. [PubMed] [Google Scholar]
- Chao A. C., de Sauvage F. J., Dong Y. J., Wagner J. A., Goeddel D. V., Gardner P. Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994 Mar 1;13(5):1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Schulman H., Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. 1989 Feb 3;243(4891):657–660. doi: 10.1126/science.2464852. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Cille Y., Deviller P., Betuel H. Guanylate cyclase activity of human lymphocytes from peripheral blood, thymus, and tonsils. A comparative study. Enzyme. 1983;29(2):86–92. doi: 10.1159/000469612. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Hadden J. W. Neurotransmitters, hormones, and cyclic nucleotides in lymphocyte regulation. Fed Proc. 1985 Jan;44(1 Pt 1):112–117. [PubMed] [Google Scholar]
- Deviller P., Cille Y., Betuel H. Guanyl cyclase activity of human blood lymphocytes. Enzyme. 1975;19(5-6):300–313. doi: 10.1159/000459005. [DOI] [PubMed] [Google Scholar]
- Efron D. T., Kirk S. J., Regan M. C., Wasserkrug H. L., Barbul A. Nitric oxide generation from L-arginine is required for optimal human peripheral blood lymphocyte DNA synthesis. Surgery. 1991 Aug;110(2):327–334. [PubMed] [Google Scholar]
- Forte L. R., Thorne P. K., Eber S. L., Krause W. J., Freeman R. H., Francis S. H., Corbin J. D. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992 Sep;263(3 Pt 1):C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Benos D. J. CFTR! Am J Physiol. 1992 Aug;263(2 Pt 1):C267–C286. doi: 10.1152/ajpcell.1992.263.2.C267. [DOI] [PubMed] [Google Scholar]
- GIBSON L. E., COOKE R. E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959 Mar;23(3):545–549. [PubMed] [Google Scholar]
- Gaston B., Drazen J. M., Loscalzo J., Stamler J. S. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Russell J. H. Cytolytic T lymphocyte effector function requires plasma membrane chloride flux. J Immunol. 1986 Apr 15;136(8):3032–3037. [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Horowitz B., Tsung S. S., Hart P., Levesque P. C., Hume J. R. Alternative splicing of CFTR Cl- channels in heart. Am J Physiol. 1993 Jun;264(6 Pt 2):H2214–H2220. doi: 10.1152/ajpheart.1993.264.6.H2214. [DOI] [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Jorens P. G., Vermeire P. A., Herman A. G. L-arginine-dependent nitric oxide synthase: a new metabolic pathway in the lung and airways. Eur Respir J. 1993 Feb;6(2):258–266. [PubMed] [Google Scholar]
- Kirk S. J., Regan M. C., Barbul A. Cloned murine T lymphocytes synthesize a molecule with the biological characteristics of nitric oxide. Biochem Biophys Res Commun. 1990 Dec 14;173(2):660–665. doi: 10.1016/s0006-291x(05)80086-5. [DOI] [PubMed] [Google Scholar]
- Knutsen A. P., Mueller K. R., Hutcheson P. S., Slavin R. G. T- and B-cell dysregulation of IgE synthesis in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Clin Immunol Immunopathol. 1990 Apr;55(1):129–138. doi: 10.1016/0090-1229(90)90074-z. [DOI] [PubMed] [Google Scholar]
- Knutsen A. P., Mueller K. R. T cell cytotoxicity in cystic fibrosis: relationship to pulmonary status. Int Arch Allergy Appl Immunol. 1990;93(1):54–58. doi: 10.1159/000235279. [DOI] [PubMed] [Google Scholar]
- Knutsen A. P., Slavin R. G. In vitro T cell responses in patients with cystic fibrosis and allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1989 Apr;113(4):428–435. [PubMed] [Google Scholar]
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993 Oct;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Krauss R. D., Bubien J. K., Drumm M. L., Zheng T., Peiper S. C., Collins F. S., Kirk K. L., Frizzell R. A., Rado T. A. Transfection of wild-type CFTR into cystic fibrosis lymphocytes restores chloride conductance at G1 of the cell cycle. EMBO J. 1992 Mar;11(3):875–883. doi: 10.1002/j.1460-2075.1992.tb05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat N., Rivlin J., Iancu T. C. Functional immunoregulatory T-cell abnormalities in cystic fibrosis patients. J Clin Immunol. 1989 Jul;9(4):287–295. doi: 10.1007/BF00918660. [DOI] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P. K., Novogrodsky A. Nitric oxide signaling: a possible role for G proteins. J Immunol. 1993 Dec 15;151(12):7182–7187. [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P., Levine D. M., Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993 Feb 15;150(4):1509–1516. [PubMed] [Google Scholar]
- Lee S. C., Price M., Prystowsky M. B., Deutsch C. Volume response of quiescent and interleukin 2-stimulated T-lymphocytes to hypotonicity. Am J Physiol. 1988 Feb;254(2 Pt 1):C286–C296. doi: 10.1152/ajpcell.1988.254.2.C286. [DOI] [PubMed] [Google Scholar]
- Levesque P. C., Hart P. J., Hume J. R., Kenyon J. L., Horowitz B. Expression of cystic fibrosis transmembrane regulator Cl- channels in heart. Circ Res. 1992 Oct;71(4):1002–1007. doi: 10.1161/01.res.71.4.1002. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Ross P. E., Cahalan M. D. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993 Jun;101(6):801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Nairn A. C., Guggino S. E. cGMP-dependent protein kinase regulation of a chloride channel in T84 cells. Am J Physiol. 1992 May;262(5 Pt 1):C1304–C1312. doi: 10.1152/ajpcell.1992.262.5.C1304. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Alley E. W., Raval P., Snowman A. M., Snyder S. H., Russell S. W., Murphy W. J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Mannick J. B., Asano K., Izumi K., Kieff E., Stamler J. S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994 Dec 30;79(7):1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. V., Nghiem P. T., Gardner P., Martens C. L. Human lymphocytes transcribe the cystic fibrosis transmembrane conductance regulator gene and exhibit CF-defective cAMP-regulated chloride current. J Biol Chem. 1992 Feb 15;267(5):3242–3248. [PubMed] [Google Scholar]
- McDonald T. V., Premack B. A., Gardner P. Flash photolysis of caged inositol 1,4,5-trisphosphate activates plasma membrane calcium current in human T cells. J Biol Chem. 1993 Feb 25;268(6):3889–3896. [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: a physiological regulator of guanosine 3',5'-monophosphate formation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4360–4364. doi: 10.1073/pnas.74.10.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mülsch A., Busse R., Liebau S., Förstermann U. LY 83583 interferes with the release of endothelium-derived relaxing factor and inhibits soluble guanylate cyclase. J Pharmacol Exp Ther. 1988 Oct;247(1):283–288. [PubMed] [Google Scholar]
- Plaut M., Marone G., Thomas L. L., Lichtenstein L. M. Cyclic nucleotides in immune responses and allergy. Adv Cyclic Nucleotide Res. 1980;12:161–172. [PubMed] [Google Scholar]
- Premack B. A., Gardner P. Role of ion channels in lymphocytes. J Clin Immunol. 1991 Sep;11(5):225–238. doi: 10.1007/BF00918180. [DOI] [PubMed] [Google Scholar]
- Prochazka G., Landon C., Dennert G. Transmembrane chloride flux is required for target cell lysis but not for Golgi reorientation in cloned cytolytic effector cells. Golgi reorientation, N alpha-benzyloxycarbonyl-L-lysine thiobenzyl ester serine esterase release, and delivery of the lethal hit are separable events in target cell lysis. J Immunol. 1988 Aug 15;141(4):1288–1294. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Riordan J. R. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Hall C., Gramates L. S., Terlecky S. R. 4,4'-Diisothiocyanatostilbene-2,2'-disulfonic acid inhibits CD3-T cell antigen receptor-stimulated Ca2+ influx in human T lymphocytes. J Biol Chem. 1988 Dec 25;263(36):19535–19540. [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Ishii K., Sheng H., Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992 Feb 7;255(5045):721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- Sessa W. C., Harrison J. K., Barber C. M., Zeng D., Durieux M. E., D'Angelo D. D., Lynch K. R., Peach M. J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992 Aug 5;267(22):15274–15276. [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Southam E., Garthwaite J. Comparative effects of some nitric oxide donors on cyclic GMP levels in rat cerebellar slices. Neurosci Lett. 1991 Sep 2;130(1):107–111. doi: 10.1016/0304-3940(91)90239-p. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Liew F. Y., Severn A., Xu D., McSorley S. J., Garside P., Padron J., Phillips R. S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994 Apr;24(4):980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- Vuorinen P. Exogenous modification of nitrovasodilator-induced cyclic GMP formation in human lymphocytes. Pharmacol Toxicol. 1992 Jun;70(6 Pt 1):463–467. doi: 10.1111/j.1600-0773.1992.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Cozens A. L., Schulman H., Gruenert D. C., Stryer L., Gardner P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature. 1991 Feb 28;349(6312):793–796. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]



