Abstract
Manganese porphyrins are a potent class of catalytic antioxidants whose activity was recently shown to be partially dependent upon flavin-containing enzymes (R. Kachadourian et al., 2004, Biochem. Pharmacol. 67, 77–85). We tested whether manganese porphyrins could redox cycle with the flavin-containing enzyme, cytochrome P450 reductase, and whether this results in the inhibition of xenobiotic metabolism. The effect of manganese porphyrins on xenobiotic metabolism in rat and human liver microsomes was assessed spectrofluorometrically by following the O-dealkylation of benzyloxy- (BROD) and methoxyresorufin (MROD). Redox cycling of manganese porphyrins with human cytochrome P450 reductase was assessed both spectrophotometrically and polarographically by following the consumption of NADPH and oxygen, respectively. The tetrakis(N-pyridinium-2-yl) meso-substituted manganoporphyrin, MnTEPyP5+, and the tetrakis(1,3-dimethyl imidazolium-2-yl) meso-substituted manganoporphyrin, MnTDMIP5+, were 40 to 100 times more potent inhibitors of rat and human liver microsomal BROD metabolism than the tetrakis(1,3-diethyl imidazolium-2-yl) meso-substituted manganoporphyrin, MnTDEIP5+, or the tetrakis(4-benzoic acid) meso-substituted manganoporphyrin, MnTBAP. A similar pattern of inhibition was also seen in β-naphthoflavone-induced rat liver microsomal MROD metabolism. This pattern of xenobiotic metabolism inhibition correlated with the compound’s ability to redox cycle with cytochrome P450 reductase where MnTEPyP5+ was more potent than MnTDEIP5+. Furthermore, redox cycling of MnTEPyP5+ with cytochrome P450 reductase was inhibited by the flavin domain inhibitor diphenyleneiodonium. Small changes to the carbon chain length on the imidazolium side groups had a large effect on this activity. It is possible that interactions between manganoporphyrins and flavin-dependent oxidoreductases can account for both adverse and beneficial effects of the compounds.
Keywords: xenobiotic metabolism, catalytic antioxidants, oxidoreductase, human, rat
A number of new metal complexes are currently under investigation as catalytic antioxidant therapeutic agents and include manganese-containing porphyrins, salens, and cyclic polyamines (Day, 2004). Manganese porphyrins possess a number of antioxidant capacities such as superoxide (Pasternack and Skowronek, 1979) and hydrogen peroxide dismutation (Day et al., 1997), inhibition of lipid peroxidation (Day et al., 1999a), and scavenging peroxynitrite (Szabo et al., 1996). These activities have been linked to their protective effects in a wide variety of in vitro and in vivo models of oxidative stress (Day, 2004). Much of the compound development of manganese porphyrins has focused on changing their redox potentials towards those of the endogenous superoxide dismutase (SOD) enzymes (Batinic-Haberle et al., 1998). Some of the newer manganese porphyrins also have similar redox potentials and fast second order rate constants for their dismutation of superoxide, >107 M−1 s−1 (Batinic-Haberle et al., 1998). The endogenous SODs have the advantage that their protein structures provide a selective channel that limits electron transfers to small molecules like superoxide (Djinovic et al., 1992). The newly developed classes of metal complexes lack this degree of selectivity and thus are likely to give and accept electrons from a wide variety of biological molecules and potentially oxidoreductase enzymes.
Cytochrome P450 reductase is an important oxidoreductase that provides electrons for xenobiotic metabolism to the cytochrome P450 enzymes and heme oxygenase (Schacter et al., 1972). NADPH supplies reducing equivalents to the flavin-domain of cytochrome P450 reductase (Vermilion and Coon, 1978). Cytochrome P450s are a superfamily of enzymes involved in the synthesis and degradation of drugs and xenobiotics and in the formation of a number of important endogenous compounds, including hormones and neurotransmitters (Gonzalez, 1992). Inhibition of cytochrome P450 reductase has the potential to disrupt a number of pathways essential to normal cellular function, such as heme oxygenase (Choi and Alam, 1996).
A number of drugs and other xenobiotics are known to redox cycle with oxidoreductases, however, many of these reactions are associated with oxidative stress and cellular toxicity (Comporti, 1989). A common mechanism proposed for these types of reactions involves electron transfer between the xenobiotic and the flavin-domain of the oxidoreductase enzyme. Paraquat is a well-characterized redox-active compound that is known to redox cycle with a number of oxidoreductases including cytochrome P450 reductase (Byczkowski and Kulkarni, 1989) and nitric oxide synthase (Day et al., 1999b). Paradoxically, a few of these types of reactions can also lead to enhanced antioxidant activity, particularly if the xenobiotic can detoxify any generated reactive oxygen species. Our laboratory has recently reported that manganoporphyrins can redox cycle with flavin-dependent cellular enzymes in rat brain homogenates and that this activity partially contributes to their ability to inhibit lipid peroxidation (Kachadourian et al., 2004). In this report, we investigated whether manganoporphyrins affect xenobiotic metabolism and whether this may be through redox cycling with cytochrome P450 reductase.
MATERIALS AND METHODS
Reagents
Chemicals and biologicals include manganese (III) meso-tetra-kis(1,3-dimethylimidazolium-2-yl) porphyrin pentachloride (MnTDMIP5+), manganese (III) meso-tetrakis(1,3-diethylimidazolium-2-yl) porphyrin penta-chloride (MnTDEIP5+), manganese (III) meso-tetrakis(N-ethylpyridinium-2-yl) porphyrin pentachloride (MnTEPyP5+), manganese (III) meso-tetrakis(4-benzoic acid) porphyrin sodium chloride salt (MnTBAP) (kind gifts from Aeolus Pharmaceuticals, Greenwood Village, CO); sterile phosphate buffered saline pH 7.4 (PBS, Invitrogen Corporation, Carlsbad, CA); 7-methoxyresorufin, 7-benzyloxyresorufin, and resorufin (Molecular Probes, Eugene, OR). Human recombinant cytochrome P450 reductase was purchased from Panvera (Madison, WI). Control and induced rat liver microsomes and pooled human liver microsomes were purchased from In Vitro Technologies (Baltimore, MD). All other chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Xenobiotic metabolism assays
Microsomal cytochrome P450 activity was assessed using two different substrates, benzyloxyresorufin (BR) and methoxyresorufin (MR) that are metabolized to resorufin by a broad range of cytochrome P450s (Burke and Mayer, 1983). Resorufin formation was followed spectrofluorometrically (RF5301 PC, Shimadzu) using an excitation wave-length of 530 nm and emission wavelength of 585 nm. Reactions were performed in buffer (2 ml) containing 0.1 M potassium phosphate (pH 7.8), 3 mM MgCl2, 1 mg/ml microsomal protein, 2.5 μM MR or BR, and 250 μM NADPH. Reactions were performed in triplicate and followed for 1 min at 25°C. The change in fluorescence/min was converted to pmol resorufin formed/min based on a resorufin standard curve that was linear over the concentration range measured for both benzyloxyresorufin O-dealkylase (BROD) and methoxyresorufin O-dealkylase (MROD) activities.
NADPH oxidase assay
Recombinant human cytochrome P450 reductase dependent NADPH oxidation to NADP+ was followed spectrophotometrically (UV2401 PC, Shimadzu) at 340 nm. Reactions were performed in buffer (1 ml) containing 0.1 M potassium phosphate (pH 7.8), 3 mM MgCl2, 2 μg/ml recombinant human cytochrome P450 reductase, and 250 lM NADPH. Reactions were followed for 1 min at 25°C. The change in absorbance/min was converted to nmol NADPH consumed/min using NADPH’s extinction coefficient (ε = 6.187 × 102 l/mol × mm). Reactions were performed in duplicate.
Oxygen redox cycling assay
The involvement of oxygen redox cycling in the inhibition of cytochrome P450 reductase was assessed using an oxygen electrode (OXEL-1 electrode and ISO2 Meter, World Precision Instruments). The analog signal was converted to a digital signal (DUO-18, World Precision Instruments) for quantification. Reactions were performed in buffer (1 ml) containing 0.1 M potassium phosphate (pH 7.8), 3 mM MgCl2, 2 μg/ml recombinant human cytochrome P450 reductase, and 250 μM NADPH and various concentrations of test compounds. Reactions were followed for 1 min at 25°C. Spontaneous oxygen consumption in the absence of cytochrome P450 reductase was subtracted from the measured rates obtained with enzyme and was less than 3% of the observed rates. Electrode signal was calibrated for Denver oxygen tension by making an oxygen-saturated buffer solution anaerobic with sodium hydrosulfite. Data was expressed as μM O2 consumed/min/mg protein. Reactions were performed in triplicate.
Data analyses
Data were presented as their means ± SE. Comparisons between multiple groups were made using a one-way analysis of variance (ANOVA) and a Tukey’s range test was employed (Prism v3, GraphPad). The acceptable level of significance was established at p < 0.05. The inhibitory concentrations of manganoporphyrins that decreased xenobiotic metabolism by 50% (IC50) were determined by fitting a sigmoidal curve with variable slope to the data (Prism v3, GraphPad).
RESULTS
In Vitro Inhibition of Liver Microsomal Metabolism by Manganoporphyrins
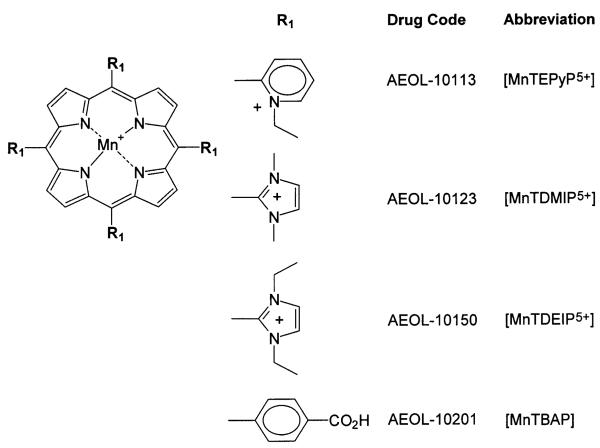
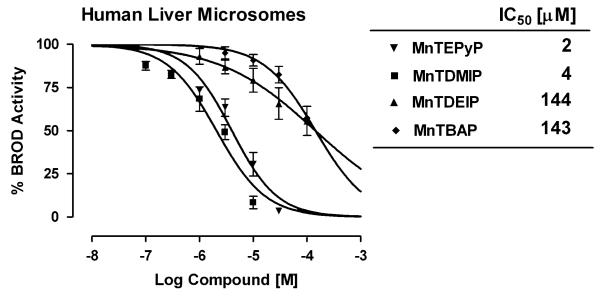
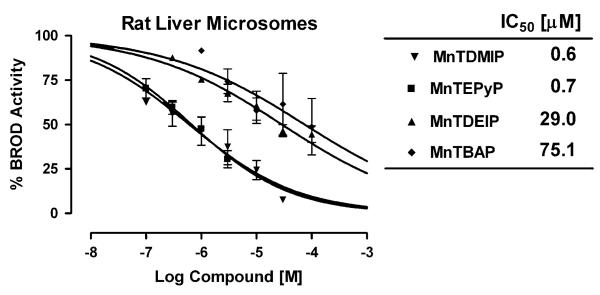
Cationic porphyrins are being pursued as catalytic antioxidants for the treatment of a variety of human diseases that involve the overproduction of reactive oxygen and nitrogen species (Fig. 1). A common concern during drug development involves the inhibition of xenobiotic metabolism that may result in drug-drug interactions. A series of manganoporphyrins were tested for their ability to inhibit liver microsomal xenobiotic metabolism. Resorufin analogs provide a convenient and sensitive fluorometric method to assess changes in xenobiotic metabolism in vitro (Lubet et al., 1990). Control rat liver microsomes were incubated with increasing concentrations of manganoporphyrins and inhibition of benzoxyresorufin O-dealkylase activity (BROD) metabolism was measured spectrofluorometrically. The inhibitory concentrations of manganoporphyrins that decreased xenobiotic metabolism by 50% (IC50) were determined by fitting a sigmoidal curve with variable slope to the data. The tetrakis(N-pyridinium-2-yl) meso-substituted manganoporphyrin, MnTEPyP5+, and the tetrakis(1,3-dimethyl imidazolium-2-yl) meso-substituted manganoporphyrin, MnTDMIP5+, were 40 to 100 times more potent inhibitors of rat liver microsomal BROD metabolism than the tetrakis(1,3-diethyl imidazolium-2-yl) meso-substituted manganoporphyrin, MnTDEIP5+, or the tetrakis(4-benzoic acid) meso-substituted manganoporphyrin, MnTBAP (Fig. 2). To test whether there was species specificity; the reaction was also examined in pooled human liver microsomes. A similar pattern of liver microsomal BROD inhibition by manganoporphyrins was also observed in the human samples (Fig. 3). The only difference between rat and human microsomal BROD inhibition was that the manganoporphyrins were about three-fold less potent. The apparent mechanism of the manganoporphyrin inhibition of xenobiotic metabolism was further investigated using β-naphthoflavone-induced rat liver microsomes and following methoxyresorufin O-dealkylase (MROD) activity spectrofluorometrically. β-Naphthoflavone induces the expression of CYP1A family of cytochrome P450 enzymes that prefers shorter alkyl chain substituted resorufin analogs (Novak and Qualls, 1989). Again the pattern of MROD inhibition by manganoporphyrins was remarkably similar to pattern observed for BROD inhibition (Fig. 4). These data suggested that the manganoporphyrins may share a common mechanism for the inhibition of xenobiotic metabolism and further studies focused on the commonly shared cyto-chrome P450 reductase that supplies electrons to the different cytochrome P450 isozymes.
FIG. 1.
The chemical structures of several manganoporphyrins: manganese (III) meso-tetrakis(1,3-dimethylimidazolium-2-yl) porphyrin (MnTDMIP5+), manganese (III) meso-tetrakis(1,3-diethylimidazolium-2-yl) porphyrin (MnTDEIP5+), manganese (III) meso-tetrakis(N-ethylpyridinium-2-yl) porphyrin (MnTEPyP5+), and manganese (III) meso-tetrakis(4-benzoic acid) porphyrin (MnTBAP) are illustrated.
FIG. 2.
In vitro inhibition of rat liver microsomal metabolism by a series of manganoporphyrins. Control rat liver microsomes are incubated with increasing concentrations of manganoporphyrins and inhibition of benzyloxy-resorufin O-dealkylase (BROD) activity is measured spectrofluorometrically. Data is presented as the percent of BROD activity in the absence of manganoporphyrins. Control BROD activity is 120 ± 10 pmol/min/mg. Each value is performed in triplicate and data are presented as their mean ± SEM. The inhibitory concentrations of manganoporphyrins that decreased xenobiotic metabolism by 50% (IC50) are determined by fitting a sigmoidal curve with variable slope to the data. Both the tetrakis(N-pyridinium-2-yl), MnTEPyP5+, and the tetrakis(1,3-dimethyl imidazolium-2-yl), MnTDMIP5+, meso-substituted manganoporphyrins are potent inhibitors of rat liver microsomal BROD metabolism while the tetrakis(1,3-diethyl imidazolium-2-yl), MnTDEIP5+, and the tetrakis(4-benzoic acid), MnTBAP, meso-substituted manganoporphyrins are weak inhibitors.
FIG. 3.
In vitro inhibition of human liver microsomal metabolism by a series of manganoporphyrins. Pooled human liver microsomes are incubated with increasing concentrations of manganoporphyrins and inhibition of benzyloxyresorufin O-dealkylase (BROD) activity is measured spectrofluoro-metrically. Data is presented as percent of BROD activity in the absence of manganoporphyrins. Control BROD activity is 60 ± 12 pmol/min/mg. Each value is performed in triplicate and data are presented as their mean ± SEM. The inhibitory concentrations of manganoporphyrins that decreased xenobiotic metabolism by 50% (IC50) are determined by fitting a sigmoidal curve with variable slope to the data. Both the MnTEPyP5+ and MnTDMIP5+ cationic manganoporphyrins are potent inhibitors of human liver microsomal BROD metabolism while the cationic MnTDEIP 5+, and the non-cationic MnTBAP, manganoporphyrins are weak inhibitors.
FIG. 4.
In vitro inhibition of rat liver β-naphthoflavone-induced microsomal metabolism by a series of manganoporphyrins. β-Naphthoflavone-induced rat liver microsomes are incubated with increasing concentrations of manganoporphyrins and inhibition of methoxyresorufin O-dealkylase (MROD) activity is measured spectrofluorometrically. Data is presented as percent of MROD activity in the absence of manganoporphyrins. Control MROD activity is 55 ± 6 pmol/min/mg. Each value is performed in triplicate and data are presented as their mean ± SEM. The inhibitory concentrations of manganoporphyrins that decreased xenobiotic metabolism by 50% (IC50) are determined by fitting a sigmoidal curve with variable slope to the data. The MnTEPyP5+ and MnTDMIP5+ cationic manganoporphyrins are potent inhibitors of rat liver β-naphthoflavone-induced microsomal metabolism while the cationic MnTDEIP5+ and the non-cationic MnTBAP manganoporphyrins are much less potent inhibitors.
Manganoporphyrins Redox Cycle with Cytochrome P450 Reductase
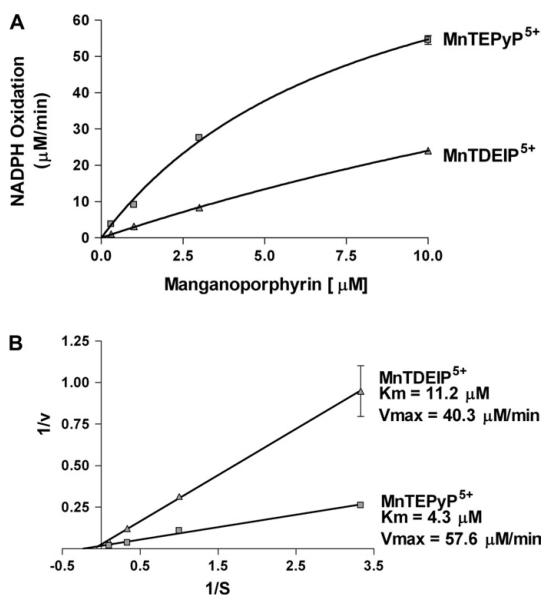
One potential explanation for manganoporphyrins’ inhibition of microsomal xenobiotic metabolism would be their ability to compete with cytochrome P450s for electrons from cytochrome P450 reductase. To test for this possibility, two manganoporphyrins with similar charge and redox potentials were assayed for their ability to stimulate NADPH utilization by human recombinant cytochrome P450 reductase. Neither compound stimulated NADPH oxidation in the absence of cytochrome P450 reductase. Both MnTEPyP5+ (a potent inhibitor of xenobiotic metabolism) and MnTDEIP5+ (weaker inhibitor of xenobiotic metabolism) stimulated NADPH utilization by human recombinant cytochrome P450 reductase in a dose-dependent manner (Fig. 5A). Kinetic analysis of the data revealed that the Km for the reaction was lower for MnTEPyP5+ than MnTDEIP5+ (Fig. 5B). The data also showed a higher Vmax for MnTEPyP5+ than MnTDEIP5+.
FIG. 5.
Cationic manganoporphyrins stimulate NADPH oxidation by human cytochrome P450 reductase. Human recombinant cytochrome P450 reductase and NADPH (250 μM) is incubated in the presence of increasing concentrations of either MnTDEIP5+ or MnTEPyP5+ and NADPH oxidation is followed spectrophotometrically at 340 nm for 1 min. Each value is performed in triplicate and data are presented as their mean ± SEM. (A) Both MnTEPyP5+ and MnTDEIP5+ stimulated cytochrome P450 reductase oxidation of NADPH. (B) Lineweaver-Burke plot of data reveals a lower Km and higher Vmax for MnTEPyP5+ than MnTDMEIP5+.
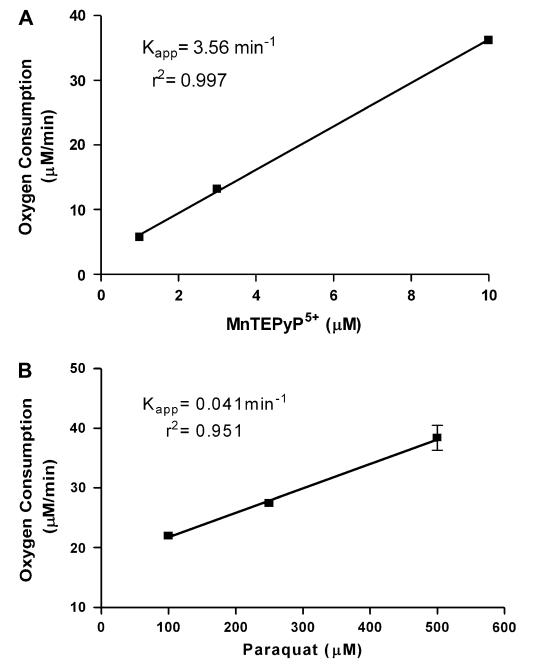
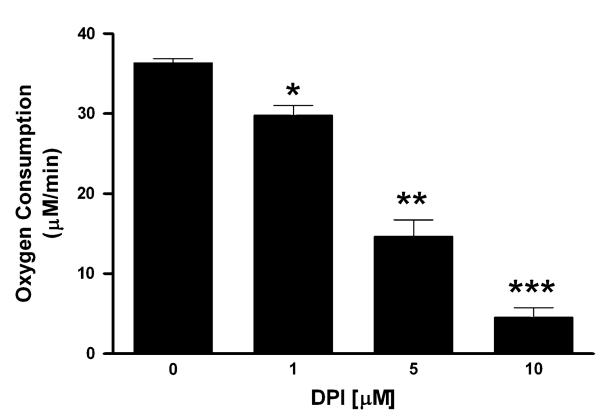
Most compounds that redox cycle with flavin-containing proteins, such as cytochrome P450 reductase, often can utilize oxygen as a terminal electron acceptor (Hochstein, 1983). To test for this possibility, oxygen utilization was assessed using an oxygen electrode in the presence of NADPH, MnTEPyP5+, and human recombinant cytochrome P450 reductase (Fig. 6A). MnTEPyP5+ stimulated oxygen consumption by human re-combinant cytochrome P450 reductase in a dose-dependent manner. MnTEPyP5+’s ability to redox cycle with human recombinant cytochrome P450 reductase was compared with another well-known redox cycler, paraquat (Fig. 6B). MnTEPyP5+ was a much more active redox cycling agent with human recombinant cytochrome P450 than paraquat. The mechanism of MnTEPyP5+ redox cycling was further investigated using a non-specific flavin domain inhibitor, diphenyleneiodonium (DPI) (McGuire et al., 1994). DPI was an effective inhibitor of MnTEPyP5+ redox cycling with human recombinant cyto-chrome P450 reductase with an IC50 of 4 μM (Fig. 7). The rank order potency of the manganoporphyrins to redox cycle with human recombinant cytochrome P450 reductase was similar to those found for the inhibition of xenobiotic metabolism. These data support the hypothesis that manganoporphyrins may inhibit xenobiotic metabolism through redox cycling with cytochrome P450 reductase.
FIG. 6.
Comparison between a cationic manganoporphyrin and paraquat in the stimulation of oxygen consumption by human cytochrome P450 reductase. Human recombinant cytochrome P450 reductase and NADPH is incubated in the presence of increasing concentrations of either (A) MnTEPyP5+ or (B) paraquat stimulated oxygen consumption is followed with an oxygen electrode for 1 min. Each value is performed in triplicate and data are presented as their mean ± SEM. The pseudo-first order rate constant for MnTEPyP5+ is two orders of magnitude higher than seen with paraquat. These data suggest that MnTEPyP5+ is a much more efficient redox cycling agent with cytochrome P450 reductase than paraquat.
FIG. 7.
Inhibition of cationic manganoporphyrin redox cycling with cytochrome P450 reductase by the flavin domain inhibitor, diphenyleneiodonium (DPI). Human recombinant cytochrome P450 reductase, NADPH (250 μM), and MnTEPyP5+ (10 μM) are incubated in the presence of increasing concentrations of DPI and oxygen consumption is followed with an oxygen electrode for 1 min. Each value is performed in triplicate and data are presented as their mean ± SEM. DPI effectively inhibited the redox cycling of MnTEPyP5+ with cytochrome P450 reductase in a dose-dependent manner with an IC50 of 4 μM. Asterisks indicate significant differences from one another, p < 0.05.
DISCUSSION
The major findings of these studies are (1) manganoporphyrins can inhibit microsomal xenobiotic metabolism; (2) the potency for this inhibition is sensitive to small changes on the meso substituents; (3) in vitro antioxidant activities do not correlate with inhibition of xenobiotic metabolism; and (4) the mechanism of microsomal xenobiotic metabolism inhibition occurs in part through redox cycling with the flavin domain of cytochrome P450 reductase. These data have been utilized in the selection and drug development of manganoporphyrins that minimize the potential for drug-drug interactions as new therapeutics for the possible treatment of a wide variety of human diseases. These studies helped in the selection of MnTDEIP5+ (AEOL 10150) that is currently in human clinical trials.
A common concern in drug development is the potential for a drug to alter xenobiotic metabolism. This can lead to altered metabolism of other drugs and/or endogenous compounds that can adversely affect the health of an individual. Drugs and other xenobiotics can alter metabolism by either inducing or inhibiting xenobiotic metabolism. These types of reactions are undesirable properties of drug candidates. During the initial development of manganoporphyrins it was observed that a series of highly active antioxidant compounds could also inhibit microsomal xenobiotic metabolism. Structure activity relationships revealed that the cationic manganoporphyrins, MnTEPyP5+ and MnTDMIP5+, were potent inhibitors of microsomal BROD and MROD activities. However, this potent inhibition was lost by chain extension of the alkyl groups of MnTDMIP5+ to form MnTDEIP5+. These data suggested that bulky alkyl substitutions on the imidazolium could be used to limit the inhibition of xenobiotic metabolism. Interestingly, both MnTDMIP5+ and MnTDEIP5+ have similar redox potentials and catalytic antioxidant activities (Kachadourian et al., 2004), so that the chain extension did not appear to affect their in vitro therapeutic activities. However, recent studies from our laboratory suggest that in vivo catalytic antioxidant activity of manganoporphyrins is partially due to redox cycling with flavin containing enzymes (Kachadourian et al., 2004). It is also possible that each flavin containing oxidoreductase has different structural requirements for efficient electron transfers and one may be able to selectively utilize some oxidoreductases while avoiding others. Therefore, it remains to be determined whether the in vivo activity of manganoporphyrins will be severely affected by bulky alkyl substitutions. It is also likely that other metal complexes besides porphyrins also share this ability to compete for electrons from endogenous flavin containing oxidoreductases. Many of these complexes also share similar redox potentials, can accept one to two electrons, and are smaller and more hydrophobic than most metalloporphyrins. However, to date, most characterized antioxidant activities for the manganosalens (Eukarion series) and polycyclic amines (Metaphore series) have been restricted to in vitro reactions devoid of flavin containing oxidoreductases (Baudry et al., 1993; Salvemini et al., 1999).
Manganoporphyrins are potent antioxidants in a wide number of in vitro, ex vivo, and in vivo models of oxidative stress (Day, 2004). The present studies are paradoxical given that most compounds identified to date that undergo redox cycling are generally considered as pro-oxidants and not antioxidants. One possible explanation for these paradoxical activities is that the manganoporphyrins can not only generate reactive species but can also consume them, unlike compounds such as paraquat that only generate reactive species. Since manganoporphyrins have been shown to strongly protect against oxidative stress, it is very likely that their in vivo scavenging activities greatly exceed their ROS generating activities. In fact, the low steady-state levels of superoxide in biological systems suggest that the reduction of manganoporphyrins is likely to occur by either endogenous low molecular weight antioxidants, like glutathi-one and ascorbate, or flavin-dependent oxidoreductases both of which are much more abundant in cells than superoxide. This concept would support the manganoporphyrins acting as super-oxide reductases that derive the first electron from a flavin-dependent oxidoreductase, which it can use to convert superoxide to hydrogen peroxide (Liochev and Fridovich, 2000). In addition, manganoporphyrins can also dismutate hydrogen peroxide to oxygen and water (Day et al., 1997). These properties probably contribute to the manganoporphyrin’s paradoxical activities of being both a potent antioxidant and redox cycling agent. However, under conditions such as elevated oxygen tensions the metalloporphyrins lose their antioxidant potency and are ineffective at preventing hyperoxia-induced lung injury (Day, unpublished data).
Flavin-dependent oxidoreductases comprise an abundant class of cellular enzymes with a wide range of important cellular functions. It is possible that inhibition of these enzymes could account for both adverse and possibly beneficial effects of the compounds. Many oxidoreductases, such as cytochrome P450 reductase, have been implicated either directly or indirectly in the endogenous production of reactive oxygen species (Bartoszek and Wolf, 1992; Rashba-Step and Cederbaum, 1994; Tindberg and Ingelman-Sundberg, 1989). Among this class of enzymes there are a number of enzymes well characterized to be involved in both oxidative and nitrosative stress. Nitric oxide synthases are well-characterized oxidoreductases that have been implicated in a number of pathologies associated with the overproduction of nitric oxide (Gross and Wolin, 1995; Rosen et al., 1995). Manganoporphyrins can inhibit nitric oxide production (Pfeiffer et al., 1998). A number of metal complexes with antioxidant activities protect against both in vitro and in vivo models of nitrosative stress (Macarthur et al., 2003; McDonald et al., 2003; Pfeiffer et al., 2001; Szabo et al., 2002; Wang et al., 2003). It remains to be tested whether metal complexes protect against nitrosative stress solely by scavenging nitric oxide and peroxynitrite or also through the inhibition of nitric oxide synthetases. Both the scavenging of reactive nitrogen species and/or inhibition of nitric oxide synthetases would provide similar protective outcomes. A number of oxidoreductases also have been characterized as sources of reactive oxygen species generators and include cytochrome P450 reductase (Kappus and Sies, 1981), cyclooxygenases (Weissmann et al., 1982), lipoxygenases (Baud and Ardaillou, 1986), xanthine oxidase (Grisham and Granger, 1988), NADPH oxidase (Forman and Torres, 2002), and several related Noxs and Duoxs (Lambeth, 2002). In fact, manganocomplexes may owe their in vivo potency by both scavenging reactive oxygen and nitrogen species and diminishing their production. Our laboratory is currently investigating these possibilities.
In summary, manganoporphyrins were found to inhibit microsomal xenobiotic metabolism that demonstrated sensitivity to small changes in carbon length on the meso-substituents. The mechanism for this inhibition appears to involve the redox cycling with cytochrome P450 reductase. The rate of redox cycling with cytochrome P450 reductase correlated with the potency of microsomal xenobiotic metabolism.
ACKNOWLEDGMENTS
These studies were supported in part by a National Institutes of Health Grant HL-31992 and a research grant from Aeolus Pharmaceuticals, Inc.
REFERENCES
- 1.Bartoszek A, Wolf CR. Enhancement of doxorubicin toxicity following activation by NADPH cytochrome P450 reductase. Biochem. Pharmacol. 1992;43:1449–1457. doi: 10.1016/0006-2952(92)90201-s. [DOI] [PubMed] [Google Scholar]
- 2.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 3.Baud L, Ardaillou R. Reactive oxygen species: Production and role in the kidney. Am. J. Physiol. 1986;251:F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- 4.Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Salen-manganese complexes are superoxide dismutase-mimics. Biochem. Biophys. Res. Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- 5.Burke MD, Mayer RT. Differential effects of phenobarbitone and 3-methylcholanthrene induction on the hepatic microsomal metabolism and cytochrome P-450-binding of phenoxazone and a homologous series of its n-alkyl ethers (alkoxyresorufins) Chem. Biol. Interact. 1983;45:243–258. doi: 10.1016/0009-2797(83)90072-8. [DOI] [PubMed] [Google Scholar]
- 6.Byczkowski JZ, Kulkarni AP. NADPH-dependent drug redox cycling and lipid peroxidation in microsomes from human term placenta. Int. J. Biochem. 1989;21:183–190. doi: 10.1016/0020-711x(89)90107-9. [DOI] [PubMed] [Google Scholar]
- 7.Choi AM, Alam J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell. Mol. Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 8.Comporti M. Three models of free radical-induced cell injury. Chem. Biol. Interact. 1989;72:1–56. doi: 10.1016/0009-2797(89)90016-1. [DOI] [PubMed] [Google Scholar]
- 9.Day BJ. Catalytic antioxidants: A radical approach to new therapeutics. Drug Discov. Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 10.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med. 1999a;26:730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 11.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 12.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1999b;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djinovic K, Coda A, Antolini L, Pelosi G, Desideri A, Falconi M, Rotilio G, Bolognesi M. Crystal structure solution and refinement of the semisynthetic cobalt-substituted bovine erythrocyte superoxide dismutase at 2.0 A resolution. J. Mol. Biol. 1992;226:227–238. doi: 10.1016/0022-2836(92)90135-7. [DOI] [PubMed] [Google Scholar]
- 14.Forman HJ, Torres M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez FJ. Human cytochromes P450: problems and prospects. Trends Pharmacol. Sci. 1992;13:346–352. doi: 10.1016/0165-6147(92)90107-h. [DOI] [PubMed] [Google Scholar]
- 16.Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig. Dis. Sci. 1988;33:6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 17.Gross SS, Wolin MS. Nitric oxide: Pathophysiological mechanisms. Annu. Rev. Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 18.Hochstein P. Futile redox cycling: Implications for oxygen radical toxicity. Fundam. Appl. Toxicol. 1983;3:215–217. doi: 10.1016/s0272-0590(83)80128-6. [DOI] [PubMed] [Google Scholar]
- 19.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem. Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Kappus H, Sies H. Toxic drug effects associated with oxygen metabolism: Redox cycling and lipid peroxidation. Experientia. 1981;37:1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- 21.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr. Opin. Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Liochev SI, Fridovich I. Copper- and zinc-containing superoxide dismutase can act as a superoxide reductase and a superoxide oxidase. J. Biol. Chem. 2000;275:38482–38485. doi: 10.1074/jbc.M007891200. [DOI] [PubMed] [Google Scholar]
- 23.Lubet RA, Syi JL, Nelson JO, Nims RW. Induction of hepatic cytochrome P-450 mediated alkoxyresorufin O-dealkylase activities in different species by prototype P-450 inducers. Chem. Biol. Interact. 1990;75:325–339. doi: 10.1016/0009-2797(90)90075-x. [DOI] [PubMed] [Google Scholar]
- 24.Macarthur H, Couri DM, Wilken GH, Westfall TC, Lechner AJ, Matuschak GM, Chen Z, Salvemini D. Modulation of serum cytokine levels by a novel superoxide dismutase mimetic, M40401, in an Escherichia coli model of septic shock: Correlation with preserved circulating catecholamines. Crit. Care Med. 2003;31:237–245. doi: 10.1097/00003246-200301000-00037. [DOI] [PubMed] [Google Scholar]
- 25.McDonald MC, d’Emmanuele di Villa Bianca R, Wayman NS, Pinto A, Sharpe MA, Cuzzocrea S, Chatterjee PK, Thiemermann C. A superoxide dismutase mimetic with catalase activity (EUK-8) reduces the organ injury in endotoxic shock. Eur. J. Pharmacol. 2003;466:181–189. doi: 10.1016/s0014-2999(03)01538-3. [DOI] [PubMed] [Google Scholar]
- 26.McGuire JJ, Anderson DJ, Bennett BM. Inhibition of the biotransformation and pharmacological actions of glyceryl trinitrate by the flavoprotein inhibitor, diphenyleneiodonium sulfate. J. Pharmacol. Exp. Ther. 1994;271:708–714. [PubMed] [Google Scholar]
- 27.Novak J, Jr., Qualls CW., Jr Effects of phenobarbital and 3-methylcholanthrene on the hepatic cytochrome P-450 metabolism of various alkoxyresorufin ethers in the cotton rat (Sigmodon hispidus) Comp. Biochem. Physiol. C. 1989;94:543–545. doi: 10.1016/0742-8413(89)90110-2. [DOI] [PubMed] [Google Scholar]
- 28.Pasternack RF, Skowronek WR., Jr. Catalysis of the disproportionation of superoxide by metalloporphyrins. J. Inorg. Biochem. 1979;11:261–267. doi: 10.1016/s0162-0134(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in mouse peritoneal macrophages activated in vitro and in vivo: Evidence against an essential role of peroxynitrite. FASEB J. 2001;15:2355–2364. doi: 10.1096/fj.01-0295com. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Mayer B. Molecular actions of a Mn(III)Porphyrin superoxide dismutase mimetic and peroxynitrite scavenger: Reaction with nitric oxide and direct inhibition of NO synthase and soluble guanylyl cyclase. Mol. Pharmacol. 1998;53:795–800. doi: 10.1124/mol.53.4.795. [DOI] [PubMed] [Google Scholar]
- 31.Rashba-Step J, Cederbaum AI. Generation of reactive oxygen intermediates by human liver microsomes in the presence of NADPH or NADH. Mol. Pharmacol. 1994;45:150–157. [PubMed] [Google Scholar]
- 32.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 33.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 34.Schacter BA, Nelson EB, Marver HS, Masters BS. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J. Biol. Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- 35.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 36.Szabo C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, Groves JT. Part I: Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: Studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol. Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 37.Tindberg N, Ingelman-Sundberg M. Cytochrome P-450 and oxygen toxicity. Oxygen-dependent induction of ethanol-inducible cyto-chrome P-450 (IIE1) in rat liver and lung. Biochemistry. 1989;28:4499–4504. doi: 10.1021/bi00436a056. [DOI] [PubMed] [Google Scholar]
- 38.Vermilion JL, Coon MJ. Identification of the high and low potential flavins of liver microsomal NADPH-cytochrome P-450 reductase. J. Biol. Chem. 1978;253:8812–8819. [PubMed] [Google Scholar]
- 39.Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, Poole BD, Bowler RP, Day BJ, Crapo JD, Schrier RW. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am. J. Physiol. Renal Physiol. 2003;284:F532–F537. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 40.Weissmann G, Serhan C, Korchak HM, Smolen JE. Neutrophils: Release of mediators of inflammation with special reference to rheumatoid arthritis. Ann. N.Y. Acad. Sci. 1982;389:11–24. doi: 10.1111/j.1749-6632.1982.tb22122.x. [DOI] [PubMed] [Google Scholar]