Abstract
Objectives.
Caregivers of dementia patients are at risk for developing cardiovascular disease (CVD), and this risk increases the longer they provide care. Greater perceptions that caregiving restricts social/recreational activities (i.e., activity restriction [AR]) has been associated with poorer health, and AR may exacerbate the relations between stress and health outcomes. The current study examined the interactive role of greater exposure to stress and increased AR on plasma catecholamine (CAT) levels: norepinephrine (NE) and epinephrine (EPI).
Method.
A total of 84 dementia caregivers completed a standard assessment battery, and a nurse collected blood, which was assayed for NE and EPI. Separate regressions for NE and EPI were used to determine whether the relations between years caregiving and CATs were greater in those with high versus low AR.
Results.
A significant interaction was found between years caregiving and AR in predicting resting EPI (p = .032) but not resting NE (p = .103). Post hoc analyses indicated that years caregiving was significantly associated with EPI when AR was high (p = .008) but not when AR was low (p = .799). Additionally, years caregiving was not significantly associated with NE when AR was high or low.
Discussion.
The subjective experience of AR can play an important role in determining risk for detrimental physical health outcomes, particularly CVD risk.
Key Words: Activity restriction, Catecholamines, SNS, Stress.
Caregivers provide assistance and care for individuals with illness, disability, or age-related restrictions. Caregiving is becoming increasingly common as the aging population continues to grow in the United States, with 71 million individuals projected to be aged 65 years and older by 2030. According to the Family Caregiver Alliance (2007), approximately 29% (65.7 million) of the adult population in the United States are providing some form of caregiving services, with 43.5 million adults providing care for a person who is 50 years and older and 14.9 million adults caring for an individual with Alzheimer’s disease (AD) or other type of dementia. As caregivers often report undergoing tremendous amounts of stress and time limitations in caring for themselves due to the burden of caregiving, studies have shown caregiving to be associated with increased risk for developing an array of potential health problems. Thus, the impact of caregiving has become a major concern in public health. Prevention is often at the forefront of intervention for both psychological and physical health promotion. Accordingly, it is important to understand the mechanisms underlying why and how caregiving puts individuals at higher risk for developing health problems. Once we identify these mechanisms and understand how they affect caregiver health, preventive and intervention efforts can use these as a target for future services.
Caring for a loved one with AD is a chronic stressor that has been associated with a number of psychological (Mausbach et al., 2012; Pinquart & Sörensen, 2003; Schulz, O’Brien, Bookwala, & Fleissner, 1995) and physical (Schulz & Beach, 1999; Vitaliano, Zhang, & Scanlan, 2003) health consequences. Caregiving in general has been associated with increased risk of cardiovascular illness (Lee, Colditz, Berkman, & Kawachi, 2003; Vitaliano et al., 2002) and all-cause mortality (Perkins et al., 2012), and this risk appears to increase the longer caregivers care for their loved ones (Capistrant, Moon, Berkman, & Glymour, 2012). For example, more years spent caregiving has been linked to cardiovascular risk markers including onset of hypertension (Capistrant, Moon, & Glymour, 2012), poorer endothelial function as measured by flow-mediated dilation (Mausbach et al., 2010, 2012), elevations in circulating concentrations of C-reactive protein (von Känel et al., 2012), interleukin-6 (Kiecolt-Glaser et al., 2003), and tissue-type plasminogen activator antigen (Mausbach, Mills, et al., 2007), increased carotid artery intima-media thickness (Roepke et al., 2012), and blunted beta 2 adrenergic receptor sensitivity (Mausbach et al., 2008).
These studies suggest a breadth of biological changes take place over a lengthy time period that may ultimately lead to the onset of cardiovascular disease (CVD). Although many of these changes may take years to develop, one of the first likely physiologic changes occurs within the sympathetic nervous system (SNS). The SNS is responsible for the body’s “fight or flight” response, becoming activated in the presence of stressors. It has been established that acute stress activates the SNS with the synaptic release of catecholamines (CATs; epinephrine [EPI] and norepinephrine [NE]) into the blood. CATs bind onto beta adrenergic receptors and are eventually cleared from the synapse through the natural processes of synaptic reuptake and enzymatic breakdown (Kalat, 2009). Although this physiologic response is believed to be adaptive for managing acute stressors, prolonged SNS activation can lead to a downregulation of beta adrenergic receptors, which may inhibit reuptake and extend the presence of CATs within the blood (Anand & Charney, 2000; Mausbach, Mills, et al., 2007, 2008). This downregulation of beta adrenergic receptors may contribute to the development of hypertension (Bertel, Buhler, Kiowski, & Lutold, 1980; de Champlain et al., 1989), possibly due to a decreased ability of blood vessels to vasodialate under stress (Fitzgerald & Goldfien, 2004). Further, greater concentrations of CATs have been associated with a host of biomarkers associated with blood coagulation (Mausbach et al., 2006; von Känel & Dimsdale, 2000), all of which are associated with increased risk of developing CVD. Thus, sustained concentrations of circulating CATs may present an early risk for the development of CVD.
Although time spent caregiving has been associated with a host of negative health outcomes, there is likely great heterogeneity within caregivers’ psychological and physical health that arise from different profiles of risk factors and protective factors (Zarit & Femia, 2008). This has led to increased attention to factors that exacerbate or attenuate this relationship. In this vein, stress and coping models often conceptualize appraisals as critical to determining whether exposure to stress results in poor health outcomes (Lazarus & Folkma, 1984). One form of appraisal is the degree of harm or loss resulting from the stressors, with higher perceived levels of harm/loss believed to result in greater negative consequences to health. Knight and Sayegh (2010) proposed a theoretical framework for stress and coping in caregivers, which hypothesizes a direct and indirect pathway through which caregiving burden leads to physical and mental health outcomes; the indirect pathway proposes that coping and social support can mediate the impact of caregiving burden appraisal on physical and mental health outcomes. Caregivers often make appraisals of their loss of leisure or recreational activities (i.e., activity restriction [AR]) as a result of their caregiving duties. A robust finding in the literature is that caregivers often report feeling restricted from engaging in pleasurable activities as a result of caregiving duties. However, according to models of resiliency, positive psychological characteristics (e.g., positive emotions) can reduce one’s risk of developing detrimental health outcomes as a result of experiencing stress and hardship (Tugade, Fredrickson, & Feldman Barrett, 2004). Most notably, caregivers who feel less restricted from engaging in leisure activities are less likely to report depressive and anxious symptoms (Mausbach, Chattillion, et al., 2011; Nieboer et al., 1998), both of which have been associated with heightened SNS activation (Mathew, Ho, Francis, Taylor, & Weinman, 1982; Mausbach et al., 2005; Sullivan et al., 1981; Wyatt, Portnoy, Kupfer, Snyder, & Engelman, 1971). These data suggest either directly or indirectly that perceiving oneself as being less restricted from leisure time may serve as a buffer against the biological risk profile that is often associated with caregiving, for example, heightened SNS activation, possibly explaining the associations between AR and poorer subjective health, poorer sleep quality, and elevated blood pressure in caregivers (Mausbach, Roepke, et al., 2012; Mausbach et al., 2011; Moore et al., 2011).
In light of a wealth of evidence that years caregiving and AR appear independently associated with negative health outcomes, we examined the direct and interactive effects of years caregiving and AR on SNS activation in a sample of 84 dementia family caregivers. We hypothesized that more years caregiving would be more strongly associated with plasma concentrations of CATs in the context of high perceived AR as opposed to low perceived AR. If there is indeed an interaction between AR and years caregiving in determining biological markers of health, one could argue for the protective role of maintaining one’s level of leisure and pleasurable activities while caregiving. These findings could inform future interventions that promote resilience in caregivers using psychosocial methods.
Method
Participants
Our sample consisted of 84 AD caregivers enrolled in the Pleasant Events Project (PEP) at the University of California San Diego (UCSD). The PEP is a randomized clinical trial examining the efficacy of behavioral activation (BA) therapy for improving psychological and physical well-being in caregivers. To be eligible for the study, all participants were required to be providing in-home care to a loved one with a physician diagnosis of AD and be at least 55 years of age at the time of enrollment. Participants were excluded if they were taking beta-blocking or anticoagulant medications, were diagnosed with a terminal illness with a life expectancy of less than 6 months, were cognitively impaired, enrolled in another intervention study to reduce caregiver distress, or had a blood pressure of greater than 200/120mm Hg. Participants were recruited through referrals from the UCSD’s Alzheimer’s Caregiver study, the UCSD Alzheimer’s Disease Research Center, community support groups, health fairs, and local senior centers. The study was approved by the Institutional Review Board at the UCSD, and all participants provided written informed consent prior to participation in the study.
Procedure
A trained research assistant administered a single structured psychosocial interview in the caregivers’ homes to assess demographic information, general physical health (e.g., level of exercise, level of adiposity) and psychological health (e.g., AR), dementia severity of the care recipient, and the degree of assistance provided by the caregiver. Following the psychosocial assessment, a research nurse collected the blood samples using a standardized procedure described elsewhere in the literature (Chattillion, Mausbach, et al., 2012).
Primary Study Measures
CAT levels.—
Resting plasma NE and EPI levels were assessed using a catechol-O-methyltransferase radioenzymatic assay. This extracts CATs from 1 ml of of plasma and removes Ca2+ and other components that inhibit the radioenzymatic assay (Kennedy & Ziegler, 1990). The construct validity of these measures as indicators of health and stress has been illustrated by several studies. A study by Cacioppo and colleagues (2006) has shown elevated levels of plasma NE in rats who experience chronic stress. These findings are complemented by a study demonstrating chronic elevations in plasma NE to be associated with both fatal and nonfatal cardiovascular events in patients with end-stage renal disease (Zoccali et al., 2002). Moreover, the study by Cacioppo and colleagues (2006) demonstrated that dementia caregivers showed elevated levels of plasma EPI and NE in response to acute stress.
Activity restriction.—
To assess AR, participants were asked to complete the Activity Restriction Scale (ARS; Williamson & Schulz, 1992). The ARS asks participants to indicate how much they have been restricted from engaging in nine social and recreational activities over the past month: (a) “caring for yourself,” (b) “caring for others” (indicating individuals other than the care recipient), (c) “doing household chores,” (d) “going shopping,” (e) “visiting friends,” (f) “working on hobbies,” (g) “sports and recreation,” (h) “going to work,” and (i) “maintaining friendships.” Responses were rated on a 5-point scale from 0 (never or seldom did this) to 4 (greatly restricted). Responses were added to create an overall score, with higher scores indicating greater AR. Cronbach’s alpha has been reported as .85 in the literature (Williamson & Schulz, 1992), and for our sample, alpha was .79.
Additional Study Measures
Demographics.—
All participants were assessed for a number of demographic characteristics including age, gender, years married, educational attainment, and years spent caregiving. Because caregivers have been shown to be at increased risk for morbidity and mortality compared with noncaregivers over time (Pinquart & Sörensen, 2003), years spent caregiving was included as a covariate in the analyses to predict caregiver health.
Caregiver level of exercise.—
The Rapid Assessment of Physical Activity (RAPA) scale (Topolski et al., 2006) was administered to all caregivers for a measure of their current physical activity levels. The RAPA is designed for older adults (aged 50 years and older) based on the Centers for Disease Control and Prevention guidelines for physical activity, which recommend at least 30 min of moderate physical activity most days of the week. Respondents answered “yes” or “no” to nine items assessing the frequency and duration of their engagement in light, moderate, and vigorous exercise. Responses were then coded as a dichotomous variable where “1” meant the guidelines were met and “0” meant they were not. Topolski and colleagues (2006) compared the RAPA with several validated questionnaires that capture physical activity and found that the RAPA was significantly positively correlated with these questionnaires, and demonstrated sensitivity and specificity (62%–80%) equal to or better than the other assessments when parsing out individuals on their level of exercise.
Caregiver adiposity.—
Participants self-reported their height and weight, and their body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. BMI is an important indicator of health status that approximates the proportion of body fat an individual has, relative to his or her height. An elevated BMI beyond a certain cutoff tends to place individuals at higher risk for developing major health problems, including diabetes, hypertension, CVD, and various other diseases (Stommel & Schoenborn, 2012).
Care recipient dementia severity.—
The Clinical Dementia Rating (CDR) scale (Morris, 1993) was used to assess caregivers’ report of their loved-ones’ dementia severity. Caregivers reported their loved-one’s level of functioning in six domains: (a) memory, (b) orientation, (c) judgment and problem solving, (d) community affairs, (e) home and hobbies, and (f) personal care, upon which a trained research assistant determined overall dementia severity on a 4-point scale: 0 (no cognitive impairment), 1 (mild), 2 (moderate), and 3 (severe). Scoring was conducted using the online tool developed at Washington University (Washington University Alzheimer’s Disease Research Center, 1999). The CDR has shown good criterion validity via demonstrating expected correlations with neuropsychological measures and rates of survival (Fillenbaum, Peterson, & Morris, 1996). Moreover, this measure has been validated with neuropathological studies showing that a CDR score of 0.5 or greater is associated with histological features characteristic of Alzheimer’s dementia, and a score of 0 is associated with the absence of Alzheimer’s-related features (Morris, 1993; Morris et al., 1996).
Years smoked.—
Caregivers were asked to report the total number of years they have smoked throughout their lives. Smoking history was included as one of the covariates in the regression models because of its established relationship with biological changes that are associated with increased risk for developing physical health problems such as high blood pressure and cardiovascular problems.
Data Analysis
Prior to our primary analysis, we screened all regression variables for normal distribution. Because NE and EPI were positively skewed, both were subjected to log10 transformation. The analytic approach was to conduct hierarchical linear regression, whereby in Step 1, we included covariates known to be predictive of CAT levels; these included age, gender, BMI, physical exercise, years smoking, and caregiver dementia severity. In Step 2, we included AR, years caregiving, and the interaction term between AR and years caregiving. Within this approach, all predictor variables in the regression model were centered, as per the recommendations of Kraemer and Blasey (2004).
In the analysis, we were primarily interested in the interaction term. Post hoc probing of simple slopes (high AR and low AR) was used to understand how AR moderated the relationships between number of years spent caregiving and CAT levels (Holmbeck, 2002). Additionally, we calculated the region of significance using the technique described by Preacher, Curran, and Bauer (2006), which improves interpretation of significant interaction effects by identifying the specific values of AR at which years caregiving becomes significantly correlated to CAT levels. This also helps make findings more clinically meaningful because readers would be informed of how restricted caregivers need to be before stress is significantly associated with heightened SNS arousal.
Results
Participant Characteristics and Zero-Order Correlations
Detailed characteristics of the sample are shown in Table 1 and zero-order correlations between variables of interest are found in Table 2. The sample had a mean age of 70.7 ± 8.4 years and had been caregiving for an average of 4.9±4.1 years. Most participants were female, Caucasian, and had a high level of formal education.
Table 1.
Sample Characteristics (n = 84)
| Variable | |
|---|---|
| Age, M (SD), years | 70.71 (8.46) |
| Gender, n (%) | |
| Male | 21 (25%) |
| Female | 63 (75%) |
| Race, n (%) | |
| Caucasian | 73 (86.9%) |
| Other | 11 (13.1%) |
| Education, n (%) | |
| High school or less | 14 (16.7%) |
| Some college | 29 (34.5%) |
| College graduate | 23 (27.4%) |
| Graduate or professional degree | 18 (21.4%) |
| Median monthly income, median (IQR), $ | $4,000.00 ($3,600.50) |
| Norepinephrine, M (SD), pg/ml | 473.86 (190.09) |
| Epinephrine, M (SD), pg/ml | 51.86 (36.68) |
| Activity restriction, M (SD) | 17.65 (5.79) |
| CDC exercise, n (%) | |
| ≥30 min per day/5 days per week | 10 (11.9) |
| <30 min per day/5 days per week | 74 (88.1) |
| BMI, M (SD) | 26.72 (5.09) |
| Care recipient dementia severity, n (%) | |
| Mild | 33 (39.3%) |
| Moderate | 46 (54.8%) |
| Severe | 5 (5.9%) |
Notes. BMI = body mass index; CDC = Centers for Disease Control and Prevention; IQR = interquartile range; M = mean; SD = standard deviation.
Table 2.
Zero-Order Correlations Between Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Epinephrine | — | |||||||||
| 2. Norepinephrine | .57** | — | ||||||||
| 3. Age | −.16 | −.11 | — | |||||||
| 4. BMI | .08 | .17 | −.16 | — | ||||||
| 5. CDC exercise | −.07 | −.17 | −.09 | −.21 | — | |||||
| 6. Years Smoked | .12 | −.05 | .25* | −.10 | .01 | — | ||||
| 7. Gender | .06 | −.02 | −.14 | −.08 | .04 | −.05 | — | |||
| 8. Years caregiving | .19 | .12 | −.02 | .15 | .08 | −.10 | −.05 | — | ||
| 9. Activity restriction | .16 | .24* | −.33** | .14 | .01 | −.26* | .02 | −.02 | — | |
| 10. Dementia severity | −.01 | .14 | .10 | .04 | .08 | −.07 | −.09 | .21* | .14 | — |
Notes. BMI = body mass index; CDC = Centers for Disease Control and Prevention; degrees of freedom = 82.
*Correlation is significant at the .05 level (two-tailed).
**Correlation is significant at the .01 level (two-tailed).
EPI Levels
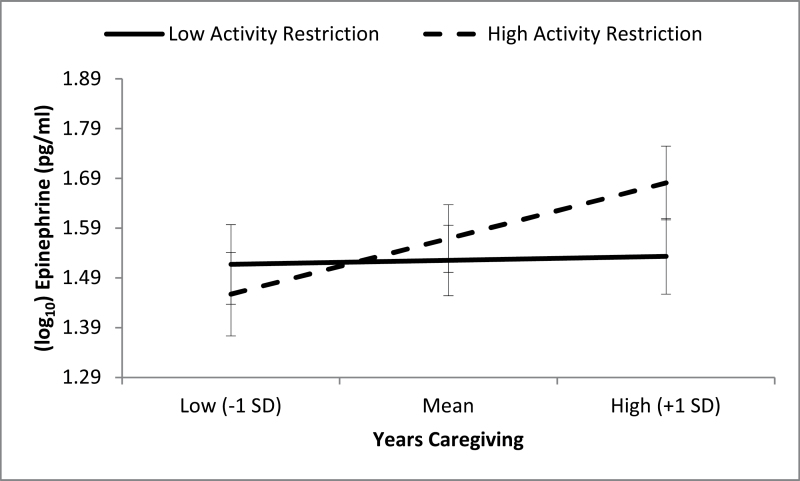
Our overall regression model accounted for 20.4% of the overall variance in EPI levels. As a set, age (t = −2.54, df = 74, p = .013), BMI (t = 0.30, df = 74, p = .767), exercise (t = −1.30, df = 74, p = .197), years smoked (t = 1.74, df = 74, p = .086), gender (t = 0.30, df = 74, p = .769), and caregiver dementia severity (t = −.03, df = 74, p = .978) accounted for 11.5% of the overall EPI variance, with age being a significant predictor of EPI. Our primary predictors years caregiving (t = 2.19, df = 74, p = .031), AR (t = 0.91, df = 74, p = .365), and the interaction term for years caregiving by AR (t = 2.18, df = 74, p = .032) accounted for an additional 9.0% of the EPI variance. We found a significant main effect of years caregiving on EPI but no significant main effect of AR, as well as a significant interaction of years caregiving by AR. Post hoc analyses indicated that among caregivers who highly restricted their activity, those with greater years caregiver also had higher EPI levels (t = 2.75, df = 75, p = .008). However, among caregivers who felt less restricted, years caregiving was not significantly associated with EPI (t = .255, df = 75, p = .799). Post hoc comparisons remained significant when the model was adjusted for age, gender, BMI, and years smoking. Results of the post hoc testing are presented in Figure 1.
Figure 1.
Moderating effect of activity restriction on the relations between years caregiving and plasma epinephrine. Error bars correspond to standard error of the mean.
NE Levels
In our final set of analyses, our full regression model accounted for 14.6% of the overall variance in NE levels. As a set, age (t = −.55, df = 74, p = .585), BMI (t = 0.89, df = 74, p = .378), exercise (t = −1.97, df = 74, p = .053), years smoked (t = 0.22, df = 74, p = .830), gender (t = 0.36, df = 74, p = .717), and caregiver dementia severity (t = 1.06, df = 74, p = .294) accounted for 8.7% of the overall variance. Of the covariates included in the model, none showed a significant relationship to NE. Our primary predictors, including years caregiving (t =.89, df = 74, p = .375), AR (t = 1.77, df = 74, p = .081), and the interaction term for years caregiving by AR (t = 1.65, df = 74, p = .103) accounted for an additional 7.2% of the NE variance. We found no significant main effect for years caregiving or AR, and no interaction of years caregiving by AR.
Although the interaction coefficient was not significant, we elected to probe the interaction effect to examine the main effect of years caregiving for caregivers who had high and low levels of AR. In these analyses, years caregiving was not significantly related to NE in caregivers who felt highly restricted (t = 1.61, df = 75, p = .113) or less restricted (t = −0.26, df = 75, p = .799).
Discussion
In summary, the current study was interested in the associations between years spent caregiving and AR in predicting levels of resting plasma CATs: EPI and NE. Previous studies have demonstrated a positive association between increased caregiving demands and elevated resting CAT levels, which may place Alzheimer’s caregivers at an increased risk for developing physical health problems such as CVD (Bhattacharyya & Steptoe, 2007; Lovallo & Thomas, 2000; Mills et al., 1997; Mills, Yu, Ziegler, Patterson, & Grant, 1999). However, there may be heterogeneity within Alzheimer’s caregivers and their psychological and physical health outcomes that arise from different profiles of risk factors and protective factors (Zarit & Femia, 2008). In particular, we found that AR moderated the relationship between years spent caregiving and resting CATs such that more years spent caregiving was associated with higher levels of resting EPI when AR was high, but when AR was low, there was no significant relationship between years caregiving and resting EPI. This finding suggests that continuing to engage in pleasant and pleasurable activities may provide a buffer against the detrimental physiological and mental health effects of chronic stress from caregiving.
Caregivers consistently report that they have significant limitations in their ability to partake in activities not related to caregiving (Roth, Perkins, Wadley, Temple, & Haley, 2009). Although many caregivers may agree that they have less time to engage in their own leisurely activities, the appraisal of this circumstance may play an important role in determining quality of life and well-being. In particular, not all caregivers will necessarily perceive themselves as being restricted by their caregiving duties. A review by Vitaliano and colleagues (2003) emphasized the need to understand the cognitive and psychological variables that may exacerbate or ameliorate the impact of chronic stressers on caregivers. Thus, identifying perceptions that may aggravate the negative effect of caregiving demands on physical health may have implications for interventions aimed at improving health in Alzheimer’s caregivers. Although previous studies have investigated the role of stress appraisal in dementia caregiver health (e.g., whether caregivers interpret care recipients’ problem behaviors as stressful), few studies have focused on caregivers’ self-perceptions of their AR. A protective factor that has shown promise in improving caregiver outcomes is engagement in leisurely or pleasurable activities. Previous studies have shown that caregivers with higher leisure satisfaction have biomarker profiles indicating lower CVD risk. For example, changes in leisure satisfaction over time corresponded to changes in flow mediated dilation, such that greater engagement in pleasant events was associated with increased arterial dilation, which indicated improved endothelial function (Mausbach et al., 2012). Moreover, a study by Chattillion, Ceglowski, et al. (2012) found that caregivers with greater engagement in pleasant events and lower perceived restriction of activities had significantly lower systolic and diastolic blood pressure. Whereas these studies emphasized the protective role of the leisure satisfaction and engagement in pleasurable activities as they relate to blood pressure, the current study sought to complement these findings by investigating whether the perception of being restricted in one’s daily activities moderates the relationship between caregiving burden and resting CAT levels.
Our study illustrated that caregivers who have had more years of caregiving tend to have higher levels of resting EPI levels (which is indicative of poorer health outcomes), but only when caregivers perceived that they are being restricted in their activities. Conversely, when AR was low, there was no relationship between years spent caregiving and resting EPI levels in caregivers. These findings support the importance of AR, or at least the perception of restriction, as a way to reduce the psychological and biological wear and tear of caregiving. Thus, interventions and preventative efforts aimed at reducing AR may be needed to mitigate the negative health effects (i.e., increase in CVD risk) of caregivers who experience more AR over time. One modality to reduce feelings of AR is to encourage engagement in leisurely and pleasurable activities through the use of BA techniques. Another intervention may be in cognitive restructuring or changing the way caregivers perceive their levels of activity. Future studies will be needed to determine the efficacy of such interventions in reducing AR, as well as its impact on CAT levels and other physical health outcomes.
Our results indicated that the interaction between AR and years caregiving was observed in EPI, but not NE; however, the direction of the effect was in the predicted direction for both types of CATs. It is possible that our analyses for NE were underpowered due to a small sample size. A correlation between NE and EPI demonstrated that the two measures were significantly and highly correlated (r = .571, p < .001), and substantial increases in total CATs have been shown to be deleterious to overall health (Lundberg, 2005; Nieboer et al., 1998).
Limitations of the current study include the fact that it is a cross-sectional study using between-subjects analyses. Although we found trends to suggest that having higher levels of AR may increase the physiological wear and tear of caregiver stress over time, our study precludes any assumptions of causality. In order to explain such trends, longitudinal analyses are required to show that fluctuations in perception of AR predate and predict levels of CATs (Piazza, Almeida, Dmitrieva, & Klein, 2010). Indeed, fluctuations in several constructs over time are expected within caregivers, and our nonsignificant correlations between years caregiving and other study variables appear to reflect the difficulty of capturing within-person fluctuations using cross-sectional data (see Table 2). This appears particularly true when considering our use of years caregiving to capture the construct of chronic stress. Other known correlates of negative caregiver outcomes, such as objective stressors (e.g., care recipient problem behaviors, dementia severity), subjective stressors (e.g., role overload), and physical strains (e.g., assisting with bed transfers) may reflect current stress, but given these fluctuate over time, cross-sectional use of these variables to indicate chronic stress is not optimal. Perhaps the best cross-sectional alternative to years caregiving is dementia severity, but our analysis did not find this variable to relate to either CAT outcome. Nonetheless, future studies should examine longitudinal levels of these variables as indicators of chronicity of stress in caregivers and examine their relationship to elevations in biomarkers.
An additional limitation to the current study is the relatively small sample size and lack of racial/ethnic diversity. Indeed, ethnicity appears to play a critical role in caregiving stress appraisals (Aranda & Knight, 1997; Knight, Silverstein, McCallum, & Fox, 2000), and it is likely that ethnicity influences which coping mechanisms caregivers use to deal with the burden of caregiving (Sayegh & Knight, 2011). Thus, the current study’s findings may not be generalizable to caregivers from other racial/ethnic groups.
A methodological limitation of the current study includes the use of plasma rather than urine CATs as an indicator of chronic stress. Cumulative levels of CATs in urine over an extended period may provide a stronger index of chronic SNS activation. Despite this limitation, there is evidence that plasma CATs can reflect chronic stress (Mansi and Drolet, 1997). Further, because we took a random sample of participants’ plasma EPI and NE levels, elevations at this random time point may indicate chronically high CAT levels. Nonetheless, future studies are needed to replicate the findings of the current study using CATs measured in urine. Furthermore, future studies should test models including several biological markers, rather than just one (Piazza et al., 2010).
In summary, this study found that spousal Alzheimer caregivers with high levels of perceived AR display a significant relationship between the numbers of years spent caregiving and their levels of CATs. Support for these findings may encourage a new approach to prevention and treatment to promote techniques focused on maintaining a low level of AR in order to lessen the negative health consequences associated with caring for one with Alzheimer’s over a long period of time.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health via grants R01 AG031090 and R01 AG15301.
References
- Anand A., Charney D. S. (2000). Norepinephrine dysfunction in depression. The Journal of Clinical Psychiatry, 61(Suppl. 10), 16–24 [PubMed] [Google Scholar]
- Aranda M. P., Knight B. G. (1997). The influence of ethnicity and culture on the caregiver stress and coping process: A sociocultural review and analysis. The Gerontologist, 37, 342–354. 10.1093/geront/37.3.342 [DOI] [PubMed] [Google Scholar]
- Bertel O., Buhler F. H., Kiowski W., Lutold B. E. (1980). Decreased beta-adrenoreceptor responsiveness as related to age, blood pressure, and plasma catecholamines in patients with essential hypertension. Hypertension, 2, 130–138. 10.1161/01.HYP.2.2.130 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya M. R., Steptoe A. (2007). Emotional triggers of acute coronary syndromes: Strength of evidence, biological processes, and clinical implications. Progress in Cardivascular Diseases, 49, 353–365. 10.1016/j.pcad.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Cacioppo J. T., Berntson G. G., Malarkey W. B., Kiecolt-Glaser J. K., Sheridan J. F., Poehlmann K. M., … Glaser R. (2006). Autonomic, neuroendocrine, and immune responses to psychological stress: The reactivity hypothesisa. Annals of the New York Academy of Sciences, 840, 664–673. 10.1111/j.1749-6632.1998.tb09605.x [DOI] [PubMed] [Google Scholar]
- Capistrant B. D., Moon J. R., Berkman L. F., Glymour M. M. (2012). Current and long-term spousal caregiving and onset of cardiovascular disease. Journal of Epidemiology and Community Health, 66, 951–956. 10.1136/jech-2011-200040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capistrant B. D., Moon J. R., Glymour M. M. (2012). Spousal caregiving and incident hypertension. American Journal of Hypertension, 25, 437–443. 10.1038/ajh.2011.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattillion E. A., Ceglowski J., Roepke S. K., von Kanel R., Losada A., Mills P. J., … Mausbach B. T. (2012). Pleasant events, activity restriction, and blood pressure in dementia caregivers. Health Psychology. 10.1037/a0029412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattillion E. A., Mausbach B. T., Roepke S. K., von Kanel R., Mills P. J., Dimsdale J. E., … Grant I. (2012). Leisure activities, caregiving demands and catecholamine levels in dementia caregivers. Psychology & Health, 27, 1134–1149. 10.1080/08870446.2011.637559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Champlain J., Gonzalez M., Lebeau R., Eid H., Petrovitch M., Nadeau R. A. (1989). The sympatho-adrenal tone and reactivity in human hypertension. Clinical and Experimental Hypertension, A11(S1), 159–171. 10.3109/10641968909045421 [DOI] [PubMed] [Google Scholar]
- Family Caregiver Alliance (2007). Selected caregiver statistics. Caregiver.org Retrieved January 10, 2013, from http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=439
- Fillenbaum G. G., Peterson B., Morris J. C. (1996). Estimating the validity of the Clinical Dementia Rating Scale: The CERAD experience. Consortium to establish a registry for Alzheimer’s disease. Aging (Milan, Italy), 8, 379 Retrieved from http://europepmc.org [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. A., Goldfien A. (2004). Adrenal medulla. In Greenspan F. S., Gardner D. G. (Eds.), Basic & clinical endocrinology (7th ed., pp. 439–477). San Francisco, CA: McGraw-Hill [Google Scholar]
- Holmbeck G. N. (Ed.). (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations [Special issue]. Journal of Pediatric Psychology, 27, 87–96. 10.1093/jpepsy/27.1.87 [DOI] [PubMed] [Google Scholar]
- Kalat J. (2009). Biological psychology. Belmont, CA: Wadsworth, Cengage Learning [Google Scholar]
- Kennedy B., Ziegler M. G. (1990). A more sensitive and specific radioenzymatic assay for catecholamines. Life Sciences, 47, 2143–2153. 10.1016/0024-3205(90)90314-H [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Preacher K. J., MacCallum R. C., Atkinson C., Malarkey W. B., Glaser R. (2003). Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America, 100, 9090–9095. 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. G., Sayegh P. (2010). Cultural values and caregiving: The updated sociocultural stress and coping model. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 5. 10.1093/geronb/gbp096 [DOI] [PubMed] [Google Scholar]
- Knight B. G., Silverstein M., McCallum T. J., Fox L. S. (2000). A sociocultural stress and coping model for mental health outcomes among African American caregivers in Southern California. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 55, P142–P150. 10.1093/geronb/55.3.P142 [DOI] [PubMed] [Google Scholar]
- Kraemer H. C., Blasey C. M. (2004). Centering in regression analyses: A strategy to prevent errors in statistical inference. International Journal of Methods in Psychiatric Research, 13, 141–151. 10.1002/mpr.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R. S., Folkman S. (1984). Stress, appraisal, and coping. New York, NY: Springer [Google Scholar]
- Lee S., Colditz G. A., Berkman L. F., Kawachi I. (2003). Caregiving and risk of coronary heart disease in U.S. women: A prospective study. American Journal of Preventative Medicine, 24, 113–119. 10.1016/S0749-3797(02)00582-2 [DOI] [PubMed] [Google Scholar]
- Lovallo W., Thomas T. (2000). Stress hormones in psychophysiological research: Emotional, behavioral, and cognitive implications. In Cacioppo J., Tassinary L., Bernston G. (Eds.), Handbook of psychophysiology (pp. 342–367). New York, NY: Cambridge University Press [Google Scholar]
- Lundberg U. (2005). Stress hormones in health and illness: The roles of work and gender. Psychoneuroendocrinology, 30, 1017–1021. 10.1016/j.psyneuen.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Mansi J. A., Drolet G. (1997). Chronic stress induces sensitization in sympathoadrenal responses to stress in borderline hypertensive rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 272, R813–R820 Retreived from http://ajpregu.physiology.org [DOI] [PubMed] [Google Scholar]
- Mathew R. J., Ho B. T., Francis D. J., Taylor D. L., Weinman M. L. (1982). Catecholamines and anxiety. Acta Psychiatrica Scandinavica, 65, 142–147. 10.1111/j.1600-0447.1982.tb00833.x [DOI] [PubMed] [Google Scholar]
- Mausbach B. T., Ancoli-Israel S., von Kanel R., Patterson T. L., Aschbacher K., Mills P. J., … Grant I. (2006). Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer disease. Sleep, 29, 1347–1352 Retrieved from http://www.journalsleep.org/ [DOI] [PubMed] [Google Scholar]
- Mausbach B. T., Aschbacher K., Mills P. J., Roepke S. K., Von Känel R., Patterson T. L., … Grant I. (2008). A 5-year longitudinal study of the relationships between stress, coping, and immune cell ß2-adrenergic receptor sensitivity. Psychiatry Research, 160, 247–255. 10.1016/j.psychres.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach B. T., Chattillion E. A., Moore R. C., Roepke S. K., Depp C. A., Roesch S. (2011). Activity restriction and depression in medical patients and their caregivers: A meta analysis. Clinical Psychology Review, 31, 900–908. 10.1016/j.cpr.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach B. T., Chattillion E., Roepke S. K., Ziegler M. G., Milic M., von Kanel R., … Grant I. (2012). A longitudinal analysis of the relations among stress, depressive symptoms, leisure satisfaction, and endothelial function in caregivers. Health Psychology, 31, 433–440. 10.1037/a0027783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach B. T., Dimsdale J. E., Ziegler M. G., Mills P. J., Ancoli-Israel S., Patterson T. L., Grant I. (2005). Depressive symptoms predict norepinephrine response to a psychological stressor task in Alzheimer’s caregivers. Psychosomatic Medicine, 67, 638–642. 10.1097/01.psy.0000173312.90148.97 [DOI] [PubMed] [Google Scholar]
- Mausbach B. T., Mills P. J., Patterson T. L., Aschbacher K., Dimsdale J. E., Ancoli-Israel S., … Grant I. (2007). Stress related reductions in personal mastery are related to reduced immune cell ß2-adrenergic receptor sensitivity. International Psychogeriatrics, 19, 935–946. 10.1017/S1041610206004364 [DOI] [PubMed] [Google Scholar]
- Mausbach B. T., Roepke S. K., Depp C. A., Moore R., Patterson T. L., Grant I. (2011). Integration of the pleasant events and activity restriction models: Development and validation of a “PEAR” model of negative outcomes in Alzheimer’s caregivers. Behavior Therapy, 42, 78–88. 10.1016/j.beth.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach B. T., Roepke S. K., Ziegler M. G., Milic M., von Känel R., Dimsdale J. E., … Grant I. (2010). Association between chronic caregiving stress and impaired endothelial function in the elderly. Journal of the American College of Cardiology, 55, 2599–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach B. T., von Känel R., Aschbacher K., Roepke S. K., Dimsdale J. E., Ziegler M. G., … Grant I. (2007). Spousal caregivers of patients with Alzheimer’s disease show longitudinal increases in plasma level of tissue-type plasminogen activator antigen. Psychosomatic Medicine, 69, 816–822. 10.1097/PSY.0b013e318157d461 [DOI] [PubMed] [Google Scholar]
- Mills P. J., Yu H., Ziegler M. G., Patterson T., Grant I. (1999). Vulnerable caregivers of patients with Alzheimer’s disease have a deficit in circulating CD62L- T lymphocytes. Psychosomatic Medicine, 61, 168–174 Retreived from http://www.psychosomaticmedicine.org [DOI] [PubMed] [Google Scholar]
- Mills P. J., Ziegler M. G., Patterson T., Dimsdale J. E., Hauger R., Irwin M., Grant I. (1997). Plasma catecholamine and lymphocyte beta 2-adrenergic receptor alterations in elderly Alzheimer caregivers under stress. Psychosomatic Medicine, 59, 251–256 [DOI] [PubMed] [Google Scholar]
- Moore R. C., Harmell A. L., Chattillion E., Ancoli-Israel S., Grant I., Mausbach B. T. (2011). PEAR model and sleep outcomes in dementia caregivers: Influence of activity restriction and pleasant events on sleep disturbances. International Psychogeriatrics, 23, 1462–1469. 10.1017/S1041610211000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. C. (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43, 2412–2414 Retrieved from http://psycnet.apa.org [DOI] [PubMed] [Google Scholar]
- Morris J. C., Storandt M., McKeel D. W., Jr., Rubin E. H., Price J. L., Grant E. A., Berg L. (1996). Cerebral amyloid deposition and diffuse plaques in “normal’’aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology, 46, 707–719. 10.1212/WNL.46.3.707 [DOI] [PubMed] [Google Scholar]
- Nieboer A. P., Schulz R., Matthews K. A., Scheier M. F., Ormel J., Lindenberg S. M. (1998). Spousal caregivers’ activity restriction and depression: A model for changes over time. Social Science & Medicine, 47, 1361–1371. 10.1016/S0277-9536(98)00214-7 [DOI] [PubMed] [Google Scholar]
- Perkins M., Howard V. J., Wadley V. G., Crowe M., Safford M. M., Haley W. E., … Roth D. L. (2012). Caregiving strain and all-cause mortality: Evidence from the REGARDS study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 10.1093/geronb/gbs084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza J. R., Almeida D. M., Dmitrieva N. O., Klein L. C. (2010). Frontiers in the use of biomarkers of health in research on stress and aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 513–525. 10.1093/geronb/gbq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M., Sörensen S. (2003). Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging, 18, 250–267. 10.1037/0882-7974.18.2.250 [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Curran P. J., Bauer D. J. (2006). Computational tools for probing interactions in multiple inear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. 10.3102/10769986031004437 [Google Scholar]
- Roepke S. K., Allison M., Von Kanel R., Mausbach B. T., Chattillion E. A., Harmell A. L., … Grant I. (2012). Relationship between chronic stress and carotid intima-media thickness (IMT) in elderly Alzheimer’s disease caregivers. Stress, 15, 121–129. 10.3109/10253890.2011.596866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. L., Perkins M., Wadley V. G., Temple E. M., Haley W. E. (2009). Family caregiving and emotional strain: Associations with quality of life in a large national sample of middle-aged and older adults. Quality of Life Research, 18, 679–688. 10.1007/s11136-009-9482-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh P., Knight B. G. (2011). The effects of familism and cultural justification on the mental and physical health of family caregivers. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66, 3. 10.1093/geronb/gbq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Beach S. R. (1999). Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association, 282, 2215–2219. 10.1001/jama.282.23.2215 [DOI] [PubMed] [Google Scholar]
- Schulz R., O’Brien A. T., Bookwala J., Fleissner K. (1995). Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. The Gerontologist, 35, 771–791. 10.1093/geront/35.6.771 [DOI] [PubMed] [Google Scholar]
- Stommel M., Schoenborn C. A. (2012). Variations in BMI and prevalence of health risks in diverse racial and ethnic populations. Obesity, 18, 1821–1826. 10.1038/oby.2009.472 [DOI] [PubMed] [Google Scholar]
- Sullivan P., Schoentgen S., DeQuattro V., Procci W., Levine D., Van der Meulen J., Bornheimer J. (1981). Anxiety, anger, and neurogenic tone at rest and in stress in patients with primary hypertension. Hypertension, 3(6 Pt. 2), II-119. 10.1161/01.HYP.3.6_Pt_2.II-119 [DOI] [PubMed] [Google Scholar]
- Topolski T. D., LoGerfo J., Patrick D. L., Williams B., Walwick J., Patrick M. B. (2006). The Rapid Assessment of Physical Activity (RAPA) among older adults. Preventing Chronic Disease, 3, A118 Retreived from http://www.ncbi.nlm.nih.gov [PMC free article] [PubMed] [Google Scholar]
- Tugade M. M., Fredrickson B. L., Feldman Barrett L. (2004). Psychological resilience and positive emotional granularity: Examining the benefits of positive emotions on coping and health. Journal of Personality, 72, 1161–1190. 10.1111/j.1467-6494. 2004.00294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliano P. P., Scanlan J. M., Zhang J., Savage M. V., Hirsch I. B., Siegler I. C. (2002). A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Medicine, 64, 418–435 Retrieved from http://www.psychosomaticmedicine.org/ [DOI] [PubMed] [Google Scholar]
- Vitaliano P. P., Zhang J., Scanlan J. M. (2003). Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin, 129, 946–972. 10.1037/0033-2909.129.6.946 [DOI] [PubMed] [Google Scholar]
- von Känel R., Dimsdale J. E. (2000). Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. European Journal of Haematology, 65, 357–369. 10.1034/j.1600-0609. 2000.065006357.x [DOI] [PubMed] [Google Scholar]
- von Känel R., Mills P. J., Mausbach B. T., Dimsdale J. E., Patterson T. L., Ziegler M. G., … Grant I. (2012). Effect of Alzheimer caregiving on circulating levels of C-reactive protein and other biomarkers relevant to cardiovascular disease risk: A longitudinal study. Gerontology, 58, 354–365. 10.1159/000334219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington University Alzheimer’s Disease Research Center (1999). Global Clinical Dementia Rating (CDR) based on CDR box scores Retrieved from http://www.biostat.wustl.edu/~adrc/cdrpgm/index.html
- Williamson G. M., Schulz R. (1992). Pain, activity restriction, and symptoms of depression among community-residing elderly adults. Journal of Gerontology, 47, P367–P372. 10.1093/geronj/47.6.P367 [DOI] [PubMed] [Google Scholar]
- Wyatt R. J., Portnoy B., Kupfer D. J., Snyder F., Engelman K. (1971). Resting plasma catecholamine concentrations in patients with depression and anxiety. Archives of General Psychiatry, 24, 65–70. 10.1001/archpsyc.1971.01750070067009 [DOI] [PubMed] [Google Scholar]
- Zarit S. H., Femia E. E. (2008). A future for family care and dementia intervention research? Challenges and strategies. Aging and Mental Health, 12, 5–13. 10.1080/13607860701616317 [DOI] [PubMed] [Google Scholar]
- Zoccali C., Mallamaci F., Parlongo S., Cutrupi S., Benedetto F. A., Tripepi G., … Seminara G. (2002). Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation, 105, 1354–1359. 10.1161/hc1102.105261 [DOI] [PubMed] [Google Scholar]