Abstract
The African protected area (PA) network has the potential to act as a set of functionally interconnected patches that conserve meta-populations of mammal species, but individual PAs are vulnerable to habitat change which may disrupt connectivity and increase extinction risk. Individual PAs have different roles in maintaining connectivity, depending on their size and location. We measured their contribution to network connectivity (irreplaceability) for carnivores and ungulates and combined it with a measure of vulnerability based on a 30-year trend in remotely sensed vegetation cover (Normalized Difference Vegetation Index). Highly irreplaceable PAs occurred mainly in southern and eastern Africa. Vegetation cover change was generally faster outside than inside PAs and particularly so in southern Africa. The extent of change increased with the distance from PAs. About 5% of highly irreplaceable PAs experienced a faster vegetation cover loss than their surroundings, thus requiring particular conservation attention. Our analysis identified PAs at risk whose isolation would disrupt the connectivity of the PA network for large mammals. This is an example of how ecological spatial modelling can be combined with large-scale remote sensing data to investigate how land cover change may affect ecological processes and species conservation.
Keywords: AVHRR, GIMMS, conservation, dispersal, NDVI, protected area network
1. Introduction
As suitable habitat declines, species’ distribution ranges become fragmented and disjointed in sparsely distributed, small populations which are characterized by an increased extinction risk [1–5]. Range fragmentation has already been experienced worldwide in densely populated areas, for example Europe [6]. In Africa, the socio-economic development of the past 30 years has led to severe habitat loss and fragmentation, resulting in an increased and unprecedented threat to wildlife [7–9]. Many large mammal populations in Africa have been declining over the same period [10]. According to future biodiversity scenarios, Africa is expected to be among the continents with the largest predicted habitat loss by 2050 [11].
In this scenario, the network of African protected areas (PAs) is expected to play a fundamental role in future biodiversity conservation. PAs can in fact host sub-populations that form the basis for meta-population dynamics [12,13], where immigrating individuals from source populations can come to the ‘rescue’ of declining sink populations (sink–source dynamics). According to the meta-population theory, the two fundamental processes, extinction and (self-)colonization rates, act in concert with patch area and distances between patches to determine the capacity of a fragmented habitat to support a viable meta-population structure [14,15]. Maximizing and maintaining connectivity between PAs in a fragmented landscape is a fundamental element of the overall population persistence [1,2,16]. Individual PAs play different roles in the maintenance of connectivity in a network, depending on their size and geographical location [17–20], and this can be translated in a measure of their irreplaceability for maintaining the overall level of connectivity of the PA network.
Continuous long-term remote-sensing series, such as those provided by the Advanced Very High-Resolution Radiometer (AVHRR), are valuable tools to analyse land-cover change over time [21,22], making it possible to monitor biodiversity trends [23,24]. In particular, they can be used to infer the land-cover dynamics of PAs [25–28], which are highly relevant for informing management and assessing PA isolation [29,30]. Though widely used for ecological applications, remote-sensing data and methods are still underexploited for conservation monitoring purposes [10,31,32].
In this paper, we combine ecological data on a large set of species of carnivores and ungulate within Africa with Earth observations, to assess the habitat change within PAs in relation to their relative importance within the network. We use a measure of connectivity among PAs based on the spatial arrangement of suitable habitat within each species’ range, to quantify individual PA contribution to the overall connectivity of the network, and hence a value for the irreplaceability of each PA. We couple this measure with an estimated rate of vegetation change inside and around PAs over the past 30 years based on the Normalized Difference Vegetation Index (NDVI). The combination of these variables identifies the African PAs at high risk of loss and high irreplaceability for the connectivity of large mammal populations that should be prioritized for conservation.
2. Material and methods
The study region encompasses 469 PAs on the African continent that intersect with at least one carnivore or ungulate geographical range. The analysis was conducted on a spatial resolution of 10 km.
(a). Protected areas and species distribution data
In total, 76 species of carnivores and ungulates occurring in Africa were used for this study (electronic supplementary material, table S1). Species distributions were derived from habitat suitability models inside species' geographical ranges according to Rondinini et al. [33]. These models were developed through expert-based species–habitat relationships. They include three suitability levels: high (corresponding with the primary habitat of the species), medium (where the species can be found but is not persisting in the absence of primary habitat) and low suitability (where the species is expected to be rarely found) [33]. The models are clipped to geographical ranges obtained from IUCN.1 Median and maximum dispersal distances were calculated from the allometric equations in Santini et al. [34]. The PA data were obtained through the World Database on Protected Areas [35].
(b). Irreplaceability analysis
An individual-based stepwise model that simulates species-specific dispersal among PAs was used to measure the irreplaceability value of each PA in maintaining the network connectivity. This model aims to analyse connectivity by incorporating ecologically relevant information into a landscape configuration analysis. This model is related to meta-population model approaches by incorporating dispersal capabilities but aims to provide a simplistic approach for range connectivity analysis. This model counts the average number of steps from emigration of the source PA to immigration in new PAs by each individual within the geographical range of the species. Immigrations into the source PA are ignored by the model. The suitability of the landscape and the species’ perceptual range was also considered in the analysis. We assigned the above-mentioned categories of habitat suitability with the following values: the ‘low suitability’ category was given high dispersal cost (100 cost units), the ‘medium suitability’ moderate cost (50) and the ‘high suitability’ was given no costs (0). In order to account for a possible impact of the habitat suitability settings, a sensitivity analysis was conducted on the dispersal costs. Therefore, two additional costing schemes were applied which reflect the relative magnitude of the landscape suitability to each other. To analyse the sensitivity concerning the differences within the chosen values, we set the values to 75, 50, 25 and 51, 50, 49 for low, medium and high suitability, respectively. The habitat suitability matrix was used to select the likelihood of the next step location for individuals to move through the landscape between two PAs. This likelihood of selecting a pixel for the next step increases with increasing suitability, owing to the preference of the animals to stay in highly suitable habitat. The default step direction was sampled from a 180° forward angle using the suitability values for the next step movement probability. Owing to the design of the model, only the connectivity is analysed and no dispersal success or post-breeding dispersal probabilities are included. To scale perceptual abilities across different species, we arbitrarily set the perceptual range to be equal to 50% of the species’ median dispersal distance. Higher differentiation of the perceptual range was not feasible because of the spatial resolution used. Note that we did not consider this as the real perceptual ability of the species. Moreover, the presence of PAs within the perceptual range was translated into a doubled probability of a step towards this direction. Individual movements were constrained in a buffer around the source PA, equal to the species’ maximum dispersal distance.
The irreplaceability value of a PA was given by the average difference in search time between the model considering all PAs and the model excluding that particular PA (when a PA does not affect search time because it is isolated, the difference is equal to zero). This analysis was iterated for each PA and computed for each species. For each PA, 100 individuals were released and the simulations were conducted until 80% of the released individuals had immigrated successfully into a PA other than the source. This percentage was set as an internal parameter for the model to terminate in order to account for individuals not emigrating into any PA.
For each species, the search times of all PAs were standardized by the maximum search time per species. The standardized values for all species were summed and assigned to each PA as its irreplaceability value. This is a proxy for the importance of PAs to support the dispersal connectivity for all studied species. The irreplaceability values were standardized for all species and summed up for all PAs. This is reflecting the importance of PAs to support the connectivity of the network.
(c). Land-cover information from remote sensing time-series
Deriving land-cover characteristics and their changes over time to account for the PA status requires a long time-series, which was provided by the Global Inventory Modelling and Mapping Studies (GIMMS) from 1982 to 2006. This dataset provides NDVI values every 10 days based on US National Oceanic and Atmospheric Administration AVHRR datasets on a spatial resolution of 8 km [36]. NDVI is a proxy for vegetation amount and its condition (photosynthetic activity) within a pixel. The NDVI ranges from −1 to 1, with vegetation values being larger than zero. Bare soil and water show values of close to zero and below, respectively. For vegetation, the value increases with the amount of vegetation present and high photosynthetic activity. We computed the mean NDVI value per year for the whole of Africa and resampled the data to 10 km to match the spatial resolution of the irreplaceability analysis.
In order to compare changes of vegetation cover inside and around PAs, we calculated the difference between the annual mean NDVI within each PA and in buffers around each PA. We used three buffers of increasing size, corresponding to the median, and first and fourth quartile of maximum species dispersal distances across all analysed species. This leads to buffers of 17, 31 and 84 km around each PA. The differences were then linearly regressed against time. A positive trend indicates growing difference of vegetation cover inside PAs when compared with their surroundings. A positive trend is therefore interpreted as a higher rate of vegetation cover change around the PA than inside; a flat trend has no change of vegetation cover inside or outside (or an identical trend inside and outside); and a negative trend has a faster change of vegetation cover inside the PA than outside or a faster increase outside the PA.
The analysis was performed in R v. 2.15.2 [37] and GRASS 6.4.2 [38,39], using the following R packages for spatial data manipulation, conversion and exchange as well as general statistical techniques: ‘spgrass6’ [40], ‘raster’ [41], ‘rgdal’ [42], ‘rgeos’ [43], ‘reshape2’ [44] and ‘rsm’ [45] as well as the ‘r.pi.searchtime.iter’ package [46] in the GRASS add-on repository for the irreplaceability and the connectivity analysis.
3. Results
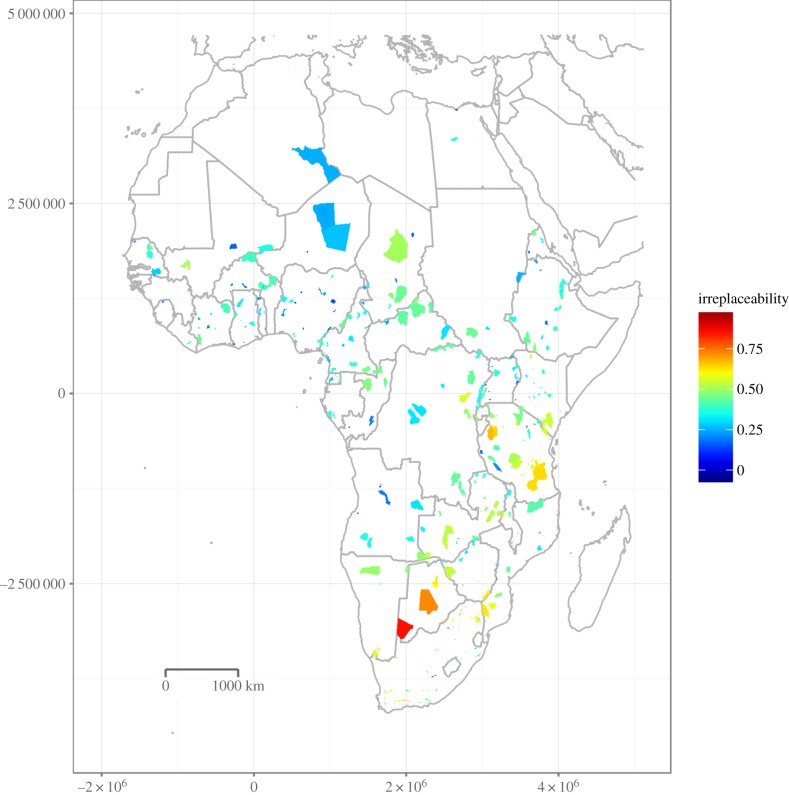
The irreplaceability values ranged from zero to unity in a unimodal distribution, with a median of 0.41 (s.d. 0.15, see the electronic supplementary material, Appendix S1, for histogram). PAs with a high irreplaceability and therefore a high importance for the connectivity or acting as stepping stones (above overall median values) occurred mainly in southern and eastern Africa, namely Namibia, Botswana, Zimbabwe, South Africa, Mozambique, Zambia, Tanzania and Kenya (figure 1). In southern Africa, Etosha (0.49), Kafue (0.53), Kalagadi (0.85) and Kalahari (0.72) feature high irreplaceability values. In East Africa, high irreplaceability values are found for Ruaha (0.54) and Selous (0.63) in Tanzania and a slightly lower value for Serengeti (0.46).
Figure 1.
Irreplaceability of African PAs to maintain connectivity of the PA network for large mammals. High values indicate a high irreplaceability of the PA and are therefore highly important for the connectivity in this area. (Online version in colour.)
Low to moderate irreplaceability (equal and below median values) was assigned to many PAs in Central and West Africa. In Central Africa, the Dzanga-Ndoki (Central African Republic) features a higher irreplaceability than surrounding areas with 0.47, which is comparable to Tai (0.44) in Ivory Coast. Areas with low irreplaceability values are Kabore-Tambie (0.17) in Burkina Faso and Marahoue (0.19) in Ivory Coast. A total of 19% of all PAs have an irreplaceability value higher than 0.5. The values for all PAs are listed in the electronic supplementary material, table S2.
The sensitivity analysis of changing land-cover suitability values did result in highly correlated results (Kendall's tau 0.76–0.87). The results differed by PAs but showed a linear pattern between each of the sensitivity models. The values of all PAs for the different suitability settings are plotted in the electronic supplementary material, figure S2. The map of overall species richness of the data used can be found in the electronic supplementary material, appendix S3.
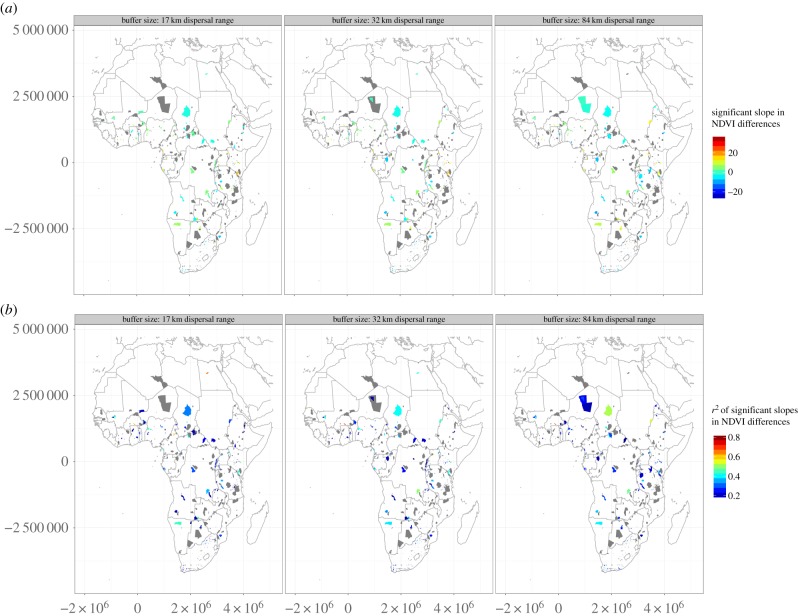
PAs with high importance in southern Africa showed similar trends in vegetation cover compared to the surrounding areas (figure 2). Several PAs in eastern Africa showed a lower rate of vegetation cover change inside PAs than outside. Some PAs lost vegetation cover at a significantly faster rate than the surroundings (21–26% of the PAs, depending on the buffer size, figure 2). In addition, 4–5% of the PAs with high irreplaceability experienced a significantly faster change of vegetation cover inside than that around them.
Figure 2.
Differences of NDVI values inside and outside PAs between 1982 and 2006 (slope values are displayed in the top row and r² values are displayed in the bottom row; values are derived from a linear regression of time against the difference in values). Grey areas experienced no significant change in differences across the time span 1982–2006. Row (a): red areas showed a decrease in the slope of the linear regression of NDVI outside, and blue areas a difference of increasing NDVI outside. Row (b): the r² values are showing high values of the linear regression fit in red, and low values in blue. (Online version in colour.)
The Serengeti National Park lost vegetation cover faster than its surrounding 84 km buffer, but not within its smaller buffers (17 km: n.s.; 32 km: n.s.; 84 km: p = 0.05). Selous or Ruaha in Tanzania showed no differences in either direction. High increase in differences for single PAs can be seen in Uganda and Kenya where the value outside the PAs decreased compared with the ones inside PAs. This is true for Mount Kenya (17 km: p = 0.02; 32 km: p = 0.004; 84 km: p < 0.008) and Matheniko (17 km: p = 0.001; 32 km: p = 0.001; 84 km: p = 0.001) in Uganda. This pattern can also be found for PAs in Central and West Africa, e.g. for the Park W complex part of Benin, the Kabore-Tambi in Burkina Faso and for the Marahoue area in Ivory Coast. Other areas such as the Tai and Comoé National Park in Ivory Coast showed no changes across this time span, as well as areas in the Sahel and Sahara or the Congo Basin.
PAs in Botswana, such as Kalahari and Kgalagadi, are shown in green (figure 2), which indicates low NDVI differences and high importance. Other PAs, for example the Matheniko in Uganda, are depicted in purple, which indicates a low importance and a high increase in NDVI differences inside and outside the PA. The overall pattern reveals that in southern and eastern Africa generally more PAs exist which have a high importance for maintaining the connectivity between species ranges, while at the same time experiencing a low difference in land-cover change within and without.
4. Discussion
Our analysis highlights the capabilities of coupling spatial modelling with large-scale ecological and remote-sensing data for a better understanding of PA status and value in broad-scale geographical domains. In this case, we used NDVI continuous data, which demonstrated an intrinsic power in discriminating among trends in vegetation cover change. Our analytical design accounted for the regional variation in vegetation cover (e.g. forest, savannah, desert), which is indeed the main source of variation among PAs [47,48].
(a). Sensitivity and assumptions of the analysis
As in any model, the results of our analysis depend on the available species distribution data, their accuracy and on the model settings [49]. The former have been evaluated, and perform better than random for ca 95% of the species for which validation data were available [33]. Our sensitivity analysis tested the effect of model parameters on the results, and the model proved robust to changes in their values. The changes between models were linear with a fit of 0.76–0.86; hence the values for the majority of PAs did not experience major differences. The connectivity of the PA network on a local scale depends on the permeability of their surroundings, which may influence population persistence [50]. The dispersal distances we used in our analysis were derived from allometric equations, fitted on dispersal distances obtained from a variety of studies [34]. Thus, they already incorporate the variable permeability of the landscape.
Remote sensing is particularly suitable for detecting land-cover changes because it allows a rapid mapping over wide regions and represents a robust and reproducible tool. This is especially useful when the objects composing land cover maps have been generated by agglomerative methods based on objective algorithms, for example image segmentation. Reviews of this issue can be found in Hay et al. [51] and Blaschke [52], while Karl & Maurer [53] provide empirical examples of their application in land-cover mapping. Various studies have used remotely sensed data successfully for landscape or animal ecology [27] and for PA effectiveness analysis [54]. Remote sensing usually monitors some threats to biodiversity, for example vegetation cover change, including localized ones such as oil spill in the Sahara ([55], this issue). While this may be a limitation for local-scale analyses, pristine vegetation cover change (and deforestation in particular) remains the biggest threat to mammals worldwide [56]. Our analysis implicitly assumes that local effects on vegetation cover change are negligible.
The observed differences inside and outside PAs in vegetation cover change relate to the accuracy in detecting land cover modifications. Loss of rainforest, for example, is easier to detect compared with habitat loss in deserts, even if differences and accuracy differ between approaches [57]. Yet, we detected positive and negative differences throughout the study area, and we assumed an equal probability to detect change unimportant for the biome.
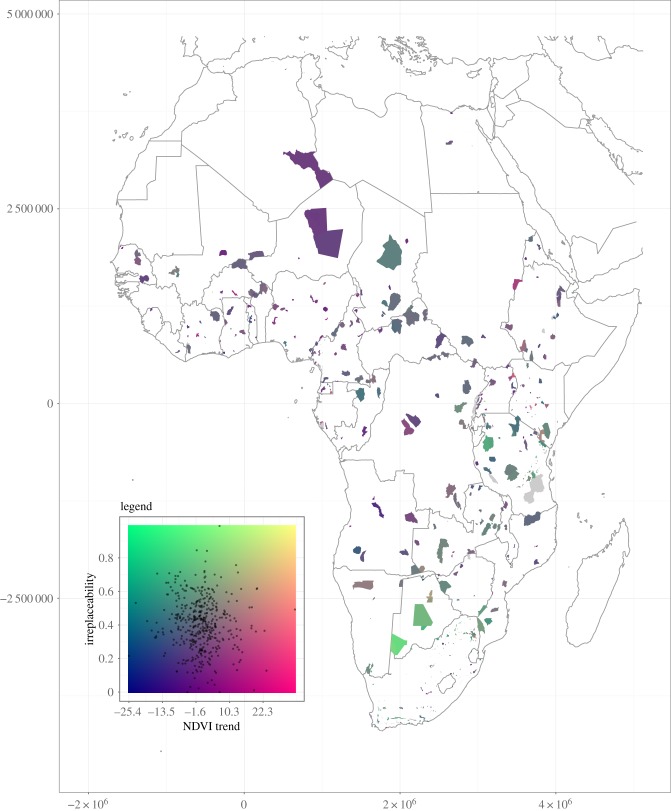
Differences inside and outside PAs may not necessarily depend solely on cover loss outside PAs, but also on cover gain inside PAs. As an example, an increased herbivore population density inside PAs might result in a changing vegetation cover [58] while the surrounding area remains stable. Hence, an increasing difference might not in all cases be the result of human-induced land-cover conversion around PAs. Also the land-cover inside and outside the PAs can change in the same manner, and therefore no differences are detected even though the PA is in a different state than before. Many PAs might have already experienced a change in their surroundings before the start of remote sensing time-series, and therefore these changes cannot be detected. Although the dynamics described above may apply to some PAs, we assumed that all differences detected could be attributed to a decrease of vegetation cover outside PAs (figure 3).
Figure 3.
Illustration of the combination of PA irreplaceability values and differences in NDVI trends inside and outside PAs. Green to yellow areas show high importance of PAs to maintain the connectivity of species ranges, blue to red colouring depicts low importance. Horizontal colouring represents the rate of vegetation cover change inside versus outside and around PAs. Blue indicates faster vegetation loss inside PAs than outside or a stronger increase outside, red indicates faster vegetation loss outside or increase inside PAs (isolation). (Online version in colour.)
(b). Protected area irreplaceability
Our results show that southern and eastern African PAs are the most irreplaceable for maintaining network connectivity. This finding complements and builds upon previous studies that report higher conservation effectiveness of southern African PAs [10]. This result is likely driven by the size of the PAs (on average larger than in West and North Africa) and additionally the higher number of large mammals in the savannah biome [59].
Our measure of irreplaceability is new to our knowledge. It is based on one specific property of the PA network (connectivity) that is not usually accounted for explicitly in conservation planning. Geldmann et al. [54] acknowledged a general shortage of understanding on PA effectiveness, and we believe that our measure can help to fill one of the existing theoretical gaps that prevent a better understanding. Alternative measures of irreplaceability generally estimate the importance of an area for the achievement of a conservation target, which is expressed in terms of number of individuals or area occupied by a species to be included in PAs [60–62]. Complementing our irreplaceability estimates with traditional irreplaceability analysis would provide a more comprehensive picture of the conservation value of African PAs for large mammals.
Mapping irreplaceable areas for connectivity is highly relevant not only for dispersing individuals, whose movements are directly related to population dynamics, but also for migratory species and to estimate the importance of PAs in climate change adaptation [63], as they could function as corridors. Thus, in addition to regional migration (sensu Thirgood et al. [64]) considered here, further analyses should also take large-scale migration into consideration, a task that will soon be feasible at the spatial and temporal extents required [65].
(c). Combining irreplaceability with vulnerability
Our analysis showed that the rate of change in vegetation change inside most African PAs was similar or lower than that in their surroundings. Across the whole African continent, an annual forest decline of 0.14–0.28% (depending on the decade) has been reported [26]. This is likely to be detected by NDVI if occurring in or around forest PAs. By contrast, desert ecosystems are rather difficult to analyse with respect to human impacts or land-cover change (see [55], this issue). Moreover, the spatial location of the PAs and their socio-economic status influence the differences. For example, many PAs in Tanzania are surrounded by conservancies (private land managed similarly to PAs) and hence have a land-cover condition and history comparable to the PA itself, while such land planning is uncommon in West Africa. Correspondingly, PAs that are located within an adjacent PA network, like the W–Arli–Pendjari complex in Benin, Burkina Faso and Niger, are less likely to report a change in NDVI differences than isolated PAs such as the Tai National Park in Ivory Coast.
Our analyses revealed that in past decades 4–5% (depending on the buffer used) of all highly irreplaceable PAs experienced an increasing difference of NDVI values compared with their surroundings, which can be interpreted as an increasing isolation of the natural habitats of these PAs from those of their surroundings. The interaction of habitat status and anthropogenic-induced changes on mammal populations, which is also discussed in detail in [66] and [67], outlines the importance of strengthening the collective contribution for biodiversity conservation. The combination of ecological and remotely sensed information proved to be a highly valuable tool. With increasing access and availability of remote sensing data, it is expected to provide further relevant information [68], with particular importance for the development of standardized measures for biodiversity monitoring [24].
Acknowledgements
We are very grateful to the anonymous reviewers; their comments improved the manuscript significantly. This study has been initialized by the CEOS Biodiversity Workshop at the German Aerospace Center in Munich, October 2012. We are grateful to N. Pettorelli and W. Turner for constructive comments on the analysis.
Endnote
IUCN spatial data download: http://www.iucnredlist.org/technical-documents/spatial-data (accessed 2012).
Funding statement
We are grateful to GEO-D and DLR for financial support of this workshop.
References
- 1.Fahrig L, Merriam G. 1985. Habitat patch connectivity and population survival. Ecology 66, 1762–1768. ( 10.2307/2937372) [DOI] [Google Scholar]
- 2.Fahrig L, Merriam G. 1994. Conservation of fragmented populations. Conserv. Biol. 8, 50–59. ( 10.1046/j.1523-1739.1994.08010050.x) [DOI] [Google Scholar]
- 3.Reed DH. 2004. Extinction risk in fragmented habitats. Anim. Conserv. 7, 181–191. ( 10.1017/S1367943004001313) [DOI] [Google Scholar]
- 4.Caughley G. 1994. Directions in conservation biology. J. Anim. Ecol. 63, 215–244. ( 10.2307/5542) [DOI] [Google Scholar]
- 5.MacArthur RH, Wilson E. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Bruinderink GG, van der Sluis T, Lammertsma D, Opdam P, Pouwels R. 2003. Designing a coherent ecological network for large mammals in northwestern Europe. Conserv. Biol. 17, 549–557. ( 10.1046/j.1523-1739.2003.01137.x) [DOI] [Google Scholar]
- 7.Brooks T, et al. 2002. Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 16, 909–923. ( 10.1046/j.1523-1739.2002.00530.x) [DOI] [Google Scholar]
- 8.Newmark WD, Hough JL. 2000. Conserving wildlife in Africa: integrated conservation and development projects and beyond. BioScience 50, 585–592. ( 10.1641/0006-3568(2000)050[0585:CWIAIC]2.0.CO;2) [DOI] [Google Scholar]
- 9.Newmark WD. 2008. Isolation of African protected areas. Front. Ecol. Environ. 6, 321–328. ( 10.1890/070003) [DOI] [Google Scholar]
- 10.Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, Green RE, Hutton JM. 2010. Large mammal population declines in Africa's protected areas. Biol. Conserv. 143, 2221–2228. ( 10.1016/j.biocon.2010.06.007) [DOI] [Google Scholar]
- 11.Visconti P, et al. 2011. Future hotspots of terrestrial mammal loss. Phil. Trans. R. Soc. B 366, 2693–2702. ( 10.1098/rstb.2011.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanski I. 1998. Metapopulation dynamics. Nature 396, 41–49. ( 10.1038/23876) [DOI] [Google Scholar]
- 13.Hanski I. 1999. Metapopulation ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Hanski I, Ovaskainen O. 2000. The metapopulation capacity of a fragmented landscape. Nature 404, 755–758. ( 10.1038/35008063) [DOI] [PubMed] [Google Scholar]
- 15.Schnell JK, Harris GM, Pimm SL, Russell GJ. 2013. Estimating extinction risk with metapopulation models of large-scale fragmenation. Conserv. Biol. 27, 520–530. ( 10.1111/cobi.12047) [DOI] [PubMed] [Google Scholar]
- 16.Kool JT, Moilanen A, Treml EA. 2012. Population connectivity: recent advances and new perspectives. Landscape Ecol. 28, 165–185. ( 10.1007/s10980-012-9819-z) [DOI] [Google Scholar]
- 17.Baranyi G, Saura S, Podani J, Jordan F. 2011. Contribution to habitat patch network connectivity: redundancy and uniqueness of topological indices. Ecol. Indic. 11, 1301–1310. ( 10.1016/j.ecolind.2011.02.003) [DOI] [Google Scholar]
- 18.Bodin Ö, Saura S. 2010. Ranking individual habitat patches as connectivity providers: integrating network analysis and patch removal experiments. Ecol. Model. 221, 2393–2405. ( 10.1016/j.ecolmodel.2010.06.017) [DOI] [Google Scholar]
- 19.Saura S, Rubio L. 2010. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 33, 523–537. [Google Scholar]
- 20.Rubio L, Saura S. 2011. Assessing the importance of individual habitat patches as irreplaceable connecting elements: an analysis of simulated and real landscape data. Ecol. Complexity 11, 28–37. ( 10.1016/j.ecocom.2012.01.003) [DOI] [Google Scholar]
- 21.Alcaraz-Segura D, Cabello J, Paruelo JM, Delibes M. 2009. Use of descriptor ecosystem functioning for monitoring a national park network: a remote sensing approach. Environ. Manage. 43, 38–48. ( 10.1007/s00267-008-9154-y) [DOI] [PubMed] [Google Scholar]
- 22.Pettorelli N, Vik JO, Mysterud A, Gaillard J-M, Tucker CJ, Stenseth NC. 2005. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol Evol. 20, 503–510. ( 10.1016/j.tree.2005.05.011) [DOI] [PubMed] [Google Scholar]
- 23.Pereira HM, Cooper HD. 2005. Towards the global monitoring of biodiversity change. Trends Ecol. Evol. 21, 123–129. ( 10.1016/j.tree.2005.10.015) [DOI] [PubMed] [Google Scholar]
- 24.Pereira HM, et al. 2013. Essential biodiversity variables. Science 339, 277–278. ( 10.1126/science.1229931) [DOI] [PubMed] [Google Scholar]
- 25.Buchanan G, Nelson A, Mayaux P, Hartley A, Donald PF. 2009. Delivering a global, terrestrial, biodiversity observation system through remote sensing. Conserv. Biol. 23, 499–502. ( 10.1111/j.1523-1739.2008.01083.x) [DOI] [PubMed] [Google Scholar]
- 26.Mayaux P, et al. 2013. State and evolution of the African rainforests between 1990 and 2010. Phil. Trans. R. Soc. B 368, 20120300 ( 10.1098/rstb.2012.0300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettorelli N, Ryan S, Mueller T, Bunnefeld N, Jedrzejewska B, Lima M, Kausrud K. 2011. The Normalized Difference Vegetation Index (NDVI): unforeseen success in animal ecology. Clim. Res. 46, 15–27. ( 10.3354/cr00936) [DOI] [Google Scholar]
- 28.Wiens J, Sutter R, Anderson M, Blanchard J, Barnett A, Aguilar-Amuchastegui N, Avery C, Laine S. 2009. Selecting and conserving lands for biodiversity: the role of remote sensing. Remote Sens. Environ. 113, 1370–1381. ( 10.1016/j.rse.2008.06.020) [DOI] [Google Scholar]
- 29.deFries R, Hansen A, Newton AC, Hansen MC. 2005. Increasing isolation of protected areas in tropical forests over the past twenty years. Ecol. Appl. 15, 19–26. ( 10.1890/03-5258) [DOI] [Google Scholar]
- 30.deFries R, Hansen A, Turner BL, Reid R, Liu J. 2007. Land use change around protected areas: management to balance human needs and ecological function. Ecol. Appl. 17, 1031–1038. ( 10.1890/05-1111) [DOI] [PubMed] [Google Scholar]
- 31.Nagendra H, Rocchini D. 2008. High resolution satellite imagery for tropical biodiversity studies: the devil is in the detail. Biodivers. Conserv. 17, 3431–3442. ( 10.1007/s10531-008-9479-0) [DOI] [Google Scholar]
- 32.Nagendra H, Lucas R, Honrado JP, Jongman RHG, Tarantino C, Adamo M, Mariota P. 2013. Remote sensing for conservation monitoring: assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 33, 45–59. ( 10.1016/j.ecolind.2012.09.014) [DOI] [Google Scholar]
- 33.Rondinini C, et al. 2011. Global habitat suitability models of terrestrial mammals. Phil. Trans. R. Soc. B 366, 2633–2641. ( 10.1098/rstb.2011.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santini L, Di Marco M, Visconti P, Baisero D, Boitani L, Rondinini C. 2013. Ecological correlates of dispersal distance in terrestrial mammals. Hystrix 24, 1–6. [Google Scholar]
- 35.IUCN and UNEP. 2010. The world database on protected areas (WDPA). Cambridge, UK: UNEP-WCMC; See http://www.protectedplanet.net. [Google Scholar]
- 36.Tucker CJ, Pinzon JE, Brown ME, Slayback D, Pak EW, Mahoney R, Vermote E, El Saleous N. 2005. An extended AVHRR 8 km NDVI data set compatible with MODIS and SPOT vegetation NDVI data. Int. J. Remote Sens. 26, 4485–5598. ( 10.1080/01431160500168686) [DOI] [Google Scholar]
- 37.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org. [Google Scholar]
- 38.GRASS Development Team. 2012. Geographic resources analysis support system (GRASS) software, v. 6.4.2 Open Source Geospatial Foundation See http://grass.osgeo.org.
- 39.Neteler M, Bowman MH, Landa M, Metz M. 2012. GRASS GIS: a multi-purpose open source GIS. Environ. Model. Softw. 31, 124–130. ( 10.1016/j.envsoft.2011.11.014) [DOI] [Google Scholar]
- 40.Bivand R. 2013. spgrass6: interface between GRASS 6 and R. See http://cran.at.r-project.org/web/packages/spgrass6/index.html. [Google Scholar]
- 41.Hijmans RJ. 2013. raster: geographic data analysis and modeling. See http://cran.r-project.org/web/packages/raster/index.html. [Google Scholar]
- 42.Bivand R, Keitt T, Rowlingson B. 2013. rgdal: bindings for the geospatial data abstraction library. See http://cran.r-project.org/web/packages/rgdal/index.html. [Google Scholar]
- 43.Bivand R, Rundel C. 2013. rgeos: interface to Geometry Engine – Open Source (GEOS). See http://cran.r-project.org/web/packages/rgeos/index.html. [Google Scholar]
- 44.Wickham H. 2007. Reshaping data with rhe reshape package. J. Stat. Softw. 21, 1–20. [Google Scholar]
- 45.Lenth RV. 2009. Response-surface methods in R, using rsm. J. Stat. Softw. 32, 1–17. [Google Scholar]
- 46.Wegmann M. 2009. r.pi software manual See http://svn.osgeo.org/grass/grass-addons/grass6/raster/r.pi.
- 47.Woodcock CE, Strahler AH. 1987. The factor of scale in remote sensing. Remote Sens. Environ. 21, 311–332. ( 10.1016/0034-4257(87)90015-0) [DOI] [Google Scholar]
- 48.Ricotta C, Avena GC, Volpe F. 1999. The influence of principal component analysis on the spatial structure of a multispectral dataset. Int. J. Remote Sens. 20, 3367–3376. ( 10.1080/014311699213712) [DOI] [Google Scholar]
- 49.De Ornellas P, Milner-Gulland EJ, Nicolson E. 2011. The impact of data realities on conservation planning. Biol. Conserv. 144, 1980–1988. ( 10.1016/j.biocon.2011.04.018) [DOI] [Google Scholar]
- 50.Cabeza M. 2003. Habitat loss and connectivity of reserve networks in probability approaches to reserve design. Ecol. Lett. 6, 665–672. ( 10.1046/j.1461-0248.2003.00475.x) [DOI] [Google Scholar]
- 51.Hay GJ, Marceau DJ, Dube P, Bouchard A. 2001. A multiscale framework for landscape analysis: object-specific analysis and upscaling. Landscape Ecol. 6, 471–490. ( 10.1023/A:1013101931793) [DOI] [Google Scholar]
- 52.Blaschke T. 2010. Object based image analysis for remote sensing. ISPRS J. Photogramm. 65, 2–16. ( 10.1016/j.isprsjprs.2009.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karl JW, Maurer BA. 2010. Multivariate correlations between imagery and field measurements across scales: comparing pixel aggregation and image segmentation. Landscape Ecol. 24, 591–605. ( 10.1007/s10980-009-9439-4) [DOI] [Google Scholar]
- 54.Geldmann J, Barnes M, Coad L, Craigie ID, Hockings M, Burgess ND. 2013. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 161, 230–238. ( 10.1016/j.biocon.2013.02.018) [DOI] [Google Scholar]
- 55.Duncan C, Kretz D, Wegmann M, Rabeil T, Pettorelli N. 2014. Oil in the Sahara: mapping anthropogenic threats to Saharan biodiversity from space. Phil. Trans. R. Soc. B 369, 20130191 ( 10.1098/rstb.2013.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schipper J, et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230. ( 10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 57.Herold M, Mayaux P, Woodcock CE, Baccini A, Schmullius C. 2008. Some challenges in global land cover mapping: an assessment of agreement and accuracy in existing 1 km data sets. Remote Sens. Environ. 112, 2538–2556. ( 10.1016/j.rse.2007.11.013) [DOI] [Google Scholar]
- 58.Asner GP, Levick SR. 2012. Landscape-scale effects of herbivores on treefall in African savannas. Ecol. Lett. 15, 1211–1217. ( 10.1111/j.1461-0248.2012.01842.x) [DOI] [PubMed] [Google Scholar]
- 59.Rondinini C, Stuart S, Boitani L. 2005. Habitat suitability models and the shortfall in conservation planning for African vertebrates. Conserv. Biol. 19, 1488–1497. ( 10.1111/j.1523-1739.2005.00204.x) [DOI] [Google Scholar]
- 60.Pressey RL, Johnson IR, Wilson PD. 1994. Shades of irreplaceability: towards a measure of the contribution of sites to a reservation goal. Biodivers. Conserv. 3, 242–262. ( 10.1007/BF00055941) [DOI] [Google Scholar]
- 61.Ferrier S, Pressey RL, Barret TW. 2000. A new predictor of the irreplaceability of areas for achieving a conservation goal, its application to real-world planning, and a research agenda for further refinement. Biol. Conserv. 93, 303–325. ( 10.1016/S0006-3207(99)00149-4) [DOI] [Google Scholar]
- 62.Margules CR, Pressey RL. 2000. Systematic conservation planning. Nature 405, 243–253. ( 10.1038/35012251) [DOI] [PubMed] [Google Scholar]
- 63.Singh NJ, Milner-Gulland EJ. 2011. Conserving a moving target: planning protection for a migratory species as its distribution changes. J. Appl. Ecol. 48, 35–46. ( 10.1111/j.1365-2664.2010.01905.x) [DOI] [Google Scholar]
- 64.Thirgood S, et al. 2004. Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Anim. Conserv. 7, 113–120. ( 10.1017/S1367943004001404) [DOI] [Google Scholar]
- 65.Wikelski M, Kays RW, Kasdin NJ, Thorup K, Smith JA, Swenson GW., Jr 2007. Going wild: what a global small-animal tracking system could do for experimental biologists. J. Exp. Biol. 210, 181–186. ( 10.1242/jeb.02629) [DOI] [PubMed] [Google Scholar]
- 66.Di Marco M, Buchanan GM, Szantoi Z, Holmgren M, Grottolo Marasini G, Gross D, Tranquilli S, Boitani L, Rondinini C. 2014. Drivers of extinction risk in African mammals: the interplay of distribution state, human pressure, conservation response and species biology. Phil. Trans. R. Soc. B 369, 20130198 ( 10.1098/rstb.2013.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Saout S, et al. 2013. Protected areas and effective biodiversity conservation. Science 342, 803–805. ( 10.1126/science.1239268) [DOI] [PubMed] [Google Scholar]
- 68.Turner W. 2013. Satellites: make data freely accessible. Nature 498, 37 ( 10.1038/498037c) [DOI] [PubMed] [Google Scholar]