Abstract
Variation is key to the adaptability of species and their ability to survive changes to the Earth's climate and habitats. Plasticity in movement strategies allows a species to better track spatial dynamics of habitat quality. We describe the mechanisms that shape the movement of a long-distance migrant bird (turkey vulture, Cathartes aura) across two continents using satellite tracking coupled with remote-sensing science. Using nearly 10 years of data from 24 satellite-tracked vultures in four distinct populations, we describe an enormous amount of variation in their movement patterns. We related vulture movement to environmental conditions and found important correlations explaining how far they need to move to find food (indexed by the Normalized Difference Vegetation Index) and how fast they can move based on the prevalence of thermals and temperature. We conclude that the extensive variability in the movement ecology of turkey vultures, facilitated by their energetically efficient thermal soaring, suggests that this species is likely to do well across periods of modest climate change. The large scale and sample sizes needed for such analysis in a widespread migrant emphasizes the need for integrated and collaborative efforts to obtain tracking data and for policies, tools and open datasets to encourage such collaborations and data sharing.
Keywords: avian scavengers, vultures, movement ecology, migration, geographical variability, remote-sensing observations
1. Introduction
Avian migration has mystified humanity for thousands of years. However, until the emergence of porro-prism binoculars in the late 1800s [1] and modern bird banding in the early 1900s [2], the study of this phenomenon was anecdotal at best. More recently, satellite tracking has allowed researchers to follow the movements of individual birds at frequent and systematic intervals, enabling analyses of dynamic environmental conditions along movement tracks [3]. Satellite tracking coupled with the emerging discipline of movement ecology offers a new working paradigm for understanding the internal and external factors that affect the movements of birds and, in turn, their behavioural ecology and conservation biology [4]. Three decades of studies involving satellite tracking have served avian ecologists well in helping to determine the general routes and destinations of free-ranging migratory birds and describing previously unknown movements and species distributions, often with important conservation implications [5–8].
Despite these incredible achievements, several gaps in knowledge still remain. First, owing to both logistical and financial constraints, most satellite-tracking studies have followed the movements of only a handful of individuals from single regional populations (notable exceptions include [9,10]). As a result, the sample sizes of most existing studies have restricted the use of robust statistics and hypothesis testing [11,12]. Additionally, the limited spatial extents involved in most of these studies may not be fully representative of a species’ broader migratory syndrome [4,13–15] (but see [3,16]). To date, moreover, research has mainly focused on ‘complete migrants’ (sensu [17]; i.e. species in which all or almost all members of the species migrate), despite the fact that most birds are ‘partial migrants’ (sensu [17]; i.e. species in which some but not all members of the species migrate) [18,19]. This is unfortunate, as studies of partial migrants offer researchers the opportunity to test the degree to which external factors affect the likelihood of migratory behaviour in these more ‘facultative’ migrants, whose geographically responsive migration syndromes are more likely to be affected by environmental variables than are those of complete migrants (see [18]).
In this study, we combine an unprecedentedly large collection of movement tracks collected over a 10 year period and including four geographically distinct populations with large-scale environmental datasets to assess the direct effects of external environmental conditions on the movement ecology of an abundant, widespread and partially migratory avian scavenger, the turkey vulture (Cathartes aura). While carrying out this assessment, we consider the movement ecology paradigm [4], which calls for comprehensive models that combine internal and external drivers of movement in predicting the consequences of movement (i.e. location, home range, survival) [4]. Such a paradigm leads us to expect that the observed movements of turkey vultures represent an energetic balance between the need to move (i.e. the energetic cost and consequences of not moving), the benefit of the outcomes of movements (i.e. better habitat as the outcome of migration or intake of food as the outcome of foraging movement), and the energetic costs of movement itself [20,21]. Under this paradigm, environmental variables directly affect the need to move by governing the amount and density of food, and also directly affect the capacity to move by governing the energetic cost of movement, which for soaring species is controlled via variation in wind speed and uplift.
We test a series of predictions drawn from two main hypotheses. First, we hypothesize that (H1) there will be significant differences in movement characteristics between populations, individuals, migration seasons and geographical locations. Specifically, we predict that (Pr1) populations engaging in long-distance migration will travel faster than those migrating shorter distances; (Pr2) vultures will travel more slowly but will demonstrate more tortuosity in their paths when not migrating than when migrating; and (Pr3) all populations will travel faster on their return migrations in spring than on the outbound migrations in autumn. Some of the variations in movement characteristics measured in (H1) should be driven by external variables; we therefore hypothesize that (H2) environmental variables that are indicative of the conditions that drive flight, and the density and availability of forage will affect the ability to fly and the need to fly (respectively) and thus will affect movement characteristics. Because parameters governing the decision not to move are key components of any movement model, an important characterization of movement ecology is time spent moving, and we predict that (Pr4) environmental conditions that reduce the capacity to fly, such as strong head winds and cross winds coupled with reduced thermal formation, will lead to more time spent roosting and less time spent moving (see [22]). We also predict that (Pr5) environmental variables that relate to the mechanistic drivers of flight, such as tailwind and thermal uplift, will affect flight speed. However, (Pr6) the strength of the effects of these environmental variables, on flight speed and time spent moving, will vary between different movement types, seasons and populations. We also expect that (Pr7) home-range size, a parameter that encompasses the results of movement at all temporal scales within a season, will show consistent variation with the environmental conditions experienced by the animals during the non-migratory seasons (i.e. breeding and non-breeding). Specifically, environmental variables that make flight more energetically cost-effective will lead to larger home ranges, whereas variables that are indicative of increased food availability will result in a reduced need to move and thus a smaller home range [23,24]. Finally, because of seasonal differences in the internal drivers of movement, we expect that (Pr8) the effect of environmental variables on home-range size will be different on the breeding versus wintering grounds.
2. Material and methods
(a). Study species: turkey vultures (Cathartes aura)
The turkey vulture is the world's most abundant and widely distributed obligate avian scavenger, and appears to be adapting to or pre-adapted to human-caused changes in the environment better than its Old World counterparts, many of whom are facing steep population declines [25]. The species' global population, which exceeds five million individuals, appears to be increasing. Overall, the species has a distributional range of more than 27 million km2 that stretches from 53° N in western Canada in North America to 55° S in Tierra del Fuego in South America as well as across most of the West Indies and the Falkland Islands. The turkey vulture is a partial migrant (sensu [18])—populations that breed north of 30° N and south of about 30° S tend to migrate equator-ward in boreal and austral winter, respectively, and populations at the more equatorial latitudes in between are largely or entirely non-migratory [26]. At least two million turkey vultures migrate annually between high-latitude breeding areas in North America and tropical wintering grounds in Central and South America [19,27]. As is true of many migratory birds, turkey vultures appear to be leap-frog migrants (sensu [18])—species in which populations breeding at high-latitudes migrate substantially farther and ‘leap over’ populations breeding at lower latitudes, thereby reversing their latitudinal relationship between seasons. As a result, although individuals in many geographical areas migrate only dozens to hundreds of miles seasonally, others at higher latitudes in the northern hemisphere are trans-equatorial, long-distance migrants [19]. Factors underlying the complex nature of this migration behaviour have yet to be studied in detail.
Turkey vultures are also obligate soaring migrants (sensu [19]) that depend upon low-cost soaring flight to complete their long-distance movements [19] and that travel in large flocks of up to tens of thousands of individuals, often along thermal corridors (sensu [18]). The scale of flocking in the species, at least in populations that migrate long distances, makes feeding during such times highly unlikely [19], and all but certainly necessitates the effective use of low-cost soaring flight, which considerable earlier work [3] indicates is possible in the species. Behavioural observations of obligate soaring migrants demonstrate that birds flying in large flocks flap less and soar more efficiently than those travelling alone or in smaller flocks [28,29]. A recent analysis of turkey vulture movements [30] indicates that the species preferentially uses thermal updrafts for soaring flight and actively seeks locations and times when available thermals are stronger.
(b). Turkey vulture dataset
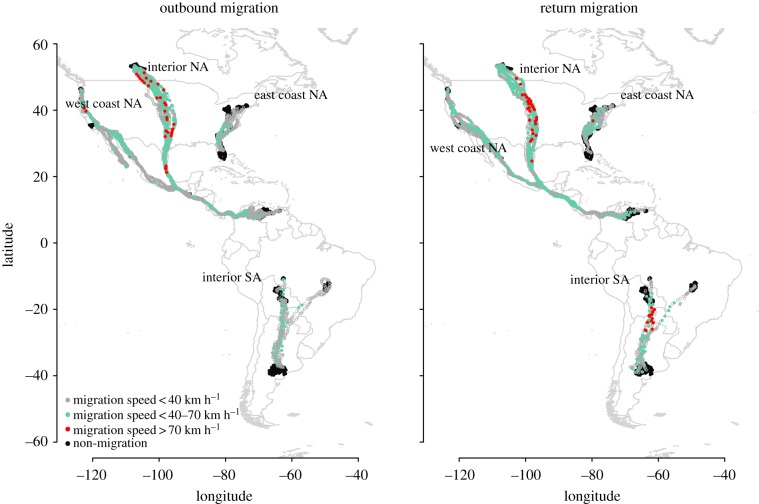
Between 2003 and 2011, we fitted 24 turkey vultures from four geographically distinct populations representing three subspecies with GPS–satellite transmitters in four distinct geographical areas across the species’ New World range (table 1). The geographical areas related to these four populations are as follows (figure 1):
P1 west coast of North America (meridionalis subspecies): migration extends over western North America (tracked during 2005–2010).
P2 east coast of North America (septentrionalis): migration extends over eastern North America (tracked during 2003–2013).
P3 interior North America (meridionalis): migration extends from Canada to South America across central regions of North America (tracked during 2009–2013).
P4 interior South America (ruficollis): migration extends over central South America (tracked during 2009–2013).
Table 1.
Summary of turkey vulture movement data used in the analysis.
| population | bird name | migration status | start time (GMT) | end time (GMT) | average sampling interval (h) | no. valid points |
|---|---|---|---|---|---|---|
| P1 (west coast of North America) | Morongo | migratory | 2006-04-10 | 2009-04-05 | 1 | 19 868 |
| Rosalie | migratory | 2006-04-11 | 2010-03-28 | 1 | 28 101 | |
| Sarkis | migratory | 2006-02-25 | 2007-07-05 | 1 | 8451 | |
| Prado | non-migratory | 2005-11-02 | 2009-07-07 | 1 | 20 967 | |
| P2 (east coast of North America) | Mary | non-migratory | 2009-10-20 | 2012-10-21 | 3 | 3299 |
| Irma | non-migratory | 2004-09-06 | 2013-03-18 | 1 | 18 314 | |
| Schaumboch | migratory | 2004-10-08 | 2006-03-29 | 1 | 3083 | |
| Disney | migratory | 2004-10-11 | 2011-10-18 | 1 | 28 578 | |
| Butterball | migratory | 2003-11-14 | 2004-03-14 | 1 | 1275 | |
| Mark | migratory | 2009-10-26 | 2012-10-03 | 3 | 5353 | |
| P3 (interior of North America) | Steamhouse 1 | migratory | 2009-09-25 | 2011-11-25 | 3 | 5655 |
| Steamhouse 2 | migratory | 2009-09-30 | 2013-03-19 | 3 | 9416 | |
| Sill | migratory | 2005-06-19 | 2005-10-31 | 1 | not valid | |
| Ranger | migratory | 2007-08-05 | 2007-12-01 | 1 | not valid | |
| Leo | migratory | 2007-09-24 | 2013-03-15 | 1 | 32 947 | |
| Blizzard | migratory | 2005-06-22 | 2005-10-07 | 1 | not valid | |
| Duck Lake | migratory | 2006-06-21 | 2006-10-27 | 1 | not valid | |
| Mac | migratory | 2007-09-27 | 2008-09-13 | 1 | 7864 | |
| P4 (interior of South America) | Young Luro | migratory | 2009-03-31 | 2012-09-19 | 3 | 7658 |
| Domingo | migratory | 2011-03-20 | 2013-04-24 | 3 | 5040 | |
| Whitey | migratory | 2011-03-26 | 2012-10-12 | 3 | 3564 | |
| Sabado | migratory | 2009-03-07 | 2009-04-14 | 3 | not valid | |
| La Pampa | migratory | 2011-04-16 | 2012-09-26 | 3 | 4032 | |
| Argentina | migratory | 2011-04-19 | 2012-09-23 | 3 | 4058 | |
| total | 19 valid tracks | 217 523 |
Figure 1.
Turkey vulture tracks and their migration speeds in four geographically distinct migratory breeding populations: west coast of North America, east coast of North America, interior of North America and interior of South America.
Four adults were trapped in California using walk-in traps in November during the non-breeding season or in February–March during return (spring) migration (see [31]). Six adults were trapped in September–November at or near their breeding sites in Pennsylvania using padded-leg hold traps and monofilament noose traps (see [31]). Eight birds (seven adults and one juvenile) were trapped in summer on their nests during the breeding season in Saskatchewan, Canada. Six birds (four adults and two juveniles) were trapped in February–March during the breeding season in La Pampa, Argentina, using walk-in and noose-string traps. All birds were fitted with tracking units and released within 45 min of capture. Transmitters were attached as backpacks using 11 mm Teflon ribbon (Bally Ribbon Mills, Bally, PA, USA) [10]. We used solar-powered GPS satellite transmitters, 14 of which were 70 g (PTT-100 models, Microwave Telemetry, Columbia, MD, USA) programmed to collect GPS locations every hour, and 10 of which were 40 g (Model 40 GPS, Northstar Science and Technology, King George, VA, USA) programmed to collect GPS locations every 3 h.
Vultures breeding on the east coast of North America are partial migrants that travel about one-third as far as those breeding along the west coast of North America and the interior of South America, and only about one-fourth as far as those breeding in the interior of North America. The abbreviated migration of east coast birds results, at least in part, from the fact that their land-restricted migration corridor ends at the tip of the Florida Peninsula and that the birds are unwilling to fly more than 100 km across the straits of Florida to overwinter in the Greater Antilles (cf. [19]). The longer distances travelled by other North American populations most likely reflect the fact that these populations use land-based corridors (i.e. Mexico and Central America) that extend farther south than does peninsular Florida, coupled with the fact that the species, like most raptors, appears to be leap-frog migrants in which northern-most populations overfly more southerly populations and winter beyond them (cf. [17]). Importantly, long-distance migrants from Canada belong to a larger subspecies (meridionalis) than the residential South American subspecies (ruficollis) with which they interact on the wintering grounds in northern South America; evidence suggests that these long-distance migrants outcompete the South American residents for food when the two feed together at carcasses [32] and that the residential subspecies moves into less productive habitats when the migrants are present [33].
(c). Definitions of seasons
Vulture movements were categorized temporally during the year, and accordingly, tracks were manually annotated as breeding season, non-breeding season, outbound migration and return migration. Breeding season was defined as the period after return migration ended and before outbound migration began. The non-breeding (or wintering) season was defined as the period after outbound migration stopped and before return migration started. Outbound migration was defined as the period when migrants moved from their breeding grounds to their non-breeding grounds [19]. Return migration was defined as the time when migrants moved from their non-breeding grounds to their breeding grounds [19].
The exact beginnings and ends of both migrations were determined by the change in an individual's movements from multi-directional ranging movements within the breeding or non-breeding grounds to geographically directed non-return movements of at least 20 km day−1, either towards the individual's breeding grounds (for return migration) or non-breeding grounds (for outbound migration).
(d). Tracking data preparation
The pre-processing of raw turkey vulture data collected from GPS tags involved truncating tracks to their valid time ranges and identifying and removing location outliers. Table 1 describes only valid tracking points that remained following data pre-processing.
(i). Truncating tracks in time
Tracking data were truncated manually to include only complete migration cycles, including finished segments of outbound and return migrations as well as complete breeding and wintering seasons. Shorter tracks and segments of incomplete migrations (owing to a bird's death or tracking-unit failures) were eliminated. Overall 32% of the original collected movement points were excluded, mostly because they represented partial tracks that do not include a full annual cycle or were collected while the tags were not deployed, and in rare cases, because they represent corrupt or outlier values (see §2d(ii)). Valid time ranges for tracks are in table 1.
(ii). Filtering outliers
A number of records in the original dataset were obvious outliers, likely the result of corrupted data transfer. In this analysis, we applied a ‘speed filter’ in which speeds greater than 109 km h−1 were considered outliers and were excluded from the analysis. This threshold value was based on the overall distribution of speed in the study. It is possible that at least some of the records above our speed threshold are valid locations of birds that were moving at high altitudes where winds, particularly tailwinds, are higher; however, the overall effect of this threshold was quite small and excluded only 38 points out of a total of 256 483 points (i.e. 0.01%) within the original tracking dataset.
(e). Environmental data annotation
Remote-sensing data and data from global weather reanalyses (i.e. models that merge simulations and ground-based and remote-sensing observations) were used to generate information about specific environmental conditions. We assume that the chosen environmental variables are effective indexes of conditions that influence movement, either because they are known to control the capacity to move directly, or (and) because they show strong correlations with many other weather and environmental variables. Table 2 summarizes a list of the relevant environmental variables that were annotated to the turkey vulture tracking data in this study.
Table 2.
Environmental variables annotated to turkey vulture movement data. The environmental-data automated track annotation (Env-DATA) system is used for annotation.
| Env-DATA data service | original data source | annotated variable | temporal resolution | spatial resolution |
|---|---|---|---|---|
| MODIS land (MYD13C1) | NASA (https://lpdaac.usgs.gov) | Normalized Difference Vegetation Index (NDVI) | 16 day | 5.6 km (0.05°) |
| ECMWF global atmospheric reanalysis | ECMWF (http://www.ecmwf.int) | 10 m U/V wind components (m s−1), 2 m temperature (K) | 6 h | 0.7° |
| derived wind variables for flight | calculated derived variables, based on ECMWF data | tailwind support and cross wind (m s−1), thermal uplift velocity (m s−1) | 6 h | 0.7° |
The Normalized Difference Vegetation Index (NDVI) is calculated as the ratio between the spectral absorbance of the land surface in the near infrared and the visible wavelength bands [34]. Extensive work has shown that NDVI provides a reasonable approximation of vegetation density [35]. NDVI is often used as an indirect measure of primary productivity, has been shown to correlate strongly with the density and abundance of herbivores [36–38], and may further be indicative of predator movements which respond to herbivore density [39]. Our expectation here is that for scavenging carrion eaters such as the turkey vulture, more productive areas would likely attract more wild herbivores or husbandry, or both, potentially increasing food availability for individuals in the areas of higher NDVI.
During migration (i.e. a time when movement is directional and feeding is minimal), we expect weather conditions and particularly tailwind speed and uplift intensity to affect performance and energetics of the vultures, with stronger tailwinds (and corresponding weaker headwinds), leading to lower cost of movement and faster migration. Temperature should also be important, because it has a major direct effect on the development of thermal uplift. Because thermals are not directly resolved by weather reanalyses models, temperature may provide a better, though indirect, indicator for movement capacity [40]. Higher temperature will lead to greater thermal uplift, and therefore correspond with improved capacity to move at any season and larger home ranges in non-migratory seasons [3,30,41]. Temperature may also have direct impact on vultures through its effects on metabolic rate and insulation, and may provide a good indicator for the general weather conditions. Particularly at seasonal scales, temperature correlates with many other environmental variables such as precipitation.
To access the environmental data used in our analysis, we used the environmental-data automated track annotation (Env-DATA) system [42] to co-register the vulture tracking data with ambient atmospheric observations and underlying landscape information from existing remote-sensing datasets and weather reanalyses. Env-DATA is a service on Movebank (http://www.movebank.org) [43], an open, online system for management, archiving, analysis and sharing of animal movement data. The NDVI data are provided by MODIS (NASA Land Processes Distributed Active Archive Center, USGS/Earth Resources Observation and Science Center, Sioux Falls, SD, USA). The European Centre for Medium-Range Weather Forecasts (ECMWF) global reanalysis dataset [44] was accessed to annotate the tracks with surface air temperature and wind velocity U and V wind components (i.e. wind blowing to the east and north, respectively), as well as thermal uplift velocity [30], a variable derived by Env-DATA using ECMWF data. Using the ECMWF wind data, tailwind support [45] was computed using the wind velocity components and the bird's movement direction (as calculated from consecutive locations). For all environmental variables, data values from the original products were annotated to the tracks using inverse weighted distance interpolation in space and time.
(f). Movement-characteristics computation
After data pre-processing, movement characteristics including speed, azimuth (i.e. movement heading) and path tortuosity (as estimated by the straightness index of the movement trajectory [46]) were computed using the sequential geographical tracking coordinates. Computational methods for path straightness and supporting R codes are in the electronic supplementary material, I.
Average movement speeds and straightness indices (i.e. the inverse of tortuosity) during each season for individuals and the four populations are shown in table 3. Table 4 summarizes, for each population, important migration-related movement parameters, including average duration of each migration track, overall distance travelled, daily activity budget, average ground speed, average straightness index, average start and end dates of each migration course, and the average start and end location of each migration course.
Table 3.
Movement speed and path straightness information for outbound and return migrations (‘s.d.’ represents standard deviation).
| population | bird name | mean ± s.d. speed (km h−1) |
max. speed (km h−1) |
mean straightness index (no unit) |
|||
|---|---|---|---|---|---|---|---|
| outbound | return | outbound | return | outbound | return | ||
| P1 (west coast of North America) | Morongo | 19.0 ± 10.2 | 24.2 ± 11.7 | 77.1 | 53.1 | 0.86 | 0.93 |
| Rosalie | 24.7 ± 12.1 | 25.7 ± 12.4 | 73.9 | 66.7 | 0.96 | 0.95 | |
| Sarkis | 30.2 ± 12.1 | 19.3 ± 8.4 | 58.1 | 39.8 | 0.97 | 0.88 | |
| P1 (all birds) | 20.8 ± 11.2 | 24.5 ± 11.9 | 77.1 | 66.7 | 0.89 | 0.94 | |
| P2 (east coast of North America) | Schaumboch | 14.2 ± 8.9 | 17.6 ± 11.8 | 51.2 | 64.8 | 0.92 | 0.93 |
| Disney | 16.2 ± 10.3 | 18.9 ± 11.4 | 56.9 | 80.4 | 0.89 | 0.91 | |
| Butterball | 14.4 ± 7.6 | 25.2 ± 17.2 | 32.8 | 61.6 | 0.93 | 0.96 | |
| Mark | 9.1 ± 2.9 | 17.3 ± 10.5 | 13.8 | 40.03 | 0.84 | 0.86 | |
| P2 (all birds) | 15.6 ± 9.8 | 19.0 ± 12.1 | 56.9 | 80.3 | 0.90 | 0.91 | |
| P3 (interior of North America) | Steamhouse 1 | 22.5 ± 14.2 | 24.9 ± 16.8 | 79.2 | 101.2 | 0.94 | 0.95 |
| Steamhouse 2 | 19.2 ± 13.1 | 22.6 ± 13.7 | 103.9 | 83.01 | 0.96 | 0.95 | |
| Leo | 26.7 ± 15.6 | 29.3 ± 16.4 | 87.5 | 108.4 | 0.95 | 0.94 | |
| Mac | 21.7 ± 15.3 | 22.5 ± 14.1 | 79.9 | 74.5 | 0.90 | 0.93 | |
| P3 (all birds) | 23.7 ± 15.9 | 26.4 ± 15.9 | 103.9 | 108.4 | 0.95 | 0.94 | |
| P4 (interior of South America) | Young Luro | 17.0 ± 10.7 | 24.7 ± 17.6 | 55.4 | 91.7 | 0.95 | 0.94 |
| Domingo | 16.9 ± 8.7 | 32.7 ± 20.6 | 45.1 | 95.7 | 0.92 | 0.97 | |
| Whitey | 17.5 ± 10.8 | 25.9 ± 19.5 | 51.5 | 87.5 | 0.91 | 0.93 | |
| La Pampa | 19.4 ± 11.2 | 19.5 ± 14.1 | 55.7 | 62.9 | 0.95 | 0.89 | |
| Argentina | 23.0 ± 14.2 | 31.4 ± 21.2 | 69.9 | 96.01 | 0.96 | 0.93 | |
| P4 (all birds) | 18.8 ± 11.4 | 25.5 ± 18.6 | 69.9 | 96.01 | 0.94 | 0.93 | |
Table 4.
Movement statistics for the four populations of turkey vultures showing mean ± standard deviation (s.d.) across bird season.
| populations |
||||
|---|---|---|---|---|
| P1: west coast of North America | P2: east coast of North America | P3: interior of North America | P4: interior of South America | |
| breeding season | ||||
| number of tracked individuals | 3 | 3 | 4 | 5 |
| number of tracks per season | 9 | 11 | 10 | 7 |
| number of years | 4 (2006–2009) | 8 (2005–2012) | 5 (2008–2012) | 4 (2009–2012) |
| mean overall distance travelled (km) | 3434.6 ± 1266.5 | 3128.6 ± 464.3 | 2071.5 ± 861.6 | 5407.7 ± 2371.0 |
| mean distance travelled per day (km) | 37.7 ± 37.5 | 20.6 ± 17.9 | 20.0 ± 20.5 | 59.0 ± 60.3 |
| mean hours actively moving per day | 5.6 ± 2.9 | 4.5 ± 2.3 | 5.0 ± 2.5 | 7.0 ± 3.0 |
| mean speed actively moving per day (km h−1) |
10.4 ± 4.2 | 8.9 ± 3.2 | 8.4 ± 4.5 | 9.5 ± 4.9 |
| mean straightness index of travel (no unit) | 0.56 ± 0.3 | 0.66 ± 0.3 | 0.46 ± 0.3 | 0.57 ± 0.3 |
| mean breeding start day | 2-Apr ± 18 days | 22-Mar ± 20 days | 5-May ± 9 days | 19-Oct ± 19 days |
| mean breeding end day | 14-Sep ± 42 days | 17-Oct ± 11 days | 29-Sep ± 7 days | 8-Apr ± 9 days |
| mean length of breeding season range (days) |
165 ± 38 | 209 ± 23 | 147 ± 13 | 171 ± 22 |
| mean breeding start latitude | 43.1 ± 4.2 | 39.5 ± 1.2 | 52.9 ± 0.6 | −37.8 ± 1.0 |
| mean breeding start longitude | −122.5 ± 1.8 | −77.9 ± 2.1 | −107.3 ± 0.3 | −64.6 ± 0.9 |
| mean breeding end latitude | 43.0 ± 4.2 | 39.4 ± 1.3 | 52.9 ± 0.6 | −37.2 ± 0.5 |
| mean breeding end longitude | −122.5 ± 1.8 | −77.9 ± 2.1 | −107.3 ± 0.3 | −64.0 ± 0.5 |
| non-breeding season | ||||
| number of tracked individuals | 3 | 4 | 4 | 5 |
| number of tracks per season | 8 | 12 | 12 | 12 |
| number of years | 4 (2006–2010) | 9 (2003–2012) | 6 (2007–2013) | 3 (2009–2012) |
| mean overall distance travelled (km) | 1380.3 ± 992.1 | 1829.8 ± 1143.9 | 3500.0 ± 2245.9 | 2887.7 ± 1493.4 |
| mean distance travelled per day (km) | 18.9 ± 18.7 | 36.2 ± 36.1 | 66.9 ± 52.8 | 43.3 ± 32.2 |
| mean hours actively moving per day | 4.4 ± 2.0 | 5.2 ± 2.2 | 6.7 ± 3.0 | 5.0 ± 2.3 |
| mean speed actively moving per day (km h−1) |
8.0 ± 3.2 | 11.0 ± 5.1 | 12.2 ± 5.2 | 8.9 ± 3.4 |
| mean straightness index of travel (no unit) | 0.52 ± 0.3 | 0.72 ± 0.3 | 0.57 ± 0.3 | 0.71 ± 0.3 |
| mean non-breeding start day | 23-Aug ± 114 days | 1-Oct ± 115 days | 27-Oct ± 90 days | 3-May ± 10 days |
| mean non-breeding end day | 12-Mar ± 14 days | 7-Mar ± 22 days | 21-Mar ± 5 days | 19-Sep ± 38 days |
| mean length of non-breeding season (days) | 110 ± 57 | 97 ± 46 | 114 ± 21 | 134 ± 38 |
| mean non-breeding start latitude | 21.6 ± 5.7 | 32.7 ± 3.5 | 8.5 ± 0.9 | −15.1 ± 2.6 |
| mean non-breeding start longitude | −101.2 ± 8.6 | −81.8 ± 2.2 | −67.8 ± 3.0 | −60.6 ± 5.5 |
| mean non-breeding end latitude | 21.6 ± 5.7 | 32.2 ± 3.9 | 8.7 ± 1.2 | −14.9 ± 2.6 |
| mean non-breeding end longitude | −101.2 ± 8.6 | −80.7 ± 2.0 | −67.5 ± 2.9 | −60.5 ± 5.4 |
| outbound migration | ||||
| number of tracked individuals | 3 | 4 | 4 | 5 |
| number of tracks per season | 8 | 12 | 14 | 12 |
| number of years | 3 (2006–2009) | 7 (2003–2010) | 5 (2007–2012) | 3 (2009–2012) |
| mean overall distance travelled (km) | 5300.1 ± 4523.8 | 1286.5 ± 618.4 | 7254.7 ± 2476.4 | 2755.7 ± 993.2 |
| mean distance travelled per day (km) | 129.0 ± 128.7 | 60.7 ± 58.8 | 245.7 ± 228.9 | 195.8 ± 181.6 |
| mean hours actively moving per day | 7.6 ± 3.1 | 5.8 ± 2.3 | 8.7 ± 3.9 | 7.7 ± 2.6 |
| mean speed actively moving per day (km h−1) |
17.6 ± 7.4 | 13.5 ± 6.7 | 20.5 ± 16.9 | 16.9 ± 6.8 |
| mean straightness index of travel (no unit) | 0.83 ± 0.2 | 0.87 ± 0.2 | 0.92 ± 0.15 | 0.94 ± 0.1 |
| mean outbound migration start day | 27-Sep ± 17 days | 19-Oct ± 12 days | 3-Oct ± 11 days | 8-Apr ± 10 days |
| mean outbound migration end day | 23-Aug ± 114 days | 5-Oct ± 119 days | 28-Oct ± 83 days | 2-May ± 9 days |
| mean length of outbound migration (days) | 56 ± 59 | 47 ± 32 | 52 ± 25 | 25 ± 9 |
| mean outbound migration start latitude | 43.8 ± 3.5 | 39.6 ± 1.3 | 59.1 ± 11.7 | −36.5 ± 1.5 |
| mean outbound migration start longitude | −122.9 ± 1.4 | −77.5 ± 2.1 | −104.8 ± 6.9 | −63.9 ± 0.5 |
| mean outbound migration end latitude | 21.7 ± 5.7 | 32.3 ± 2.6 | 11.1 ± 9.1 | −15.1 ± 2.5 |
| mean outbound migration end longitude | −101.3 ± 8.7 | −81.1 ± 1.5 | −69.9 ± 8.7 | −60.6 ± 5.4 |
| return migration | ||||
| number of tracked individuals | 3 | 4 | 4 | 5 |
| number of tracks per season | 8 | 12 | 10 | 10 |
| number of years | 3 (2007–2010) | 7 (2004–2011) | 4 (2008–2012) | 3 (2009–2012) |
| mean overall distance travelled (km) | 3730.4 ± 1392.1 | 1210.7 ± 469.1 | 8315.0 ± 252.4 | 2755.9 ± 914.4 |
| mean distance travelled per day (km) | 239.0 ± 170.7 | 101.4 ± 91.2 | 315.6 ± 236.8 | 224.2 ± 195.9 |
| mean hours actively moving per day | 9.6 ± 3.8 | 6.9 ± 2.9 | 10.2 ± 3.1 | 9.1 ± 3.0 |
| mean speed actively moving per day (km h−1) |
22.2 ± 8.1 | 17.2 ± 9.0 | 24.1 ± 10.11 | 24.1 ± 12.2 |
| mean straightness index of travel (no unit) | 0.89 ± 0.2 | 0.90 ± 0.1 | 0.93 ± 0.1 | 0.92 ± 0.1 |
| mean return migration start day | 12-Mar ± 14 days | 6-Mar ± 23 days | 21-Mar ± 5 days | 30-Sep ± 16 days |
| mean return migration end day | 4-Apr ± 13 days | 26-Mar ± 13 days | 4-May ± 9 days | 13-Oct ± 19 days |
| mean length of return migration (days) | 23 ± 15 | 19 ± 17 | 44 ± 8 | 14 ± 5 |
| mean return migration start latitude | 21.7 ± 5.7 | 32.1 ± 2.9 | 8.8 ± 1.1 | −14.9 ± 2.8 |
| mean return migration start longitude | −101.3 ± 8.7 | −81.1 ± 1.5 | −67.8 ± 2.8 | −59.9 ± 5.8 |
| mean return migration end latitude | 43.9 ± 3.5 | 39.6 ± 1.2 | 52.8 ± 0.6 | −35.9 ± 6.1 |
| mean return migration end longitude | −122.9 ± 1.5 | −77.5 ± 2.3 | −107.3 ± 0.5 | −63.5 ± 3.7 |
We also computed the home-range areas of turkey vultures at their breeding and non-breeding grounds using the R package ‘adehabitatHR’ [47]. The ranging areas are computed as a 95% minimum convex polygon (MCP) (after the removal of 5% of extreme points). The MCP is widely used to estimate the area traversed by an animal during its normal activities of foraging, mating and caring [48]. A summary of turkey vulture breeding season and overwintering ranging areas of all four populations per season is provided in the electronic supplementary material, II.
(g). Relating movement and environment
For each population, we assessed correlations between vulture movement patterns and the annotated environmental conditions separately for the four seasons of their annual cycle: outbound migration, return migration, breeding grounds and non-breeding grounds. Within migration segments, we first extracted records with a computed ground speed above 5 km h−1 to distinguish flying from non-flying behaviour [14,40,49]. This speed threshold is the first quartile of the computed movement speed of all records obtained from GPS locations. Here, we classified records with ground speeds higher than 5 km h−1 (from the previous point) as high-activity moments (i.e. movements in which birds were active and their movement more likely aerial). We classified records with speeds less than 5 km h−1 as low-activity moments. Characteristics of spatial exploration patterns (i.e. daily range, daily activity hours, speed and path tortuosity) for both migration and non-migration segments were investigated in relation to external environmental and geographical factors such as latitude, NDVI, air temperature, thermal uplift and tailwind support. Owing to large individual differences in the sizes of breeding and non-breeding range areas, we used a non-parametric ordinal logistic fit to test the effects of temperature and NDVI on these range areas.
3. Results
(a). Characterizing turkey vultures’ movement (H1, Pr1–3)
We found little variation in the spread of the migration routes within populations among individuals and years (H1). The migration routes were relatively narrow and repeatable, though migrants from the interior of North America travelled along a longitudinally ‘thinner’ corridor in the United States and Canada on return migration than on outbound migration as well as in the Mesoamerica Land Corridor [19] south of the US border with Mexico (figure 1).
However, turkey vultures breeding in the four distinct geographical regions exhibited substantial differences in a number of migration parameters. A significant variation was found in flight speed and straightness index across seasons and among individuals, but surprisingly not between populations (Pr1, tables 4 and 5). There were significant interactions between population and seasonal response, indicating that the differences between populations are limited to specific seasons (table 6). East Coast vultures travelled at substantially lower speeds (65–85% slower) while migrating than did vultures in the other three populations (Pr1, table 4). Movement during migratory seasons was, on average, faster than non-migratory season, whereas movement during non-migratory seasons was less directional and composed of daily tracks with higher tortuosity (Pr2, table 4). Migrants in all four populations travelled faster and engaged in migratory flight longer each day on their return migrations than on their outbound migrations (Pr3, tables 3–5). As a result of population differences in the speed of travel, the actual time taken to complete outbound and return migrations differed relatively little among the four populations considering the overall travelled distances (tables 4 and 5).
Table 5.
ANOVA table for the tests of significance of variance of flight speed and flight path straightness among seasons, populations and individuals. Individuals were included as a random effect, nested within populations to prevent pseudo-replication. The interaction between season and population was included as a random effect. p < 0.05 was considered significant.
| Source | d.f. num | speed (prob > F) | straightness (prob > F) |
|---|---|---|---|
| season | 3 | <0.0001 | 0.0002 |
| population | 3 | 0.8028 | 0.7911 |
| individual (nested within population) [random effect] | 11 | <0.0001 | <0.0001 |
| season × population (random effect) | 9 | <0.0001 | <0.0001 |
Table 6.
Statistical summary of ordinal logistic models of hourly movement speed as a function of population, environmental variables and their interaction with population, per season. Only points where movement speed was larger than 5 km h−1 were considered. During migration seasons, where the movement is across large ranges of latitudes, the latitude range (in bins of 10 degrees at absolute value, i.e. away from the equator) was included as an ordinal effect. As latitude is correlated with temperature, NDVI, uplift and wind, these continuous environmental variables were replaced in models of migration seasons by the residuals from their least-square fit model with latitude range (indicated using a superscript R after the variable name). Coefficients (slope of the relationship) were included only for significant continuous variables. p < 0.05 was considered significant.
| effects on flight speed | coefficient | d.f. | log-ratio χ2 | probability (>χ2) |
|---|---|---|---|---|
| season–outbound migration | ||||
| population | 3 | 374.99 | <0.0001 | |
| latitude range | 5 | 360.52 | <0.0001 | |
| thermal upliftR | 4.30 | 1 | 79.04 | <0.0001 |
| temperatureR | −0.2 | 1 | 19.70 | <0.0001 |
| NDVIR | 5.69 | 1 | 8.34 | 0.0039 |
| tailwindR | 1 | 0.12 | 0.7254 | |
| population × tailwindR | 3 | 2.99 | 0.3930 | |
| population × thermal upliftR | 3 | 69.55 | <0.0001 | |
| population × temperatureR | 3 | 28.45 | <0.0001 | |
| population × NDVIR | 3 | 21.26 | <0.0001 | |
| season–return migration | ||||
| population | 3 | 126.25 | <0.0001 | |
| latitude range | 5 | 115.61 | <0.0001 | |
| thermal upliftR | 2.70 | 1 | 42.44 | <0.0001 |
| temperatureR | 1.08 | 1 | 320.82 | <0.0001 |
| NDVIR | 1 | 0.12 | 0.7245 | |
| tailwindR | 1 | 0.01 | 0.9160 | |
| population × tailwindR | 3 | 1.26 | 0.7382 | |
| population × thermal upliftR | 3 | 2.150 | 0.5418 | |
| population × temperatureR | 3 | 45.48 | <0.0001 | |
| population × NDVIR | 3 | 23.50 | <0.0001 | |
| season–breeding grounds | ||||
| population | 3 | 95.83 | <0.0001 | |
| thermal uplift | 0.83 | 1 | 23.04 | <0.0001 |
| temperature | 0.01 | 1 | 0.44 | 0.5095 |
| NDVI | −7.23 | 1 | 90.34 | <0.0001 |
| tailwind | 1 | 0.003 | 0.9534 | |
| population × tailwind | 3 | 2.52 | 0.4713 | |
| population × thermal uplift | 3 | 11.82 | 0.0080 | |
| population × temperature | 3 | 17.36 | 0.0006 | |
| Population × NDVI | 3 | 43.50 | <0.0001 | |
| season–overwintering grounds | ||||
| population | 3 | 67.49 | <0.0001 | |
| thermal uplift | 0.13 | 1 | 0.24 | 0.6234 |
| temperature | 0.22 | 1 | 25.77 | <0.0001 |
| NDVI | 4.00 | 1 | 12.41 | 0.0004 |
| tailwind | 1 | 0.01 | 0.9203 | |
| population × tailwind | 3 | 1.38 | 0.7100 | |
| population × thermal uplift | 3 | 25.32 | <0.0001 | |
| population × temperature | 3 | 13.72 | 0.0033 | |
| population × NDVI | 3 | 18.52 | 0.0003 | |
(b). Environmental drivers of time spent flying (H2, Pr4–5)
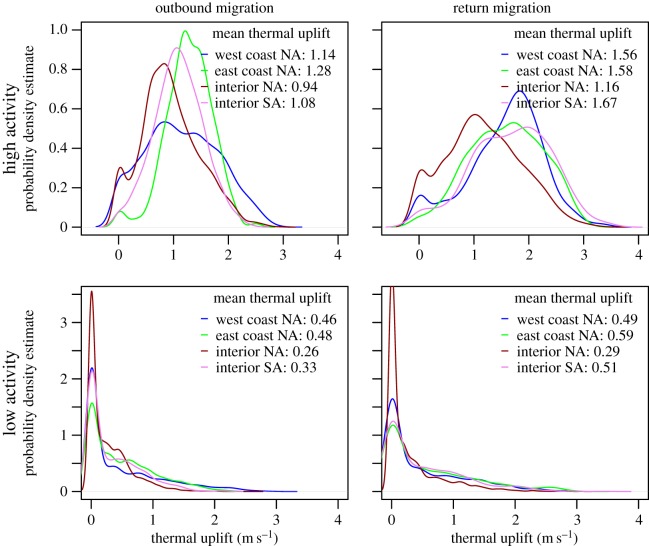
Because turkey vultures are obligate soaring migrants (sensu [19]), we were interested in the conditions they choose for flight versus resting during their migratory journeys (H2). To evaluate this, we measured the association between thermal uplift (sensu [30]) and behaviour (flying versus resting) during their migratory trips (figure 2). The differences between distribution functions of migration-season uplift availability during flight and non-flight periods suggest that vultures use thermal uplift to gain speed for their flights (mean thermal uplift greater than 0.90), and when thermal uplift is low (mean less than 0.60) they are less likely to be flying (speed less than 5 km h−1; Pr4–5).
Figure 2.
Thermal uplift experienced by four populations of turkey vultures during migration periods of active flights and non-flying periods (flying: speed ≥5 km h−1; non-flying: speed <5 km h−1).
(c). Environmental effects on migratory movement (H2, Pr5–6)
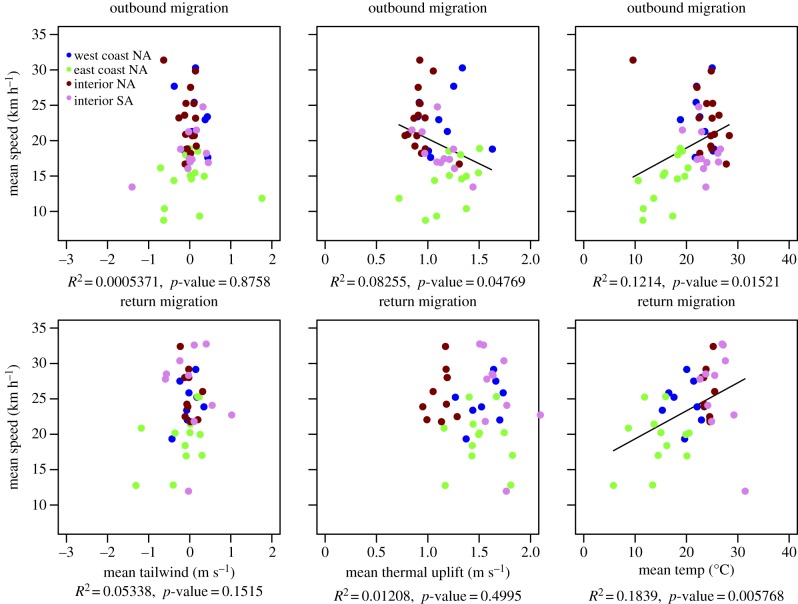
Significant correlations were found between migration flight speeds and thermal uplift, ambient temperature and NDVI at all seasons (Pr5, table 6). Figure 1 and table 6 also suggest that overall speed varies at different latitudes, with birds in the interior of North America population travelling faster at higher latitudes on both their outbound and return movements (Pr6). This may be driven, in part, by longer days in the more northern areas of the migration path during the late spring and early autumn. Thermal uplift (or its residual from the latitudinal prediction) showed significant positive effects on movement at all seasons, but during the migration seasons its coefficients were roughly 3–30 times larger than in the non-migratory seasons (Pr5–6, table 6). The results suggested a significant interaction between thermal uplift and population, indicating that uplift had different effects in different populations (Pr6). Similarly, temperature had a significant direct effect and significant interaction with populations at all seasons (Pr6). NDVI was positively correlated with flight speed during outbound migration and the wintering season, and it was strongly negatively correlated with flight speed during the breeding season. However, NDVI did not show a significant effect on flight speed during the return migration. Surprisingly, tailwinds did not have any significant effect on flight speed.
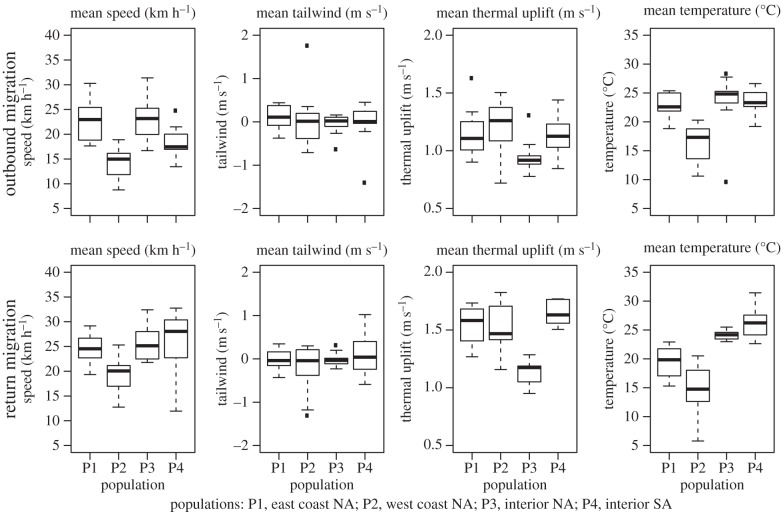
We assessed the seasonal mean flight speed per individual bird during migratory seasons (i.e. when movement is directional) and the home-range size during non-migratory seasons (i.e. when movement is non-directional and centred around one or a few central nest and roost points). When aggregating the flight characteristics to a seasonal scale, different patterns emerge. Years in which the mean seasonal uplift was lower were characterized by faster movement during the outbound migration and years with higher temperature were characterized by faster flight during both migratory seasons (Pr6, figure 3). Overall, these relationships were stronger during outbound migration, when our analyses showed that the vultures were travelling more slowly (table 4 and figure 4) because of less optimal conditions for soaring (less uplift, figures 2 and 4).
Figure 3.
Associations between migration ground speeds (km h−1) and weather conditions during flight for 46 outbound and 40 return migration trips made by 19 vultures from four populations.
Figure 4.
Boxplots of (from left to right) mean flight speed, mean tailwind (m s−1), mean thermal uplift and mean air temperature (°C) annotated along turkey vulture tracks in outbound migration (top) and return migration (bottom) segments. Means are calculated per bird per year.
(d). Environmental effects on home ranges during non-migratory seasons (H2, Pr7–8)
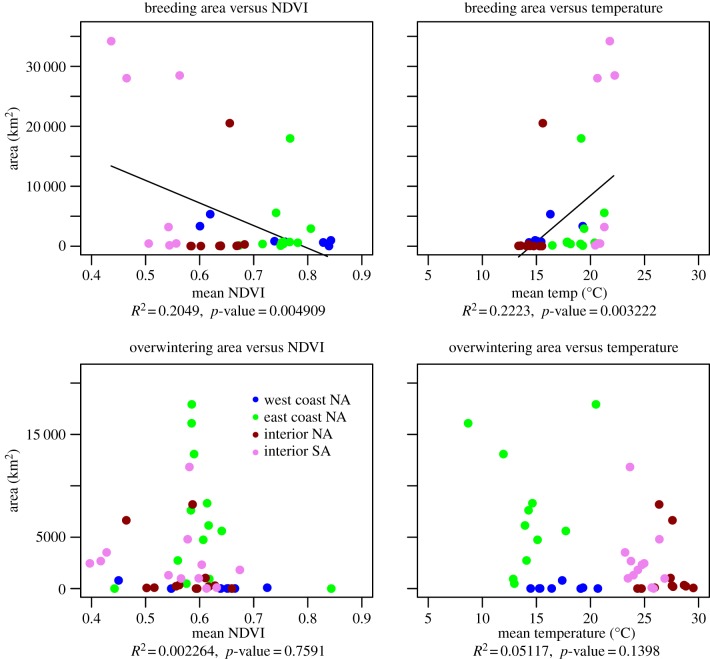
As was hypothesized (H1), there was considerable variability in both breeding and non-breeding range size among the four geographical populations, with west coast North American birds having the smallest ranges in both seasons, South American birds showing exceptionally large breeding-season ranges, and east coast North American birds using larger non-breeding ranges than breeding ranges (figure 5). The presence of this variability allows us to search for environmental drivers that explain these differences between populations and seasons (H2). Except for the birds that spent their breeding seasons in interior North America, we do not know the actual breeding status of any of the birds. However, in this population, the home ranges of known active breeders varied considerably, with two known breeders having ranges of 75 km2 or less and two others ranges of more than 895 km2 [50]. This suggests that high variability in breeding-season ranges among individuals does not simply reflect their breeding status.
Figure 5.
Plots of turkey vulture home-range areas versus (on the left) mean NDVI (no unit) and (on the right) mean air temperature (°C) in the breeding (top) and overwintering (non-breeding) grounds (bottom). Each point represents data for one individual bird for 1 year.
To study what affects the sizes of seasonal home ranges, we characterized the environments used by individuals with three remote-sensing datasets: mean seasonal NDVI (i.e. an indirect indicator for food availability), mean seasonal uplift availability and seasonally averaged ambient temperature (table 2). Using a stepwise approach to minimize the effects of cross-correlation between the environmental variables, we found that mean seasonal temperature was significantly and positively correlated with the sizes of the breeding-season ranges of individual birds, with warmer and uplift-rich conditions associated with larger individual breeding ranges (Pr7, figure 5 and table 7). NDVI was significantly and negatively correlated with home-range size. In contrast to the effects we found during the breeding season, none of these environmental variables was correlated with the home-range size during the non-breeding season (Pr8).
Table 7.
Statistical summary of a stepwise regression between seasonal mean environmental conditions—NDVI, temperature and uplift—in each bird home range and the log of the area of the home range of each bird during the breading and wintering seasons. Only significant effects (marked with *) entered the model.
| F-ratio | AICc | coefficient | probability (>F) | |
|---|---|---|---|---|
| breading season | full model R2 = 0.31 | |||
| mean NDVI | 4.495 | 775.239 | −26 969.33 | 0.041* |
| mean temperature | 5.357 | 773.167 | 1153.24 | 0.027* |
| mean uplift | 0.497 | 0.485 | ||
| wintering | full model R2 < 0.00001 | |||
| mean NDVI | 0.095 | 0.759 | ||
| mean temperature | 2.265 | 0.139 | ||
| mean uplift | 0.078 | 0.781 | ||
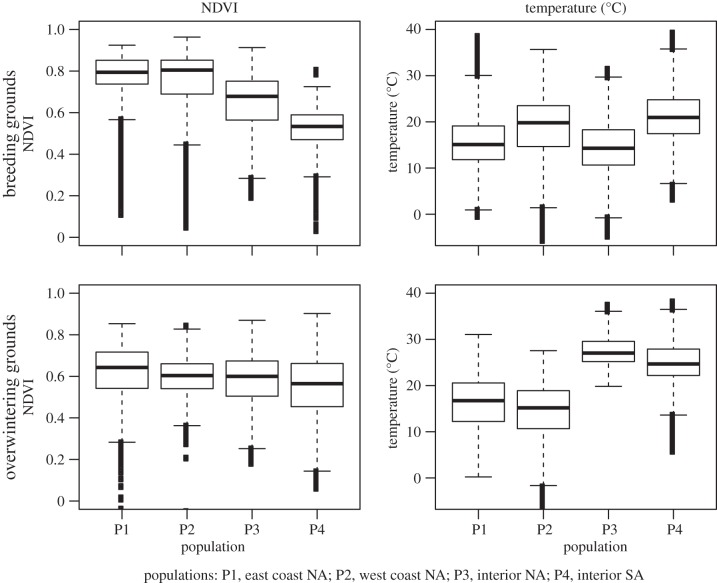
Our comparisons of seasonal differences in NDVI experienced by the same individuals suggest a motivation for long-distance migration in that most populations of vultures inhabit breeding areas that are more productive than their non-breeding areas (figure 6). Likely explanations for why vultures are able to overwinter successfully in less productive areas include the fact that they are not raising young and are therefore less limited by forage availability, as they are during the breeding season. Temperature comparisons (figure 6) show that, except for two east coast birds, which both bred and overwintered in the temperate zone, our tracked vultures overwintered in subtropical and tropical areas that were warmer than their temperate zone breeding areas. Additional on-the-ground fieldwork is needed to assess the demographic consequences, if any, of the east coast population's cooler wintering area.
Figure 6.
Boxplots of NDVI (on the left) and air temperature (°C; on the right) annotated along turkey vulture tracks in breeding grounds (top) and overwintering (non-breeding) grounds (bottom).
4. Discussion
(a). Variability in turkey vultures’ movements characteristics
Using nearly 10 years of data from 24 satellite-tracked Turkey vultures in four distinct populations, we demonstrate that the need to move for partial migrants is driven largely by a need to reach different foraging grounds and climatic zones, whereas during non-migratory periods it is driven mostly by the need to find food. Furthermore, our results show that foraging needs for such species may be highest in the breeding grounds, where adults birds are feeding young and moulting both their body and flight feathers [51].
We suggest that the longitudinal thinning in the migration corridors of migrants travelling through Mesoamerica (figure 1) reflects the fact that flock size among migrants swells enormously in Mesoamerica as migrants are increasingly attracted to one another. Why this is so becomes obvious when one recognizes the hourglass shape of North America in subtropical and tropical latitudes (i.e. Mesoamerica). This geographical feature increases the density of migrants considerably, all but eliminating opportunities for effective foraging behaviour and, in turn, reducing significantly the use of metabolic energy to fuel the flight. Flocking in obligate soaring migrants helps individuals find and use updrafts more efficiently, presumably via increased social facilitation [19,52].
(b). Variability in the speed of outbound versus return migration
As can be seen in tables 3 and 4, most vultures migrate faster on return versus outbound migration. This is typical of many species of birds including many raptors [19]. Historically, explanations for this were driven by differences in the need to move between the seasons and included the fact that outbound migration occurs at the end of the breeding season after the time when many populations of potential prey have reproduced and are relatively more available as a food resource for the migrants, whereas return migration occurs in spring before most potential prey have reproduced. Many outbound migrants are therefore better able to fuel their journeys while feeding en route than are return migrants and, as such, are travelling more slowly while migrating [53]. Other explanations include that outbound migration includes large numbers of inexperienced and slower travelling juvenile migrants, whereas return migration includes only experienced birds that tend to migrate faster than first-time migrants [19], and that adults are rushing to the breeding grounds to secure the best territories [54]. The former appears to be an unlikely explanation for this dataset in that all but three of the birds involved were adults at the time of capture. Moreover, there could be a time constraint for birds migrating overall longer distances to reach the breeding grounds. Thus, they are expected to be more time-selected than short-distance migrant species and obliged to travel faster, achieving higher daily distances [55–57]. Our data offer some support for this hypothesis as the highest mean migration speeds are observed in the populations with the longest migrations (tables 3 and 4). One factor that is not likely to affect the faster rate of travel on return versus outbound migration is the so-called ‘urge to reproduce’. It makes little sense for vultures to migrate at anything other than their optimal rate of travel, given environmental circumstances and their own internal physical states (cf. [58]), and there is no evidence that reproductive urges induce them to do so. A forced march back to the breeding grounds in spring is counterproductive, particularly when arrival there in appropriate body condition is likely to be important for territorial establishment and subsequent successful breeding. On the other hand, an earlier departure from the wintering grounds is likely to allow migrants to arrive proportionately earlier on the breeding grounds in appropriate condition [19].
(c). Environmental drivers of migratory movement
Our analyses of the timing of return versus outbound migration, together with differences in the strength of thermal updrafts during the two periods, strongly support a fourth possibility related to the capacity to move, or cost of movement: vultures migrate longer distances each day and complete their migrations in fewer days (table 4) on return migration versus outbound migration (table 4), because longer daytime hours of travel coupled with stronger thermals [40] in spring versus autumn (figure 2) enable more energetically cost-effective flight during return migration. Overall, vultures regularly completed their return migrations more rapidly than their outbound migrations, and periods of high migratory movement were enhanced by the presence of thermal uplift, corroborating previous studies [30,40]. However, it is not clear why thermals are stronger during return versus outbound migration. One possibility, which remains to be tested, is that vegetation is drier and photosynthetically less active during spring versus return migration, which would enhance surface heating via solar radiation in spring and therefore the formation of thermal updrafts. Another possibility, that birds are travelling closer to summer solstice in spring than in autumn and thus enjoy more thermally active daytime hours, does not seem to be responsible. Although return migration in both interior North American and interior South American populations does occur more than a month closer to summer solstice (table 4)—when thermals tend to be strongest—than outbound migration, the same is not true for either North American west coast or east coast populations, whose return migrations actually are about 10 days farther from summer solstice than are their outbound migrations.
(d). Environmental drivers of non-migratory movement affects home-range size
With a few notable exceptions (see [59]), previous studies of satellite-tracked birds have focused largely on migratory movements rather than on movements during the breeding and non-breeding seasons. Here, we highlight several findings from our turkey vulture dataset that suggest opportunities for future study of movements during non-migration periods. Unlike the relatively fast and directed movements engaged in by vultures during their migrations, breeding and non-breeding season movements are characterized by shorter and slower multi-directional flight segments around one or several central points (table 4). Nevertheless, on a daily basis, vultures spent similar amounts of time in the air during migratory and non-migratory periods, and in two populations (east coast of North America and interior of South America) individuals actually travelled greater average distances on their breeding grounds and in their overwintering areas than on their briefer outbound and return migrations in autumn and spring (table 4). We believe that the extraordinary amount of daily movement during the non-migratory seasons is best explained by the species’ extremely efficient soaring behaviour, which reduces the cost considerably [3].
We suggest that the relationship between range size and NDVI is due to the likelihood of reduced carcass availability in less productive breeding areas and, therefore, the need for larger searching areas, a relationship that also occurs in Old-World vultures [23,24]. Furthermore, as increased temperature correlates positively with thermal uplifts in both breeding areas (p < 0.001, R2 = 0.08, d.f. = 80 680) and non-breeding areas (p < 0.001, R2 = 0.07, d.f. = 56 294), we believe the relationship between range size and temperature is due to greater opportunities for low-cost soaring in warmer areas, which is in accordance with previous findings for other soaring migratory raptors [40]. In fact, in a very real sense, vultures are solar-powered flyers whose metabolic costs of flight are inversely related to the availability of thermal updrafts, which, in turn, are positively correlated with daily temperature. That temperature correlates with home range better than thermal uplift velocity suggests that temperature has an additional effect on movement and home-range size, the nature of which is not yet clear. One possibility is that the vultures we studied expended less metabolic energy at high temperature than at low temperatures and that these savings allowed the birds to fly more frequently. We believe this dependency on environmental conditions as indicated by NDVI and temperature reflects differences in food available to each individual during the breeding season coupled with the species’ low-cost soaring flight that allows it to range widely while searching for food. Presumably, NDVI influences the need to move, with high vegetation productivity indicating high forage density and a lower need to move, whereas uplift and temperature affect their capacity for flight (higher temperatures reflecting more thermals). This is consistent with the energy landscape hypothesis [60]—that movement speed and extent are strongly influenced by the spatial and temporal variation in available environmental energy that can be harvested for movement. Intriguingly, the home-range areas on the breeding grounds were dependent on climate, whereas this was not the case in non-breeding seasons, possibly because the feeding needs during that season are smaller and the birds do not need to maximize their foraging ranges.
The correlations we found between mean temperature and home-range size allow us to hypothesize that climate change resulting in warmer summers will increase the sizes of breeding home ranges in both North and South America. The extent to which this will affect overall population densities will depend on how regional climate change affects primary productivity and, therefore, carcass availability. Higher productivity, driven by precipitation, for example, may decrease the need for large home ranges and offset any changes caused by higher temperatures, perhaps even leading to increased population density. Alternatively, decreases in primary productivity driven by drought may increase the need for larger home ranges leading to increased competition, which may reduce the population density. That said, the enormous variability we have uncovered in the movement ecology of this species suggests that it is likely to do well in the face of global change, and that unless other human-related threats [25], including environmental toxicants or increased direct human persecution, intervene, the species is likely to remain widespread and abundant across much of its current range. Whether other partial migrants will fare as well is likely to depend on the extent of variability in their migration syndromes.
5. Conclusion
Our study used satellite-tracking data to evaluate quantitatively the effects of biologically relevant environmental conditions on turkey vulture movement ecology in a wide variety of landscapes across much of their range [4]. Specifically, we used the new Env-DATA system to annotate tracking data of 24 turkey vultures from four populations in North and South America, collected over 10 years, including 90 migration events. We suggest that our approach and methods are applicable to other species’ movement datasets in terms of movement ecology predictions related to environmental conditions. We discovered a number of consistent patterns that characterize the response of turkey vultures to environmental conditions, and allow us to generate predictions as to the home range and migration speeds of future populations under alternative future climate scenarios. We also found that populations in different areas of the species range exhibited the phenomenon of avian migration differently, and that enormous geographical variability in movement ecology in the species may, in part, explain the species’ high abundance and widespread distribution in the New World.
A key prediction of macroecology is that abundant and widespread species such as turkey vultures should be more broadly tolerant of environmental conditions than more rare and uncommon species [61]. The variability we have documented in turkey vulture movement ecology supports that prediction. Overall, our study indicates that the pre-adaptation or ‘key innovation’ (sensu [62]) of light wing loading coupled with a dihedral wing configuration results in hyper-efficient flight [3] and allows the species to modify its movements to suit local conditions more easily than if its movements were more expensive metabolically. Although the degree to which this variability occurs at the individual versus the population level remains uncertain, our findings that flexibility in movement ecology occurs at both levels suggests that evolution has selected both for different movement strategies in different populations as well as for increased phenotypic plasticity in the species.
We note, however, that even with a dataset as large as the one presented above, many questions remain unanswered owing to a lack of adequate sample size. Consequently, predictions remain somewhat speculative, and our findings cannot be used to infer population-level conclusions with high confidence (see [11]). Our results illustrate the need to move beyond accumulating large numbers of small datasets and analysing them in isolation. Standardization of analytical and statistical methods, and substantial increases to dataset size by combining individual datasets and conducting long-term, single-species studies rather than starting new ones are needed to advance our understanding of factors affecting the movement ecology of birds and other organisms. Owing to limitations, including funding, site access, permit requirements, etc., few research groups in the field can accomplish this alone. Thus, we call for fully integrated collaborations and policies that encourage and reward data sharing. Researchers should work to find collaborators with whom to coordinate data collection, and also should become more comfortable with the idea of sharing data shortly after collection, so that it could be leveraged for further collaboration. As datasets and collaborations grow, it will be necessary to find the time and expertise needed to manage, analyse and describe data using methods that move beyond simple maps and descriptive statistics. In our turkey vulture example, this will include path-annotation analysis [41] and comparative investigations of Old-World vultures (family Accipitridae) being tracked by us and other researchers [23,24,40]. Many resources currently exist to support these goals. Free online databases for collecting, managing and sharing animal tracking data such as Movebank (http://www.movebank.org), http://www.seaturtle.org and WRAM (http://www.slu.se/WRAM) already are used by many researchers and serve as places to identify existing data and potential collaborators, compile datasets for analysis and implement analysis tools that can be shared with others.
As we move forward, journals, universities and funding agencies should develop policies to facilitate collaborative research, such as supporting and incentivizing archiving, curating and managing datasets during and following research efforts, thus ensuring that multiple data owners can be properly credited in publications, and at the same time, driving increasingly creative and sophisticated ways to maximize the scientific benefit of deploying tracking devices on wild animals and meet ever increasing data needs for future ecological predictions.
Acknowledgements
This is Conservation Science contribution number 240 from Hawk Mountain Sanctuary. We thank the Acopian Family for providing Hawk Mountain Sanctuary with funds to study turkey vultures for the past decade.
Data accessibility
The data used in this study are available on Movebank (http://www.movebank.org) and are published in the Movebank Data Repository (doi:10.5441/001/1.46ft1k05).
Funding statement
This research was supported by NASA under grant no. NNX11AP61G.
References
- 1.Best B. 2008. Binoculars and people. Otley, UK: Biosphere. [Google Scholar]
- 2.Jespersen P, Tåning Å. 1950. Studies in bird migration, being the collected papers of H. Chr. C. Mortensen 1856–1921. Washington, DC: Academy of Natural Sciences, Philadelphia, and American Ornithologists’ Union, Washington. [Google Scholar]
- 3.Mandel JT, Bildstein KL, Bohrer G, Winkler DW. 2008. Movement ecology of migration in turkey vultures. Proc. Natl Acad. Sci. USA 105, 19 102–19 107. ( 10.1073/pnas.0801789105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince PA, Wood AG, Barton T, Croxall JP. 1992. Satellite tracking of wandering albatrosses (Diornedea exulans) in the South Atlantic. Antarct. Sci. 4, 31–36. ( 10.1017/S0954102092000075) [DOI] [Google Scholar]
- 6.BirdLife International. 2004. Tracking ocean wanderers: the global distribution of albatrosses and petrels. In Results from the Global Procellariiform Tracking Workshop, Gordon's Bay, South Africa, 1–5 September, 2003. [Google Scholar]
- 7.Seegar W, Cutchis P, Fuller M, Suter JJ, Bhatnagar V, Wall JG. 1996. Fifteen years of satellite tracking development and application to wildlife research and conservation. Johns Hopkins APL Tech. Digest 17, 401–411. [Google Scholar]
- 8.Meyburg B, Scheuer W, Meyburg C. 1995. Migration and wintering of the lesser spotted eagle (Aquila pornarina): a study by means of satellite telemetry. J. Ornithol. 136, 401–422. ( 10.1007/BF01651588) [DOI] [Google Scholar]
- 9.Petersen M, Larned W, Douglas D. 1999. At-sea distribution of specified eiders: a 120-year-old mystery resolved. Auk 116, 1009–1020. ( 10.2307/4089681) [DOI] [Google Scholar]
- 10.Fuller M, Seegar W, Schueck L. 1998. Routes and travel rates of migrating Peregrine falcons Falco peregrinus and Swainson's hawks Buteo swainsoni in the western hemisphere. J. Avian Biol. 29, 433–440. ( 10.2307/3677162) [DOI] [Google Scholar]
- 11.Lindberg MS, Walker J. 2007. Satellite telemetry in avian research and management: sample size considerations. J. Wildl. Manage. 71, 1002–1009. ( 10.2193/2005-696) [DOI] [Google Scholar]
- 12.Hebblewhite M, Haydon DT. 2010. Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Phil. Trans. R. Soc. B 365, 2303–2312. ( 10.1098/rstb.2010.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O. 2008. Trends and missing parts in the study of movement ecology. Proc. Natl Acad. Sci. USA 105, 19 060–19 065. ( 10.1073/pnas.0800483105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limiñana R, Soutullo A, Urios V. 2006. First description of the autumn migration of Montagu's harriers tracked by satellite telemetry. Ibis 284, 1–19. [Google Scholar]
- 15.Higuchi H. 2010. Satellite tracking the migration of birds in East Asia. Br. Birds 103, 284–302. [Google Scholar]
- 16.Polovina JJ, Howell E, Kobayashi DR, Seki MP. 2001. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 49, 469–483. ( 10.1016/S0079-6611(01)00036-2) [DOI] [Google Scholar]
- 17.Newton I. 2008. The migration ecology of birds. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 18.Berthold P. 2001. Bird migration: a general survey. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Bildstein KL. 2006. Migrating raptors of the world: their ecology and conservation. Ithaca, NY: Cornell University Press. [Google Scholar]
- 20.Spiegel O, Getz WM, Nathan R. 2013. Factors influencing foraging search efficiency: why do scarce lappet-faced vultures outperform ubiquitous white-backed vultures? Am. Nat. 181, E102–E115. ( 10.1086/670009) [DOI] [PubMed] [Google Scholar]
- 21.Spiegel O, Harel R, Getz WM, Nathan R. 2013. Mixed strategies of griffon vultures’ (Gyps fulvus) response to food deprivation lead to a hump-shaped movement pattern. Mov. Ecol. 1, 5 ( 10.1186/2051-3933-1-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bildstein K, Bechard M, Farmer C, Newcomb L. 2009. Narrow sea crossings present major obstacles to migrating griffon vultures Gyps fulvus. Ibis 151, 382–391. ( 10.1111/j.1474-919X.2009.00919.x) [DOI] [Google Scholar]
- 23.Margalida A, Colomer MÀ. 2012. Modelling the effects of sanitary policies on European vulture conservation. Sci. Rep. 2, 1–7. ( 10.1038/srep00753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monsarrat S, Benhamou S, Sarrazin F, Bessa-Gomes C, Bouten W, Duriez O. 2013. How predictability of feeding patches affects home range and foraging habitat selection in avian social scavengers? PLoS ONE 8, e53077 ( 10.1371/journal.pone.0053077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogada DL, Keesing F, Virani MZ. 2012. Dropping dead: causes and consequences of vulture population declines worldwide. Ann. NY Acad. Sci. 1249, 57–71. ( 10.1111/j.1749-6632.2011.06293.x) [DOI] [PubMed] [Google Scholar]
- 26.Kiff LF. 2000. The current status of North American vultures. In Raptors at risk (eds Chancellor RD, Meyburg BU.), pp. 175–189. Blaine, WA: Hancock House. [Google Scholar]
- 27.Ferguson-Lees J, Christie DA. 2001. Raptors of the world. London, UK: Christopher Helm. [Google Scholar]
- 28.Ferland-Raymond B, Bachand M, Thomas D, Bildstein KL. 2005. Flapping rates of migrating and foraging turkey vultures Cathartes aura in Costa Rica. Vulture News 53, 4–9. [Google Scholar]
- 29.Careau V, Therrien J-F, Porras P, Thomas D, Bildstein KL. 2006. Soaring and gliding flight of migrating broad-winged hawks: behavior in the Nearctic and Neotropics compared. The Wilson J. Ornithol. 118, 471–477. ( 10.1676/05-140.1) [DOI] [Google Scholar]
- 30.Bohrer G, Brandes D, Mandel JT, Bildstein KL, Miller TA, Lanzone M, Katzner T, Maisonneuve C, Tremblay JA. 2012. Estimating updraft velocity components over large spatial scales: contrasting migration strategies of golden eagles and turkey vultures. Ecol. Lett. 15, 96–103. ( 10.1111/j.1461-0248.2011.01713.x) [DOI] [PubMed] [Google Scholar]
- 31.Bloom PH, Clark WS, Kidd JW. 2007. Capture techniques. In Raptor research and management techniques (eds Bird DM, Bildstein KL.), pp. 193–219. Surrey, British Columbia, Canada: Hancock House. [Google Scholar]
- 32.Kirk DA, Gosler AG, Url S. 2013. Body condition varies with migration and competition in migrant and resident South American vultures. Auk 111, 933–944. ( 10.2307/4088825) [DOI] [Google Scholar]
- 33.Kirk DA, Houston DC. 1995. Social dominance in migrant and resident turkey vultures at carcasses: evidence for a despotic distribution? Behav. Ecol. Sociobiol. 36, 323–322. ( 10.1007/BF00167793) [DOI] [Google Scholar]
- 34.Didan K, Huete A. 2006. MODIS vegetation index product series collection 5 change summary. TBRS Lab, The University of Arizona. See http://landweb.nascom.nasa.gov/QA_WWW/forPage/MOD13_VI_C5_Changes_Document_06_28_06.pdf. [Google Scholar]
- 35.Sellers PJ, Berry JA, Collatz GJ, Field CB, Hall FG. 1992. Canopy reflectance, photosynthesis, and transpiration. III. A reanalysis using improved leaf models and a new canopy integration scheme. Remote Sens. Environ. 42, 187–216. ( 10.1016/0034-4257(92)90102-P) [DOI] [Google Scholar]
- 36.Bartlam-Brooks HLA, Beck PSA, Bohrer G, Harris S. 2013. In search of greener pastures: using satellite images to predict the effects of environmental change on zebra migration J. Geophys. Res. Biogeosci. 118, 1427–1437. ( 10.1002/jgrg.20096) [DOI] [Google Scholar]
- 37.Pettorelli N, Bro-Jørgensen J, Durant SM, Blackburn T, Carbone C. 2009. Energy availability and density estimates in African ungulates. Am. Nat. 173, 698–704. ( 10.1086/597379) [DOI] [PubMed] [Google Scholar]
- 38.Pettorelli N, Ryan S, Mueller T, Bunnefeld N, Jedrzejewska B, Lima M, Kausrud K. 2011. The Normalized Difference Vegetation Index (NDVI): unforeseen successes in animal ecology. Clim. Res. 46, 15–27. ( 10.3354/cr00936) [DOI] [Google Scholar]
- 39.Squires JR, DeCesare NJ, Olson LE, Kolbe JA, Hebblewhite M, Parks SA. 2013. Combining resource selection and movement behavior to predict corridors for Canada lynx at their southern range periphery. Biol. Conserv. 157, 187–195. ( 10.1016/j.biocon.2012.07.018) [DOI] [Google Scholar]
- 40.Mellone U, et al. 2012. Interspecific comparison of the performance of soaring migrants in relation to morphology, meteorological conditions and migration strategies. PLoS ONE 7, e39833 ( 10.1371/journal.pone.0039833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandel JT, Bohrer G, Winkler DW, Barber DR, Houston CS, Bildstein KL. 2011. Migration path annotation: cross-continental study of migration-flight response to environmental conditions. Ecol. Appl. 21, 2258–2268. ( 10.1890/10-1651.1) [DOI] [PubMed] [Google Scholar]
- 42.Dodge S, et al. 2013. The environmental-data automated track annotation (Env-DATA) system: linking animal tracks with environmental data. Mov. Ecol. 1, 3 ( 10.1186/2051-3933-1-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kranstauber B, Cameron A, Weinzerl R, Fountain T, Tilak S, Wikelski M, Kays R. 2011. The Movebank data model for animal tracking. Environ. Model. Softw. 26, 834–835. ( 10.1016/j.envsoft.2010.12.005) [DOI] [Google Scholar]
- 44.Dee DP, et al. 2011. The ERA-Interim reanalysis: configuration and performance of the data assimilation system. Q. J. Royal Meteorol. Soc. 137, 553–597. ( 10.1002/qj.828) [DOI] [Google Scholar]
- 45.Safi K, Kranstauber B, Kays R, Weinzerl R, Griffin L, Cruz S, Takekawa J, Wikelski M, Bohrer G. 2013. Flying with the wind. Mov. Ecol. 01, 4 ( 10.1186/2051-3933-1-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benhamou S. 2004. How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity, or fractal dimension? J. Theor. Biol. 229, 209–220. ( 10.1016/j.jtbi.2004.03.016) [DOI] [PubMed] [Google Scholar]
- 47.Calenge C. 2011. Home range estimation in R: the adehabitatHR package See http://cran.r-project.org/web/packages/adehabitatHR/.
- 48.Burt W. 1943. Territoriality and home range concepts as applied to mammals. J. Mammal 24, 346–352. ( 10.2307/1374834) [DOI] [Google Scholar]
- 49.Strandberg R, Klaassen RHG, Olofsson P, Alerstam T. 2009. Daily travel schedules of adult Eurasian hobbies Falco subbuteo: variability in flight hours and migration speed along the route. Ardea 97, 287–295. ( 10.5253/078.097.0304) [DOI] [Google Scholar]
- 50.Houston CS, McLoughlin PD, Mandel JT, Bechard MJ, Stoffel MJ, Barber DR, Bildstein KL. 2011. Breeding home ranges of migratory turkey vultures near their northern limit. The Wilson J. Ornithol. 123, 472–478. ( 10.1676/10-090.1) [DOI] [Google Scholar]
- 51.Kirk D, Mossman M. 1998. Turkey vulture (Cathartes aura). The Birds North Am. 339 ( 10.2173/bna.339) [DOI] [Google Scholar]
- 52.Kerlinger P. 1989. Flight strategies of migrating hawks. Chicago, IL: University of Chicago Press. [DOI] [PubMed] [Google Scholar]
- 53.O'Reilly K, Wingfield J. 1995. Spring and autumn migration in Arctic shorebirds: same distance, different strategies. Am. Zool. 35, 222–233. [Google Scholar]
- 54.Kokko H. 1999. Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. ( 10.1046/j.1365-2656.1999.00343.x) [DOI] [Google Scholar]
- 55.Alerstam T. 2006. Strategies for the transition to breeding in time-selected migration. Ardea 94, 347–357. [Google Scholar]
- 56.Alerstam T. 2003. Bird migration speed. In Avian migration (eds Berthold P, Gwinner E, Sonnenschein E.), pp. 253–267. Berlin, Germany: Springer. [Google Scholar]
- 57.Strandberg R, Alerstam T, Hake M, Kjellén N. 2009. Short communication short-distance migration of the common buzzard Buteo buteo recorded by satellite tracking. Ibis 151, 200–206. ( 10.1111/j.1474-919X.2008.00890.x) [DOI] [Google Scholar]
- 58.Izhaki ID, Maitav A. 1995. Blackaps Sylvia atricapilla stopping over at the desert edge; physiological state and flight-range estimates. Ibis 140, 223–233. ( 10.1111/j.1474-919X.1998.tb04383.x) [DOI] [Google Scholar]
- 59.López-López P, Benavent-Corai J, García-Ripollés C, Urios V. 2013. Scavengers on the move: behavioural changes in foraging search patterns during the annual cycle. PLoS ONE 8, e54352 ( 10.1371/journal.pone.0054352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson RP, Quintana F, Hobson VJ. 2012. Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc. R. Soc. B 279, 975–980. ( 10.1098/rspb.2011.1544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown JH. 1995. Macroecology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 62.Bock WJ. 1965. The role of adaptive mechanisms in the origin of higher levels of organization. Syst. Biol. 14, 272–287. ( 10.2307/sysbio/14.4.272) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available on Movebank (http://www.movebank.org) and are published in the Movebank Data Repository (doi:10.5441/001/1.46ft1k05).