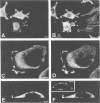
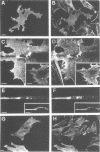
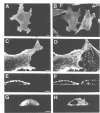
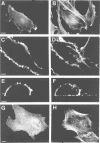
Abstract
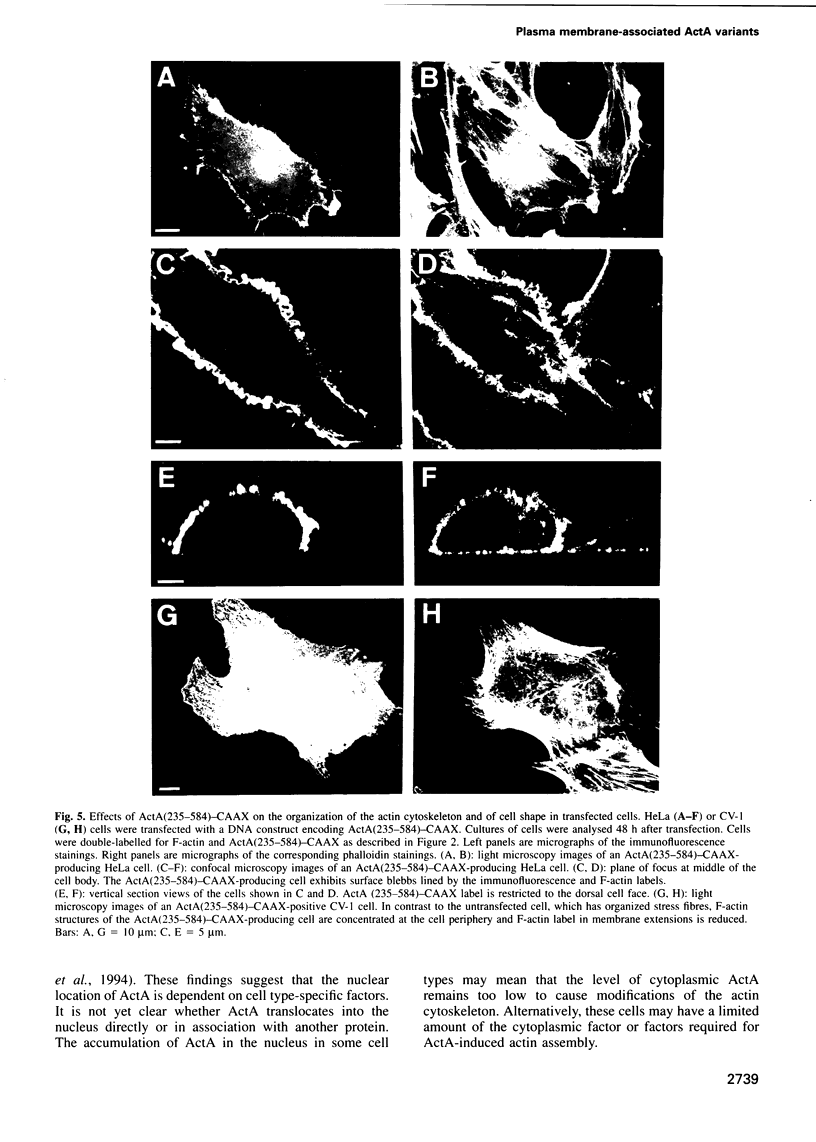
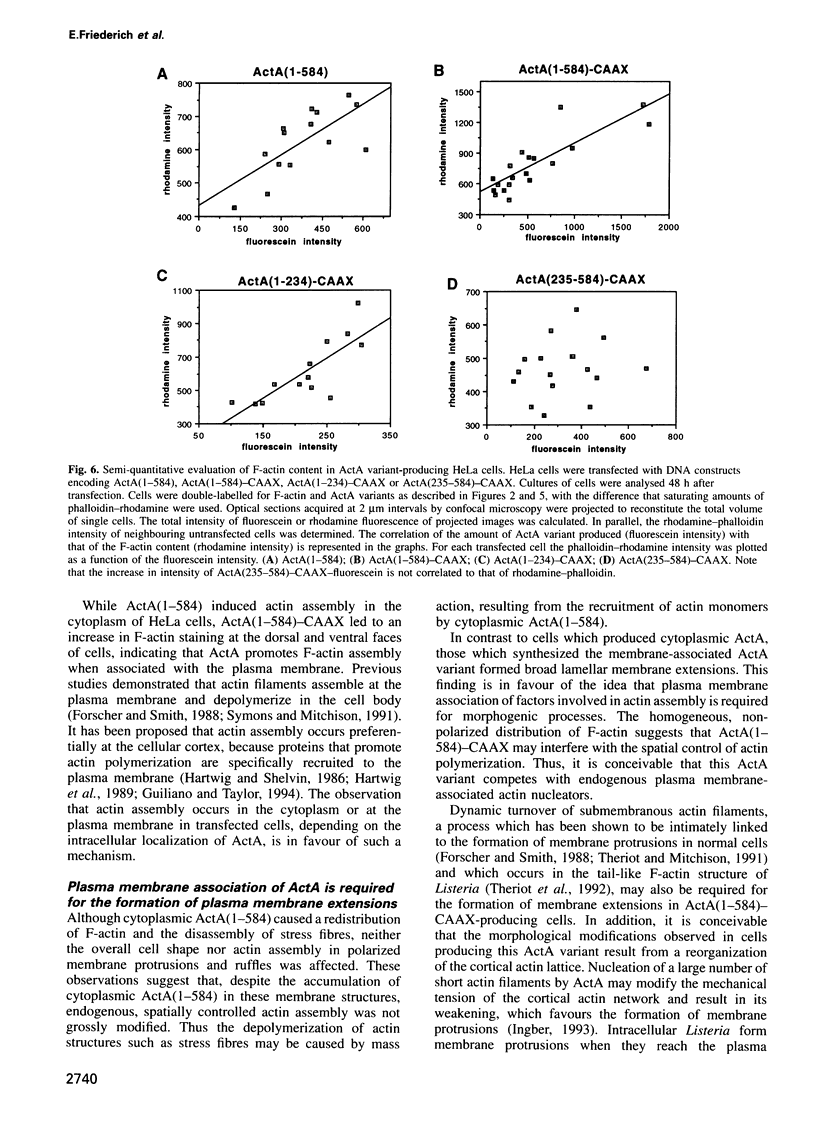
Actin assembly on the surface of Listeria monocytogenes in the cytoplasm of infected cells provides a model to study actin-based motility and changes in cell shape. We have shown previously that the ActA protein, exposed on the bacterial surface, is required for polarized nucleation of actin filaments. To investigate whether plasma membrane-associated ActA can control the organization of microfilaments and cell shape, variants of ActA, in which the bacterial membrane signal had been replaced by a plasma membrane anchor sequence, were produced in mammalian cells. While both cytoplasmic and membrane-bound forms of ActA increased the F-actin content, only membrane-associated ActA caused the formation of plasma membrane extensions. This finding suggests that ActA acts as an actin filament nucleator and shows that permanent association with the inner face of the plasma membrane is required for changes in cell shape. Based on the observation that the amino-terminal segment of ActA and the remaining portion which includes the proline-rich repeats cause distinct phenotypic modifications in transfected cells, we propose a model in which two functional domains of ActA cooperate in the nucleation and dynamic turnover of actin filaments. The present approach is a new model system to dissect the mechanism of action of ActA and to further investigate interactions of the plasma membrane and the actin cytoskeleton during dynamic changes of cell shape.
Full text
PDF

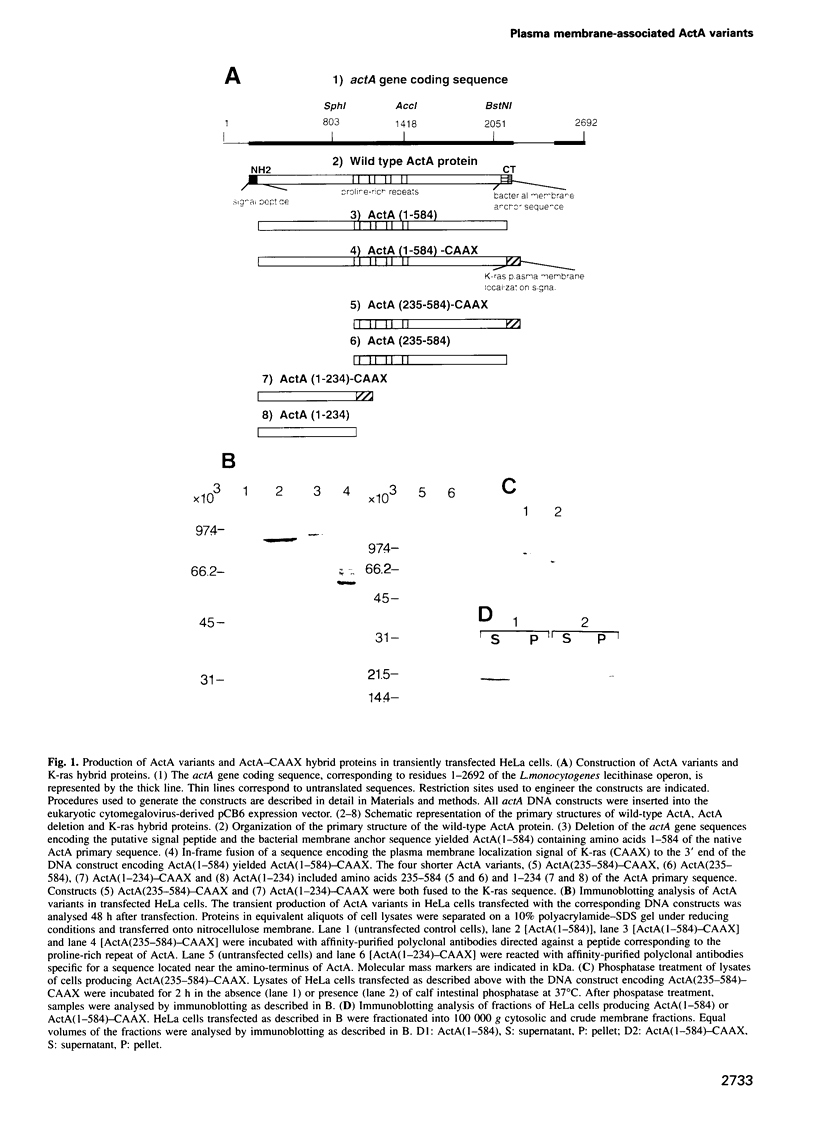

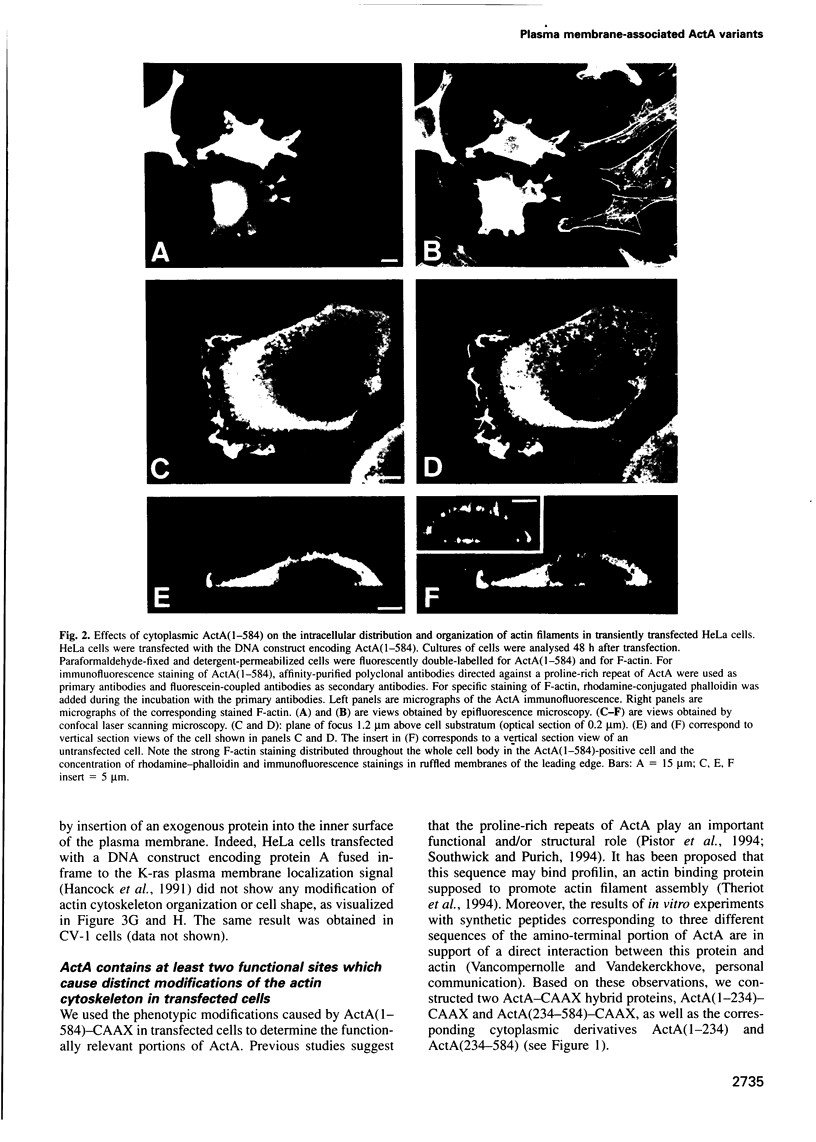
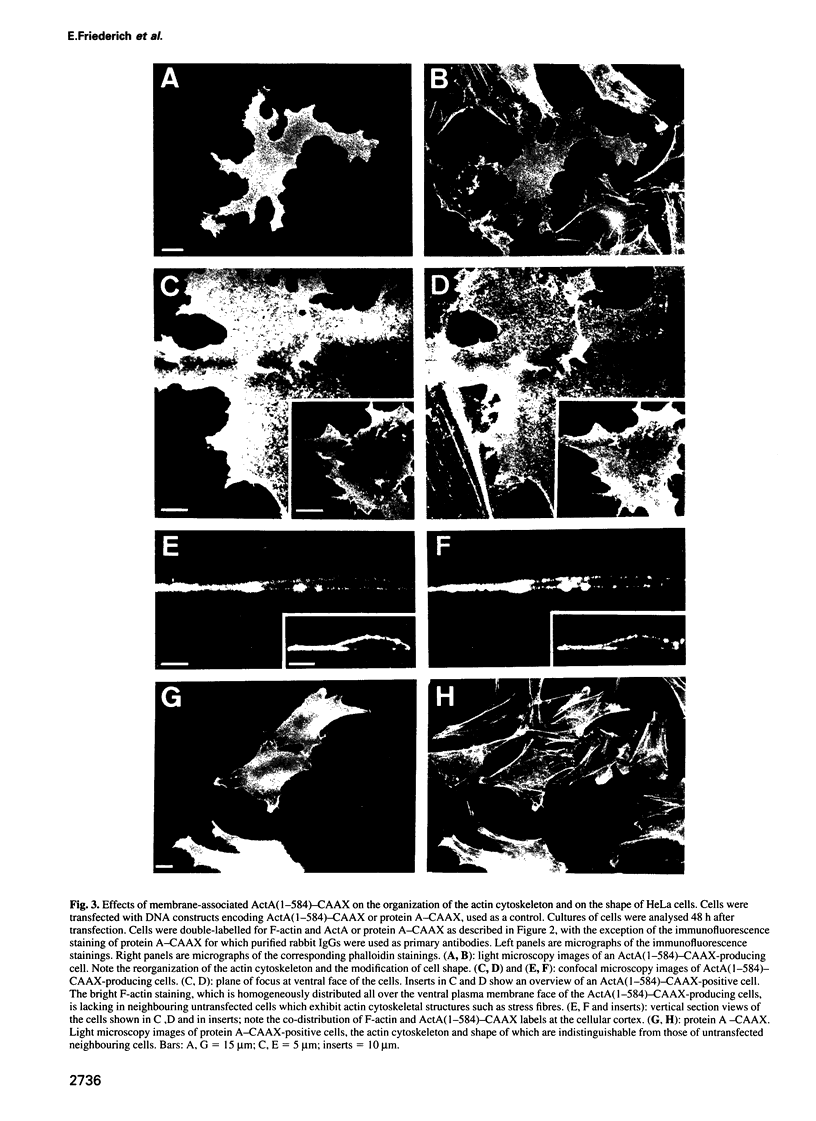
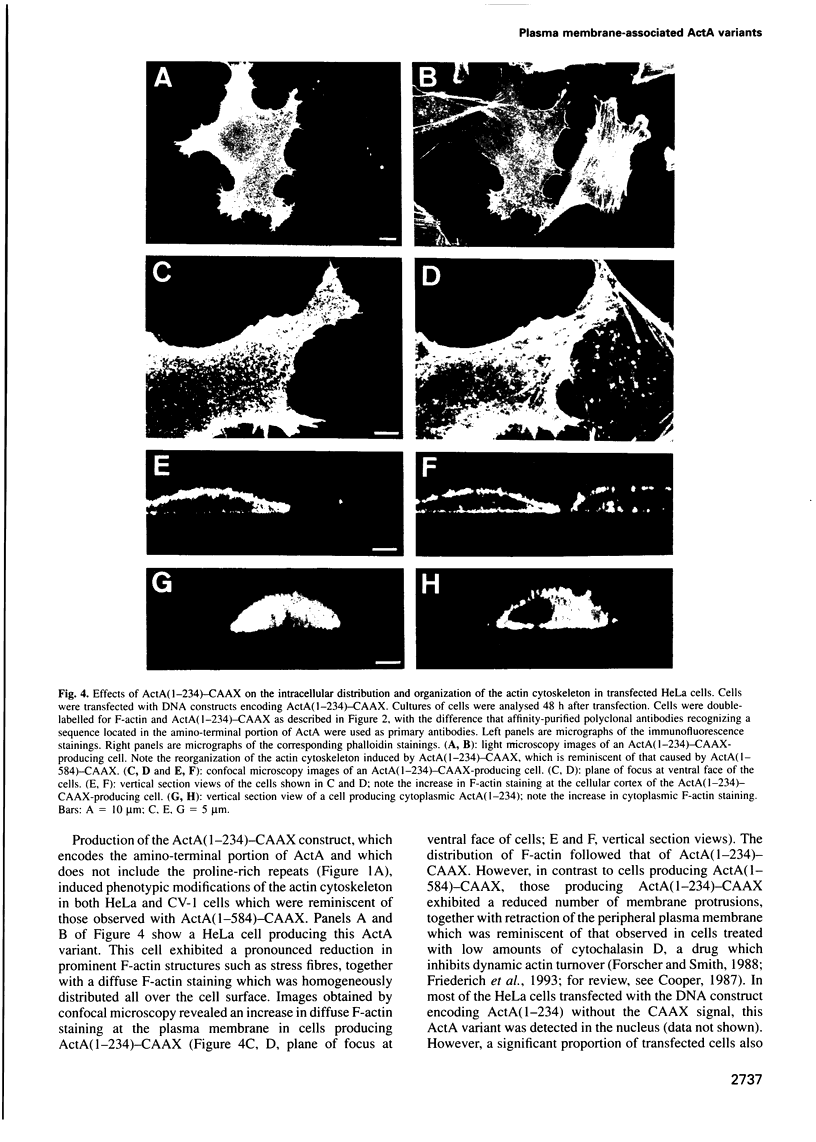





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brundage R. A., Smith G. A., Camilli A., Theriot J. A., Portnoy D. A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cao L. G., Babcock G. G., Rubenstein P. A., Wang Y. L. Effects of profilin and profilactin on actin structure and function in living cells. J Cell Biol. 1992 Jun;117(5):1023–1029. doi: 10.1083/jcb.117.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P. Actin-based bacterial motility. Curr Opin Cell Biol. 1995 Feb;7(1):94–101. doi: 10.1016/0955-0674(95)80050-6. [DOI] [PubMed] [Google Scholar]
- Cossart P., Kocks C. The actin-based motility of the facultative intracellular pathogen Listeria monocytogenes. Mol Microbiol. 1994 Aug;13(3):395–402. doi: 10.1111/j.1365-2958.1994.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. C., Gorlin J. B., Kwiatkowski D. J., Hartwig J. H., Janmey P. A., Byers H. R., Stossel T. P. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992 Jan 17;255(5042):325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasio R. L., Wang L. L., Fisher G. W., Taylor D. L. The dynamic distribution of fluorescent analogues of actin and myosin in protrusions at the leading edge of migrating Swiss 3T3 fibroblasts. J Cell Biol. 1988 Dec;107(6 Pt 2):2631–2645. doi: 10.1083/jcb.107.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold F. G., Sanger J. M., Sanger J. W. Intact alpha-actinin molecules are needed for both the assembly of actin into the tails and the locomotion of Listeria monocytogenes inside infected cells. Cell Motil Cytoskeleton. 1994;28(2):97–107. doi: 10.1002/cm.970280202. [DOI] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Theriot J. A., Dise K. R., Tomaselli G. F., Goldschmidt-Clermont P. J. Dynamic actin structures stabilized by profilin. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1510–1514. doi: 10.1073/pnas.91.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Lin C. H., Thompson C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature. 1992 Jun 11;357(6378):515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Forscher P., Smith S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988 Oct;107(4):1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E., Huet C., Arpin M., Louvard D. Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell. 1989 Nov 3;59(3):461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Friederich E., Kreis T. E., Louvard D. Villin-induced growth of microvilli is reversibly inhibited by cytochalasin D. J Cell Sci. 1993 Jul;105(Pt 3):765–775. doi: 10.1242/jcs.105.3.765. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Taylor D. L. Fluorescent actin analogs with a high affinity for profilin in vitro exhibit an enhanced gradient of assembly in living cells. J Cell Biol. 1994 Mar;124(6):971–983. doi: 10.1083/jcb.124.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B. K., Lillie S. H., Adams A. E., Magdolen V., Bandlow W., Brown S. S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990 Jan;110(1):105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Paterson H., Marshall C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991 Dec;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Chambers K. A., Hopcia K. L., Kwiatkowski D. J. Association of profilin with filament-free regions of human leukocyte and platelet membranes and reversible membrane binding during platelet activation. J Cell Biol. 1989 Oct;109(4 Pt 1):1571–1579. doi: 10.1083/jcb.109.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Shevlin P. The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J Cell Biol. 1986 Sep;103(3):1007–1020. doi: 10.1083/jcb.103.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt A. L., Hartwig J. H., Luna E. J. Ponticulin is the major high affinity link between the plasma membrane and the cortical actin network in Dictyostelium. J Cell Biol. 1994 Sep;126(6):1433–1444. doi: 10.1083/jcb.126.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt A. L., Lu T. H., Luna E. J. Ponticulin is an atypical membrane protein. J Cell Biol. 1994 Sep;126(6):1421–1431. doi: 10.1083/jcb.126.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Ma A. Isolation of rat hepatocyte plasma membranes. II. Identification of membrane-associated cytoskeletal proteins. J Cell Biol. 1983 Jan;96(1):230–239. doi: 10.1083/jcb.96.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993 Mar;104(Pt 3):613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Kocks C., Hellio R., Gounon P., Ohayon H., Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J Cell Sci. 1993 Jul;105(Pt 3):699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Poland T. D. Profilin as a potential mediator of membrane-cytoskeleton communication. Trends Cell Biol. 1993 Nov;3(11):381–385. doi: 10.1016/0962-8924(93)90087-h. [DOI] [PubMed] [Google Scholar]
- Matthias P. D., Bernard H. U., Scott A., Brady G., Hashimoto-Gotoh T., Schütz G. A bovine papilloma virus vector with a dominant resistance marker replicates extrachromosomally in mouse and E. coli cells. EMBO J. 1983;2(9):1487–1492. doi: 10.1002/j.1460-2075.1983.tb01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S., Hirokawa N. Incorporation and turnover of biotin-labeled actin microinjected into fibroblastic cells: an immunoelectron microscopic study. J Cell Biol. 1989 Oct;109(4 Pt 1):1581–1595. doi: 10.1083/jcb.109.4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistor S., Chakraborty T., Niebuhr K., Domann E., Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 1994 Feb 15;13(4):758–763. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio H., Webster P., Louvard D. Use of immunocytochemical techniques in studying the biogenesis of cell surfaces in polarized epithelia. Methods Enzymol. 1983;98:379–395. doi: 10.1016/0076-6879(83)98166-1. [DOI] [PubMed] [Google Scholar]
- Sanders M. C., Wang Y. L. Exogenous nucleation sites fail to induce detectable polymerization of actin in living cells. J Cell Biol. 1990 Feb;110(2):359–365. doi: 10.1083/jcb.110.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff A., Luna E. J. Dictyostelium discoideum plasma membranes contain an actin-nucleating activity that requires ponticulin, an integral membrane glycoprotein. J Cell Biol. 1990 Mar;110(3):681–692. doi: 10.1083/jcb.110.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Isenberg G., Celis J. E. Polarity of actin at the leading edge of cultured cells. Nature. 1978 Apr 13;272(5654):638–639. doi: 10.1038/272638a0. [DOI] [PubMed] [Google Scholar]
- Sohn R. H., Goldschmidt-Clermont P. J. Profilin: at the crossroads of signal transduction and the actin cytoskeleton. Bioessays. 1994 Jul;16(7):465–472. doi: 10.1002/bies.950160705. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Purich D. L. Arrest of Listeria movement in host cells by a bacterial ActA analogue: implications for actin-based motility. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5168–5172. doi: 10.1073/pnas.91.11.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Symons M. H., Mitchison T. J. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991 Aug;114(3):503–513. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temm-Grove C. J., Jockusch B. M., Rohde M., Niebuhr K., Chakraborty T., Wehland J. Exploitation of microfilament proteins by Listeria monocytogenes: microvillus-like composition of the comet tails and vectorial spreading in polarized epithelial sheets. J Cell Sci. 1994 Oct;107(Pt 10):2951–2960. doi: 10.1242/jcs.107.10.2951. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. Actin microfilament dynamics in locomoting cells. Nature. 1991 Jul 11;352(6331):126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J., Tilney L. G., Portnoy D. A. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Rosenblatt J., Portnoy D. A., Goldschmidt-Clermont P. J., Mitchison T. J. Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell. 1994 Feb 11;76(3):505–517. doi: 10.1016/0092-8674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Portnoy D. A. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol. 1990 Dec;111(6 Pt 2):2979–2988. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992 Jul;118(1):71–81. doi: 10.1083/jcb.118.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., DeRosier D. J., Weber A., Tilney M. S. How Listeria exploits host cell actin to form its own cytoskeleton. II. Nucleation, actin filament polarity, filament assembly, and evidence for a pointed end capper. J Cell Biol. 1992 Jul;118(1):83–93. doi: 10.1083/jcb.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranter M. P., Sugrue S. P., Schwartz M. A. Evidence for a direct, nucleotide-sensitive interaction between actin and liver cell membranes. J Cell Biol. 1989 Dec;109(6 Pt 1):2833–2840. doi: 10.1083/jcb.109.6.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland J. A., Kocks C., Dramsi S., Ohayon H., Geoffroy C., Mengaud J., Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992 Jan;60(1):219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuestehube L. J., Luna E. J. F-actin binds to the cytoplasmic surface of ponticulin, a 17-kD integral glycoprotein from Dictyostelium discoideum plasma membranes. J Cell Biol. 1987 Oct;105(4):1741–1751. doi: 10.1083/jcb.105.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]