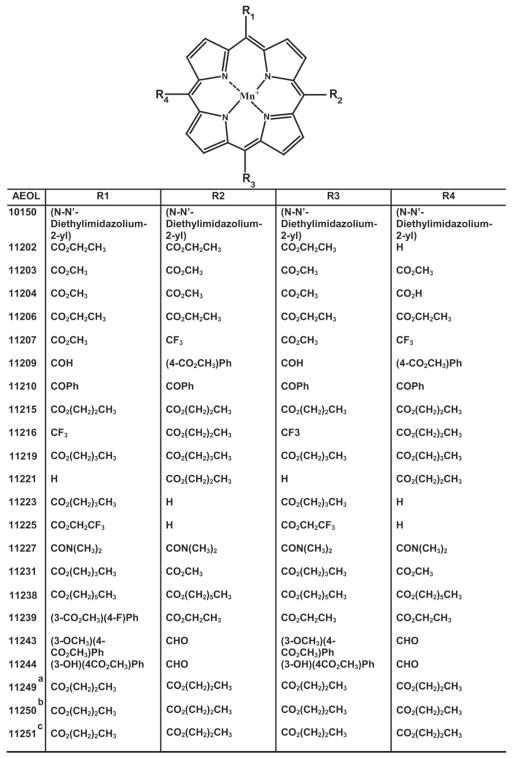
Abstract
Many studies have established a role for oxidative stress and mitochondrial dysfunction as an important mechanism in the pathogenesis of neuronal disorders. Metalloporphyrins are a class of catalytic antioxidants that are capable of detoxifying a wide range of reactive oxygen species. The AEOL112 series of glyoxylate metalloporphyrins were designed with increased lipid solubility for better oral bioavailability and penetration of the blood-brain barrier. The goal of this study was to develop an in vitro assay using rat brain mitochondria to reliably detect endogenously released hydrogen peroxide (H2O2) and identify glyoxylate metalloporphyrins based on rank order of potency for removal of physiologically relevant H2O2. A polarographic method was established for the sensitive, accurate, and reproducible detection of low levels of H2O2. The assay identified several potent glyoxylate metalloporphyrins with H2O2 scavenging potencies (IC50) in the nanomolar range. These results provide a simplified in vitro model system to detect physiologically generated mitochondrial H2O2 as a screening tool to predict the biological efficacy of potential therapeutic entities.
Oxidative stress is strongly implicated as a mediator of neuronal damage in diverse acute and chronic neuronal disorders (Lin and Beal, 2006). Metalloporphyrins are a class of catalytic antioxidants that are capable of detoxifying a wide range of reactive oxygen species (ROS), such as superoxide ( ), H2O2, peroxynitrite, and lipid peroxyl radicals (Patel and Day, 1999). Several water-soluble metalloporphyrin compounds, including manganese (III) meso-tetrakis (4-carboxyphenyl or benzoic acid) porphyrin (MnTBAP), AEOL10150, and AEOL10113, have been shown to be efficacious in animal models of central nervous system disorders, including status epilepticus, (Liang et al., 2000), stroke (Mackensen et al., 2001), and amyotrophic lateral sclerosis (Crow et al., 2005). Previous work has also demonstrated that manganic porphyrins can protect mature neuronal cultures from excitotoxic injury by scavenging intracellular (Patel et al., 1996; Li et al., 2001). These compounds contain a manganese center that catalytically dismutes both and H2O2 (Pasternack and Skowronek, 1979; Day et al., 1997) Previous meso-substituted porphyrin rings contained positively charged pyridyl (AEOL10113) or imidazole (AEOL10150) groups to electrostatically facilitate reaction with negatively charged (Batinic-Haberle et al., 1998; Kachadourian et al., 2004). However, the charged nature of the water-soluble pyridine- and imidazole-substituted metalloporphyrins makes them less efficient in crossing lipid membranes. To overcome these issues, a series of novel glyoxylate metalloporphyrins (AEOL112 series) with improved lipid solubility have been developed and chemically characterized (Trova et al., 2003) to improve the potential for in vivo therapeutic use in neurological disorders characterized by increased ROS levels and oxidative stress. This newly developed glyoxylate series of metalloporphyrins (Fig. 1) have been shown to dismute H2O2 in a catalase-like reaction to generate O2 and inhibit lipid peroxidation in cell-free systems (Kachadourian et al., 2003, 2004; Trova et al., 2003; Liang et al., 2007).
Fig. 1.
Chemical structure of AEOL compounds. The structure of hydrophobic glyoxylate manganoporphyrins with the indicated side groups (R1–R4) is shown. a, AEOL11249, Mn2+ was substituted by Zn2+; b, AEOL11250, Mn2+ was substituted by Fe2+; c, AEOL11251, Mn2+ was substituted by Co2+.
A major initial step toward determining the in vivo efficacy of glyoxylate (AEOL112 series) metalloporphyrins is the pre-selection of lead compounds in a simple yet physiologically relevant in vitro system. Cell-free antioxidant assays can be used for this purpose and have the advantage of allowing accurate assessment of antioxidant potencies without interference from cellular components. However, the lack of endogenous factors renders these systems less predictive of in vivo efficacy. These issues may be overcome by using simplified in vitro model systems that recapitulate more physiologically relevant conditions and therefore serve as better screening tools to predict the biological efficacy of potential therapeutic entities. We have recently demonstrated the mechanism of net ROS production from purified rat brain mitochondria by the redox-cycling agent paraquat (PQ2+) using a polarographic assay (Castello et al., 2007). The goals of this study were to 1) develop an in vitro assay that generated physiologically relevant H2O2 levels and 2) identify lead metalloporphyrin compounds based on rank order of potency for scavenging endogenously generated H2O2.
Materials and Methods
Materials
Metalloporphyrins with >97% purity were provided by Aeolus Pharmaceuticals (Laguna Niguel, CA). With the exceptions indicated, all the other drugs used in these studies were obtained from Sigma-Aldrich (St. Louis, MO).
Isolation of Purified Rat Brain Mitochondria
Animal housing was conducted in compliance with University of Colorado at Denver and Health Sciences Center (Denver, CO) procedures. Mitochondria were isolated from adult male Sprague-Dawley rats using Percoll gradient density centrifugation as described previously (Anderson and Sims, 2000) with minor modifications (Castello et al., 2007). The purity of mitochondrial fractions was assessed using Western blotting techniques. In brief, denatured protein fractions of cytosol, mitochondria, and whole-cell homogenate were separated by electrophoresis on a 10% polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membrane. Membrane blots were incubated with primary antibodies against lactate dehydrogenase (LDH) (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or cytochrome c oxidase subunit IV (COX), (1:1000; Molecular Probes, Eugene, OR). LDH and COX membranes were incubated with horse-radish peroxidase-conjugated anti-goat or anti-mouse secondary antibodies, respectively. Membranes were developed using an ECL Western blotting detection reagent (GE Healthcare, Buckinghamshire, UK). Figure 2 shows that COX was undetectable in cytosolic fractions and robustly expressed in mitochondrial fractions. In contrast, LDH was undetectable in mitochondrial samples indicating highly purified isolated fractions (Fig. 2).
Fig. 2.
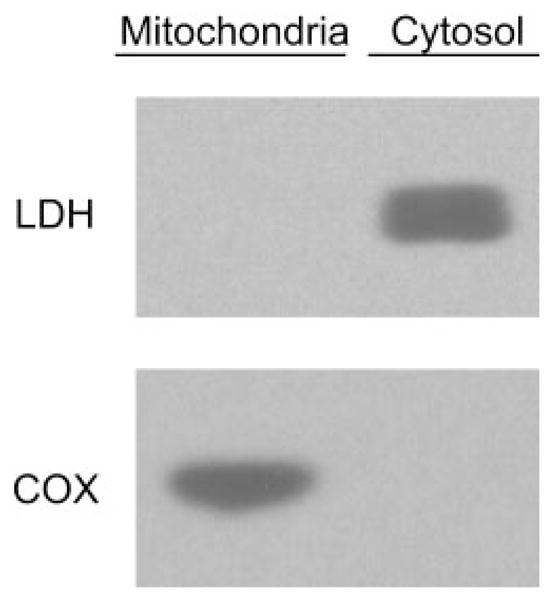
Purity of brain mitochondria fraction. Cytosolic and mitochondrial fractions of rat brain were isolated as described under Materials and Methods and subjected to Western blot analysis for cytosolic and mitochondrial protein markers, LDH and COX, respectively. Mitochondria samples robustly expressed COX, whereas LDH was undetectable, indicating highly purified mitochondrial fractions.
Polarographic Measurement of Net H2O2 Production
H2O2 net production by isolated brain mitochondria was measured using an Apollo 4000 Free Radical Analyzer (WPI, Sarasota, FL) equipped with a 100-μm H2O2 sensor. The measurements were conducted in a thermostatted open chamber at 30°C with a final reaction volume of 2 ml. Each measurement was started with the addition of reaction buffer (100 mM KCl, 75 mM mannitol, 25 mM sucrose, 10 mM Tris-Cl, and 10 mM KH2PO4, pH 7.4) to the chamber. Once the output signal stabilized, the following were consecutively added: respiration substrate (2.5 mM malate + 5 mM pyruvate) and mitochondrial protein (200 μg of protein). The output signal was allowed to stabilize subsequent to each addition, followed by the addition of 250 μM PQ2+ to the chamber, and the trace was recorded. After 2 to 3 min of recording, vehicle [1 μl of dimethyl sulfoxide (DMSO)] or tested compound (dissolved in 1 μl of DMSO) was added. The anti-oxidant activity of the metalloporphyrins was evaluated by measuring the change in the rate of H2O2 net production after the addition of the tested compound. The activity was expressed by the percentage of decrease in the rate of H2O2 net increase by mitochondria after the addition of the compound.
Fluorometric Detection of H2O2
H2O2 was measured using the horseradish peroxidase (HRP)-linked fluorometric assay (Amplex Ultra Red; Invitrogen, Carlsbad, CA). Brain mitochondria (10 μg) were added to a 96-well plate containing 100 μl of reaction buffer containing 0.1 U/ml HRP, 50 μM Amplex UltraRed and 2.5 mM malate + 5 mM pyruvate, 250 μM PQ2+, and 1 μl of DMSO control or tested compound dissolved in 1 μl of DMSO. Resorufin fluorescence was followed by a Gemini fluorescence microplate reader (Molecular Devices, Sunnyvale, CA). Superoxide dismutase (SOD) and catalase were added as controls at concentrations of 500 and 40 U/ml, respectively. The antioxidant activity of the compounds was expressed by the percentage of inhibition of the rate of H2O2 net increase by mitochondria in the presence of the compound.
SOD Screening Assay
The SOD-like activities were measured using the xanthine/xanthine oxidase system as a source of and ferricytochrome c as its indicating scavenger. was produced at the rate of 1.2 μM/min, and reduction of ferricytochrome c was followed at 550 nm. Assays were conducted in the presence of 0.1 mM EDTA in 0.05 M phosphate buffer, pH 7.8, at 25°C. Some metalloporphyrins that were analyzed interfered with the activity of xanthine oxidase, as checked by following urate production at 295 nm in the absence of cytochrome c, or they reoxidized cytochrome c at concentrations necessary to measure SOD activity.
Lipid Peroxidation Screening Assay
The concentration of thiobarbituric acid (TBA) reactive species (TBARS) in rat brain homogenates was used as an index of lipid peroxidation (Bernheim et al., 1948). Malondialdehyde (MDA) standards were obtained by adding 8.2 μl of 1,1,3,3-tetramethoxypropane in 10 ml of 0.01 M HCl and mixing for 10 min at room temperature. This stock was further diluted in water to give standards that ranged from 0.25 to 25 μM. Samples or standards (200 μl) were acidified with 200 μl of 0.2 M phosphoric acid in 1.5 ml of locking microfuge tubes. The color reaction was initiated by the addition of 25 μl of a 0.11 M thiobarbituric acid solution, and samples were placed in a 90°C heating block for 45 min. TBARS were extracted with 0.5 ml of n-butanol by vortexing samples for 3 min and chilling on ice for 1 min. The samples were then centrifuged at 12,000g for 3 min, and 150 μl of aliquots of the n-butanol phase were placed in each well of a 96-well plate and read at 535 nm in a plate-reader (Spectramax 340PC; Molecular Devices) at 25°C. Sample absorbances were converted to MDA equivalencies (micromolar) by extrapolation from the MDA standard curve. None of the antioxidants at concentrations used in these studies affected the reaction of MDA standards with TBA, and reactions without TBA were used as subtraction blanks.
Statistical Analysis
All experimental data shown were derived from at least two or three independent experiments. Nonlinear regression was performed with GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA) adjusting a sigmoidal dose (concentration)-response equation (variable slope).
Results
A Polarographic Assay for Evaluation of H2O2-Scavenging Activity
To develop a biologically relevant in vitro assay for detection of H2O2-scavenging activities, a polarographic method of H2O2 detection was used. Unlike other methods of H2O2 detection based on endpoint readings, the use of polarographic sensors allows for the real-time measurement of steady-state H2O2 concentrations that takes into account the contributions of both production and consumption of H2O2 in the system under study. We have previously shown that the addition of PQ2+ to isolated rat brain mitochondria supplemented with respiration substrates results in a rapid and robust net production/increase of H2O2 (Castello et al., 2007). The consistent and stable increase in H2O2 allows for the analysis of antioxidant activity of added compounds over a short period of time. To validate this method as a tool for identifying antioxidant molecules, we analyzed the effect of externally added endogenous antioxidant enzymes, catalase, and SOD. SOD had no effect on net H2O2 production at a saturating concentration of 500 U/ml, whereas catalase produced a concentration-dependent inhibition of PQ2+-induced mitochondrial H2O2 (Figs. 3, A and B, and 4).
Fig. 3.
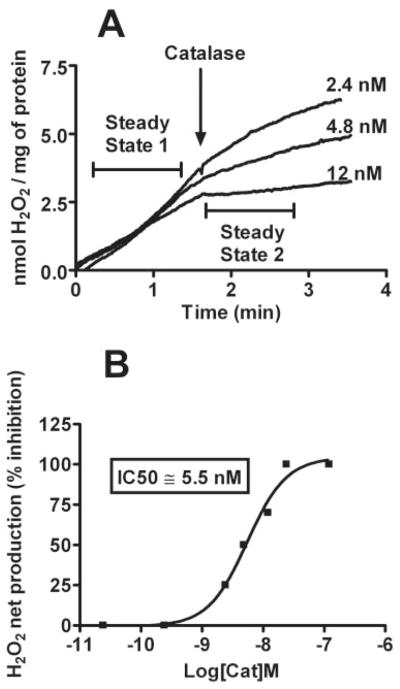
Change in the steady-state PQ2+-induced mitochondrial H2O2 and effect of catalase. A, polarographic detection of H2O2 net production from isolated mitochondria with the addition of 250 μM PQ2+. Rates of H2O2 net production before and after the addition of catalase (steady state 1 and 2, respectively) were compared to obtain the percentage of inhibition under each condition. B, concentration-response curve demonstrating inhibition of PQ2+-induced H2O2 by catalase, indicating an IC50 value of 5.5 nM.
Fig. 4.
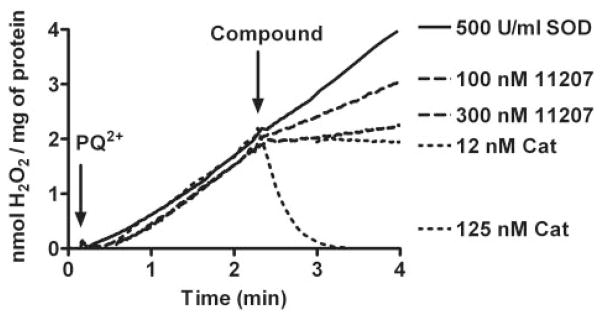
Inhibition of PQ2+-induced mitochondrial H2O2 by a representative glyoxylate metalloporphyrin. After the addition of PQ2+, H2O2 net production in mitochondria was monitored with addition of AEOL11207 (100 and 300 nM). AEOL11207 demonstrates a dose-dependent inhibition of mitochondrial net H2O2 production. Although exogenously added SOD at a saturating concentration (500 U/ml) had no effect on H2O2 net production, catalase is capable of scavenging H2O2, thus serving as a positive control. Similar results were obtained with other glyoxylate metalloporphyrins.
Because polarographic measurement of H2O2 is a novel, electrode-based methodology, a series of experiments were performed with the aim of identifying possible interferences. Control studies with the following treatments showed no changes over baseline H2O2 signal: 1) reaction buffer, 2) reaction buffer + mitochondria, 3) reaction buffer + mitochondria + PQ2+, 4) reaction buffer + mitochondria + malate + pyruvate, and 5) reaction buffer + PQ2+ + malate + pyruvate. Upon addition of PQ2+ to reaction buffer + mitochondria + malate + pyruvate, H2O2 was generated at the rate of 0.75 to 1 nmol/mg/min. Overall, these controls indicate that the polarographic measurement using an H2O2 electrode is not affected by the majority of the components used in the assays.
Inhibition of PQ2+-Induced Mitochondrial H2O2 by AEOL112 Compounds
Using the polarographic method described above, we were able to measure the H2O2-scavenging activities of metalloporphyrin compounds. Figure 4 shows an example of the results using AEOL11207. After the addition of PQ2+, H2O2 levels increased at a steady rate for several minutes at which point compounds were added to determine inhibitory effects. Catalase at a concentration of 12 nM was chosen as a positive control and was considered to represent 100% inhibition of the H2O2 net increase. This concentration of catalase caused no significant change in levels of H2O2, indicating a balance between the production and removal of H2O2 in the system. Increasing concentrations of catalase (125 nM) led to a decrease in H2O2 signal corresponding with the enzymatic removal of endogenously generated H2O2 in the system. Addition of SOD at saturating concentrations had no effect on the rate of H2O2 net production. A full range of inhibition curves was determined for different concentrations of AEOL112 compounds (Fig. 5). Figure 5 represents the percentage of inhibition of steady state of PQ2+-induced mitochondrial H2O2 as a function of the logarithm concentration of each compound. Using non-linear regression, the best fit for each concentration-response curve was obtained to determine IC50 values for each compound. Compounds were divided into two groups based on their observed H2O2-scavenging activities: 1) those compounds exhibiting a strong concentration-response relationship (Fig. 5; Table 1) and 2) those compounds exhibiting a less optimal concentration-response relationship (Table 1). The former group included compounds showing canonical concentration-response relationship with IC50 values obtained after nonlinear regression of the activity data. The latter contains compounds that do not demonstrate any inhibition of H2O2 net increase and/or have an IC50 value higher than 3 μM. Table 1 shows the IC50 values for the compounds that exhibited a strong concentration-response relationship. A notable exception is AEOL10150, which interfered with the polarographic detection. To ascertain whether the observed inhibition of H2O2 net production by metalloporphyrins was not due to a change in the sensitivity of the electrode for the H2O2 after addition of the compound, the effect of exogenously added H2O2 was determined. No change in signal was observed after the addition of the AEOL112 series compounds listed in Table 1 to the reaction buffer + mitochondria + malate + pyruvate + 2 μM H2O2. The most potent metalloporphyrins identified with IC50 < 1 μM are as follows: AEOL11209 (IC50 = 17 nM) > AEOL11216 (IC50 = 93 nM) ≥ AEOL11207 (IC50 = 104 nM) > AEOL11215 (IC50 = 206 nM) > AEOL11223 (IC50 = 408) > AEOL11210 (IC50 = 725 nM) > AEOL11202 (IC50 = 1642 nM).
Fig. 5.
Concentration-response curves of the inhibition of PQ2+-induced mitochondrial H2O2 by glyoxylate metalloporphyrins. The ability of glyoxylate metalloporphyrins to inhibit PQ2+-induced mitochondrial H2O2 net production was evaluated. Compounds that demonstrated a strong concentration-response relationship are shown. Compounds that exhibited a less optimal concentration-response relationship are indicated in Table 1. Each point represents the mean values from independent duplicate experiments.
TABLE 1.
Inhibition of PQ2+-induced H2O2 and catalase activity of selected glyoxylate metalloporphyrins
| Compound | IC50 | % Catalase Activity |
|---|---|---|
|
| ||
| nM | ||
| SOD | N.A. | N.A. |
| Catalase | 5.5 | 100 |
| AEOL10150 | (3000)* | (0.2)* |
| AEOL11209a | 17 | 32.4 |
| AEOL11216 | 93 | 5.9 |
| AEOL11207 | 104 (30)* | 5.3 (18.3)* |
| AEOL11215 | 206 | 2.2 |
| AEOL11223 | 408 | 1.3 |
| AEOL11210 | 725 | 0.8 |
| AEOL11202b | 1642 | 0.3 |
| AEOL11203 | >3000 | >0.2 |
| AEOL11204 | >3000 | >0.2 |
| AEOL11206 | >3000 | >0.2 |
| AEOL11219 | >3000 | >0.2 |
| AEOL11227 | >3000 | >0.2 |
| AEOL11238 | >3000 | >0.2 |
| AEOL11239 | >3000 | >0.2 |
| AEOL11243 | >3000 | >0.2 |
| AEOL11244 | >3000 | >0.2 |
| AEOL11249 | >3000 | >0.2 |
| AEOL11250 | >3000 | >0.2 |
| AEOL11251 | >3000 | >0.2 |
N.A., not applicable.
Activity determined using Amplex Red assay.
AEOL112 compounds in italics exhibit a strong concentration-response relationship.
AEOL112 compounds in regular font exhibit a less optimal concentration-response relationship.
Comparison of the Inhibition of PQ2+-Induced Mitochondrial H2O2 by AEOL10150 and AEOL112 Series
To validate the polarographic method and overcome its interference with AEOL10150, a HRP-linked fluorometric method (Amplex Red assay) was used. The values obtained were used to construct the concentration-response curve shown in Fig. 6. To compare the IC50 values obtained using the fluorometric assay with the polarographic method, AEOL11207 was used as a positive control. AEOL11207 showed a concentration-response relationship comparable to that obtained using the polarographic method. The IC50 values of AEOL11207 using the fluorometric and the polarographic assays were 30 and 104 nM, respectively. AEOL10150 showed a concentration-response relationship with an IC50 value of 3 μM, using the fluorometric assay.
Fig. 6.
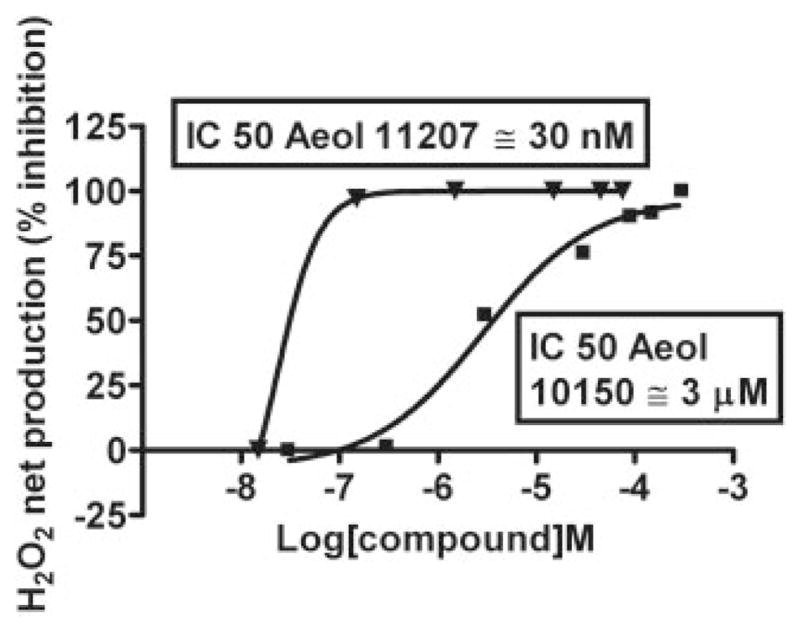
Concentration-response curves of inhibition of PQ2+-induced mitochondrial H2O2 by AEOL11207 and AEOL10150. H2O2 was fluorometrically detected using the HRP-linked Amplex Red assay. Rates of H2O2 net production were measured in the presence of different final concentrations of AEOL11207 and AEOL10150. The percentage of inhibition of H2O2 net production was determined for each concentration and used to calculate IC50 values for each compound. Each point is the mean of values from duplicate experiments.
Structure Activity Relationships of the AEOL112 Series with Their Ability to Inhibit Net Production of H2O2
The most potent inhibitors of H2O2 net production in the AEOL112 series were meso-substituted with electron-withdrawing groups such as aldehydes, as seen in the bis-substituted AEOL11209 compound and trifluoromethyl groups in the bis-substituted AEOL11207 and AEOL11216 compounds. It was interesting to note that n-alkyl-substituted ester groups also correlated with the ability of the compounds to inhibit the net production of H2O2 as seen with AEOL11215 that has a meso-tetrakis propyl ester substitution, but activity drops off substantially as one lengthens the alkyl chain as seen in the meso-tetrakis hexyl ester-substituted compound AEOL11238.
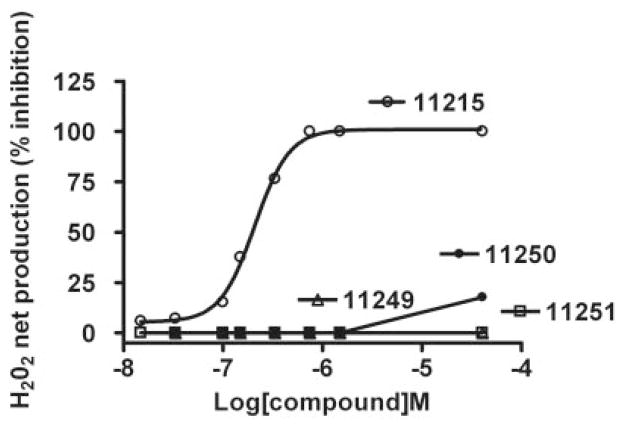
To determine whether inhibition of PQ2+-induced H2O2 by metalloporphyrins showing an optimal concentration-response relationship was due to the manganese moiety, the effects of metal-substituted analogs of AEOL11215 (AEOL11249, Zn2+ analog; AEOL11250, Fe2+ analog; AEOL11251, Co2+ analog) were evaluated. Figure 7 shows that none of these analogs was able to inhibit the net production of H2O2 in a broad range of concentrations. These studies suggest that manganese is the optimal metal to support H2O2-scavenging activity.
Fig. 7.
Concentration-response curves by metal-substituted analogs of AEOL11215. The ability of metal-substituted analogs of AEOL11215 to inhibit PQ2+-induced mitochondrial H2O2 net production was evaluated. These data show that none of the metal-substituted analogs was able to inhibit the net production of H2O2 over a broad range of concentrations, suggesting that manganese is critical in H2O2-scavenging activity. AEOL11215, Mn+; AEOL11249, Zn2+ analog; AEOL11250, Fe2+ analog; AEOL11251, Co2+ analog.
Antioxidant Properties of AEOL112 Compounds
Table 2 shows the antioxidant activity of hydrophobic metalloporphyrins evaluated as SOD-like activity and inhibition of lipid peroxidation (TBARS). Overall, the data indicate that AEOL112 compounds have very low SOD-like activity and very high lipid peroxidation inhibition activity. These properties, together with the high capacity to dismute H2O2, suggest that these compounds have an alternative antioxidant mechanism compared with that observed in known SOD mimetics.
TABLE 2.
Structures and activities of selected glyoxylate metalloporphyrins Each column contains the structure of the side chains (R1–R4) indicated in Fig 1.
| AEOL | Product No.a | SOD Activity | TBARS (IC50) |
|---|---|---|---|
|
| |||
| U/mg | μM | ||
| 10150 | N.L. | 13,658 | 0.1 |
| 11202 | 30 | N.A. | 0.1 |
| 11203 | 26 | N.A. | 0.725 |
| 11204 | 27 | N.A. | 0.259 |
| 11206 | 28 | 5.62 | 3.35 |
| 11207 | 37 | N.A. | 8.2 |
| 11209 | N.L. | 183 | 0.2 |
| 11210 | N.L. | 81 | 9 |
| 11215 | 32 | 129.5 | 6.65 |
| 11216 | 39 | 98 | 4 |
| 11219 | 33 | Interferes | 8.2 |
| 11221 | 45 | Red cytochrome c | 8.4 |
| 11223 | 47 | Interferes | 4.4 |
| 11225 | 43 | Interferes | 4.7 |
| 11227 | 36 | 4.5 | 30 |
| 11231 | 54 | 67.4 | 7.1 |
| 11238 | 35 | Red cytochrome c | 306 |
| 11239 | 52 | 6.5 | 0.91 |
| 11243 | N.L. | Interferes | N.A. |
| 11244 | N.L. | 84 | 36 |
| 11249 | N.L. | Inactive | Inactive |
| 11250 | N.L. | Inactive | Inactive |
| 11251 | N.L. | inactive | Inactive |
N.L., not listed; N.A., not applicable.
Former product number taken from (Gauuan et al., 2002; Trova et al., 2003).
Discussion
In this study, we developed a novel in vitro screening assay using polarographic detection of H2O2 endogenously generated in isolated rat brain mitochondria treated with PQ2+. Using this assay, we identified several potent antioxidant compounds belonging to a novel class of lipophilic glyoxylate metalloporphyrins.
A polarographic method was established for the sensitive, accurate, and reproducible detection of H2O2 scavenging by AEOL compounds in a physiologically relevant in vitro model involving rat brain mitochondrial H2O2. The method is rapid and sensitive, with low level of interferences, and has the potential for high-throughput analysis. The assay uses respiring mitochondria and PQ2+ to generate endogenous H2O2. The system is specific for H2O2 as shown by the high sensitivity to the addition of catalase but not SOD (Fig. 4). The ability of catalase to inhibit the H2O2 signal is due to the ability of intramitochondrially generated H2O2 to cross mitochondrial membranes, which then can be readily dismuted by catalase. The lack of effect with SOD is probably due to the inability of this large protein to penetrate mitochondrial membranes to dismute intramitochondrial , which is short-lived and not very permeable to biological membranes. Once the steady-state net increase of H2O2 was induced by PQ2+ in mitochondria, the addition of either several metalloporphyrins or catalase (but not SOD) changed the steady-state net production of H2O2. The velocity of H2O2 net production in the lower steady state was used as an indicator of compound potency. This approach has the advantage of using the initial steady state as a control, eliminating the random differences between different measurements and preparations in which the initial steady state may vary.
It is worth mentioning that in vitro H2O2 concentrations previously used in the measurement of catalase activity of AEOL compounds was 1 mM (Day et al., 1997). This concentration is orders of magnitude higher than H2O2 steady-state concentrations in physiological systems that are in the nanomolar range (Chance et al., 1979). Because catalase activity is assumed to follow pseudo-first-order kinetics, the use of such high amounts of H2O2 can result in an overestimation of the pseudo-first-order rate constant and, therefore, of catalase activity. Moreover, it has been reported that high amounts of H2O2 can inactivate the metalloporphyrins (Day et al., 1997). This new assay presents several advantages over the established ones. First, it uses concentrations of H2O2 (~1–100 nM) that may be achieved physiologically. Steady-state concentrations of H2O2 are estimated to 10−9 to 10−7 M (Chance et al., 1979; Gardner et al., 2006). Second, it uses H2O2 produced by brain mitochondria, which are an important cellular source of ROS contributing to neurodegeneration and aging. Third, it is based in an in vitro system using the redox-cycling agent PQ2+, an environmental toxin implicated in the etiology of Parkinson’s disease (Di Monte, 2003).
The results presented in Table 1 reveal the following order of potencies of the metalloporphyrins tested in this study that showed an IC50 < 1 μM: AEOL11209 (IC50 = 17 nM) > AEOL11216 (IC50 = 93 nM) > AEOL11207 (IC50 = 104 nM) > AEOL11215 (IC50 = 206 nM) > AEOL11223 (IC50 = 408) > AEOL11210 (IC50 = 725 nM) > AEOL11202 (IC50 = 1642 nM). The potencies of the compounds obtained in our in vitro assay have been validated in the in vivo setting by the demonstration that orally administered AEOL11207 achieving brain concentrations of ~200 nM inhibited oxidative stress indices and neuronal damage in a mouse model of mitochondrial oxidative stress (Liang et al., 2007).
The ability of manganese-substituted, but not zinc-, cobalt-, and iron-substituted, metalloporphyrins to inhibit the net production of H2O2 illustrates the importance of manganese as the optimal metal in the H2O2-scavenging effects of the compounds. Although the glyoxylate metalloporphyrins are lipid-soluble, the control studies described under Results suggest that most AEOL compounds do not inhibit the net production of H2O2 by interfering with the redox-cycling mechanism of PQ2+ in the mitochondria. Their ability to remove H2O2 is probably not caused by scavenging of intramitochondrial . This observation is based on their low SOD activity in cell-free assays (Trova et al., 2003), which renders the compounds less suitable as SOD mimetics.
Comparison of the data obtained in this study (Table 1) with previously published values for the AEOL112 series (Gauuan et al., 2002; Trova et al., 2003) demonstrates that compounds exhibiting a strong concentration-response relationship (Fig. 5; Table 1) have an average catalase activity ~145% higher than the average activity of the compounds exhibiting a less optimal concentration-response relationship (Table 1). On the other hand, the compounds demonstrating a strong concentration-response relationship (Fig. 5; Table 1) display average TBARS levels that are eight times lower than the average of the compounds exhibiting a less optimal concentration-response relationship, indicating a greater ability to remove lipid peroxides (Table 2). Together, these results conclude that the grouping of compounds according to their antioxidant properties was reflected not only by the current method but also by other previous screening methods.
One interesting finding that has emerged from several in vitro models of neuronal injury is a discrepancy between anti-oxidant potency and neuroprotective efficacy of metalloporphyrins. For example, although the water-soluble metalloporphyrin MnTE-2-PyP (AEOL10113) has at least 20 times more SOD activity in cell-free assays compared with MnTBAP, it was only two to three times more potent in its efficacy as a neuroprotective agent in Sod2−/− cultures (Patel, 2003). This paradoxical difference between antioxidant activities and neuroprotective efficacy has also been observed in other in vitro models involving glutamate excitotoxicity and oxygen-glucose deprivation injury (Li et al., 2001). These observations suggest that high SOD activity in a cell-free assay per se may not be sufficient to predict neuroprotection in vivo (cells or animals) and provides the rationale for the development of metalloporphyrins with broad antioxidant properties other than antioxidant activities derived from cell-free assays. The glyoxylate (AEOL112) series of metalloporphyrins have modest SOD activity but show high potencies as inhibitors of lipid peroxidation and cell and tissue injury (Choudhary et al., 2001; Kachadourian et al., 2003, 2004; Trova et al., 2003; Liang et al., 2007).
The ability of metalloporphyrins to scavenge mitochondrially generated H2O2 is highly significant based on previous demonstrations that mitochondrial overexpression of catalase provides protection against menadione toxicity, a chemical agent that preferentially generates intramitochondrially (Gurgul et al., 2004). Moreover, median and maximal life spans were increased the most in animals overexpressing mitochondrial catalase due to a reduction in mitochondrial generated oxidative stress (Schriner et al., 2005). Although the overexpression of mitochondrial catalase is difficult to achieve, the particular scavenging of mitochondrial H2O2 by AEOL compounds opens a new paradigm in the therapeutic treatment of neuronal diseases in which mitochondrial oxidative stress is a major contributor.
Acknowledgments
We gratefully acknowledge Wenfang (Wendy) Ji for technical assistance.
This work was supported by NINDS, National Institutes of Health Grants RO1NS045748, R01NS039587, and R21NS053548 (to M.P.) and Aeolus Pharmaceuticals (to M.P. and B.J.D.).
ABBREVIATIONS
- ROS
reactive oxygen species
- MnTBAP
manganese (III) meso-tetrakis (4-carboxyphenyl or benzoic acid) porphyrin
- AEOL10150
manganese (III) meso-tetrakis (N,N′-diethylimidazolium-2-yl) porphyrin
- AEOL10113
manganese (III) meso-tetrakis (N-ethyl pyridinium-2-yl) porphyrin
- PQ2+
paraquat
- LDH
lactate dehydrogenase
- COX
cytochrome c oxidase
- DMSO
dimethyl sulfoxide
- HRP
horseradish peroxidase
- SOD
superoxide dismutase
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive species
- MDA
malondialdehyde
- MnTE-2-PyP
manganese tetrakis-(N-ethyl-2-pyridyl) porphyrin
Footnotes
B.J.D. is a consultant for and holds equity in Aeolus Pharmaceuticals, which is developing catalytic antioxidants as therapeutic agents.
References
- Anderson MF, Sims NR. Improved recovery of highly enriched mitochondrial fractions from small brain tissue samples. Brain Res Brain Res Protoc. 2000;5:95–101. doi: 10.1016/s1385-299x(99)00060-4. [DOI] [PubMed] [Google Scholar]
- Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese (III) meso tetrakis (N-methylpyridinium-2-yl) porphyrin (MnTM-2-PyP5+) a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- Bernheim F, Bernheim MLC, Wilbur KM. The reaction between thiobarbituric acid and the oxidation products of certain lipids. J Biol Chem. 1948;174:257–264. [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Keshavarzian A, Yong S, Wade M, Bocckino S, Day BJ, Banan A. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig Dis Sci. 2001;46:2222–2230. doi: 10.1023/a:1011975218006. [DOI] [PubMed] [Google Scholar]
- Crow JP, Calingasan NY, Chen J, Hill JL, Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- Di Monte DA. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Gardner R, Moradas-Ferreira P, Salvador A. Why does superoxide dismutase overexpression often increase hydrogen peroxide concentrations? An alternative explanation. J Theor Biol. 2006;242:798–800. doi: 10.1016/j.jtbi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Gauuan PJ, Trova MP, Gregor-Boros L, Bocckino SB, Crapo JD, Day BJ. Superoxide dismutase mimetics: synthesis and structure-activity relationship study of MnTBAP analogues. Bioorg Med Chem. 2002;10:3013–3021. doi: 10.1016/s0968-0896(02)00153-0. [DOI] [PubMed] [Google Scholar]
- Gurgul E, Lortz S, Tiedge M, Jorns A, Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes. 2004;53:2271–2280. doi: 10.2337/diabetes.53.9.2271. [DOI] [PubMed] [Google Scholar]
- Kachadourian R, Flaherty MM, Crumbliss AL, Patel M, Day BJ. Synthesis and in vitro antioxidant properties of manganese(III) β-octabromo-meso-tetrakis(4-carboxyphenyl)porphyrin. J Inorg Biochem. 2003;95:240–248. doi: 10.1016/s0162-0134(03)00135-1. [DOI] [PubMed] [Google Scholar]
- Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Li QY, Pedersen C, Day BJ, Patel M. Dependence of excitotoxic neurodegeneration on mitochondrial aconitase inactivation. J Neurochem. 2001;78:746–755. doi: 10.1046/j.1471-4159.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- Liang L-P, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27:4326–4333. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Mackensen GB, Patel M, Sheng H, Calvi CL, Batinic-Haberle I, Day BJ, Liang LP, Fridovich I, Crapo JD, Pearlstein RD, et al. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack RF, Skowronek WR., Jr Catalysis of the disproportionation of superoxide by metalloporphyrins. J Inorg Biochem. 1979;11:261–267. doi: 10.1016/s0162-0134(00)80022-7. [DOI] [PubMed] [Google Scholar]
- Patel M. Metalloporphyrins improve the survival of Sod2-deficient neurons. Aging Cell. 2003;2:219–222. doi: 10.1046/j.1474-9728.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- Patel M, Day BJ. Metalloporphyrin class of therapeutic catalytic antioxidants. Trends Pharmacol Sci. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Trova MP, Gauuan PJ, Pechulis AD, Bubb SM, Bocckino SB, Crapo JD, Day BJ. Superoxide dismutase mimetics. Part 2: synthesis and structure-activity relationship of glyoxylate- and glyoxamide-derived metalloporphyrins. Bioorg Med Chem. 2003;11:2695–2707. doi: 10.1016/s0968-0896(03)00272-4. [DOI] [PubMed] [Google Scholar]