Abstract
In the cell, mRNAs and non-coding RNAs exist in association with proteins to form ribonucleoprotein (RNP) complexes. Regulation of RNP stability and function is achieved by alterations to the RNP through poorly understood mechanisms into which recent studies have now begun to provide insight. This emerging body of work identifies chemical modifications of RNPs at the RNA or protein level and ATP-dependent RNP remodeling by RNA helicases/RNA-dependent ATPases as central events that dictate RNA fate. Some RNP modifications serve as tags for recruitment of regulatory proteins, with RNP modifiers and recruited proteins analogous to the writers and readers of chromatin modification, respectively. This review highlights examples of in which RNP modification and ATP-dependent remodeling play key roles in the control of eukaryotic RNA fate, suggesting that we are only at the beginning of uncovering the multitude of ways in which RNP modification and remodeling impact RNA regulation.
Keywords: RNA tailing, uridylation, ribonucleotidyltransferase, RNA modification, RNP modification, post-translational modification, RNA helicase, RNA decay
RNAs function in, and are regulated as, RNPs
Life depends on the proper decoding of information contained within DNA into the creation of functional molecules, and maintenance of those molecules at levels that meet cellular needs and changing environmental stimuli. The immediate product of DNA decoding is RNA, which serves critical cellular and developmental functions either directly, as non-coding RNAs, or indirectly, as protein-coding messenger (m)RNAs. Each step in the life of a eukaryotic RNA - from transcriptional birth to processing to function - involves the dynamic organization and reorganization of RNA structures with proteins to form ribonucleoprotein (RNP) complexes [1]. The biological importance of RNA-binding proteins (RBPs) is underscored by the fact that many human diseases result from RBP malfunction [2–4].
It is within the context of structured RNPs that the activity and stability of many RNAs are regulated post-transcriptionally. The composition of an RNP dictates RNA fate, reflecting aberrations that subject the RNA to quality control degradation pathways [5] or enabling the specific recognition of an RNA as a target by regulatory machineries. The latter is perhaps best understood for RNP complexes containing mRNAs (mRNPs), in which different RNA binding proteins mediate the initiation of translation, translational repression and storage, or the recruitment of RNA degradation enzymes, and fate switches for a given mRNA are the result of changes to the complement of associated RNA binding proteins [6]. In RNA decay, restructuring an RNP is not only important for the recruitment of degradation machineries, but also critical to permit nuclease access to the RNA itself. For example, it is known that proteins bound to the 5′ mRNA cap are inhibitory to decapping factors that remove the cap during mRNA degradation [7], while the poly(A)-binding protein inhibits some deadenylases that degrade the poly(A) tail while activating others [8].
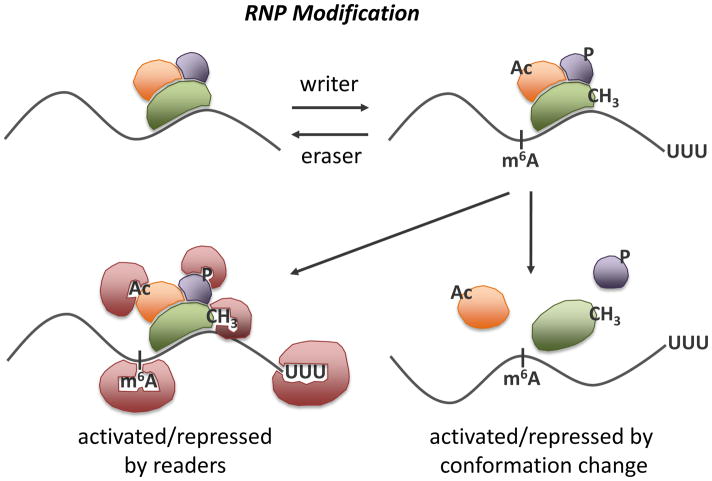
Thus, an important, and poorly understood, question is how these fate-determining RNP transitions – between an active state and an inactive state, from stability to instability – are mediated. Here we review key examples that are beginning to highlight RNP modification – the covalent addition of a chemical moiety to RNA or protein components of RNPs (Figure 1) - and ATP-dependent RNP remodeling (Figure 2) as important mechanisms that alter RNP composition and thereby regulate RNA fate.
Figure 1. Modification of RNA and protein components of RNPs controls RNA fate.
The RNA within RNPs is represented by a black line and proteins are represented by spheres. The terms writer, reader, and eraser are borrowed from the chromatin field [70] and refer to proteins that add, bind to, and remove RNP modifications, respectively. m6A, N6-methyl-adenosine; CH3, methyl group, Ac; acetyl group, P, phosphate group; UUU, oligouridylation.
Figure 2. RNP remodeling by ATP-dependent RNA helicases regulates RNA function.
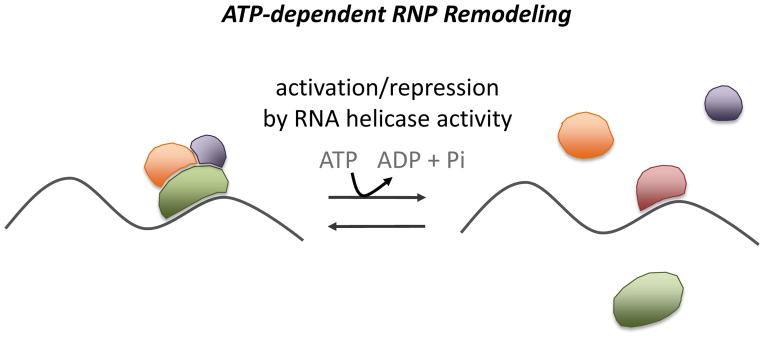
ATP, adenosine triphosphate; ADP, adenosine diphosphate; Pi, inorganic phosphate.
Complexes that combine RNP modification and remodeling activities are key players in RNA degradation pathways in bacteria and eukaryotic nuclei
In bacteria, it has long been known that RNP modification and remodeling activities come bundled as one package in the degradosome, which is a multisubunit complex that targets mRNAs and misfolded structural RNAs for decay [9]. Within the degradosome, there exists a poly(A) polymerase, which tails RNA with a string of 3′ adenosines, and an RNA helicase, which resolves RNP structures. Together, these RNP modifying and remodeling activities render the targeted RNAs unstable by creating a single-stranded RNA tail that serves as a tag accessible to the 3-to-5′ exonucleases which carry out RNA degradation.
It has only been in the past decade that a conserved multisubunit complex similar in composition to the bacterial degradosome was identified in eukaryotic nuclei [10]. What is now known as the Trf4p/Air2p/Mtr4p polyadenylation (TRAMP) complex contributes to a nuclear quality control pathway responsible for degrading a broad range of RNA species that are aberrantly processed, in excess, or are otherwise nonfunctional. Like it’s bacterial counterpart, eukaryotic TRAMP contains Trf4p, an adenosine-specific ribonucleotidyltransferase (rNTr), and Mtr4, an RNA helicase [11–14]. Promotion of nuclear decay is achieved at least in part by increasing the accessibility of 3′ RNA ends to 3′-to-5′ exonucleases through RNP structure resolution and/or the addition a 3′ oligoadenosine tail [12–18]. In addition, 3′ tailing may stimulate the activity of the helicase component of TRAMP itself by creating an optimal RNA tag for helicase binding [19–21].
Interestingly, given the huge variety of RNPs degraded by nuclear quality control, RNP modification and remodeling activities by TRAMP may be employed “on-demand”, modulated in response to a given target RNP or the degree of local structure encountered during degradation of a single RNP. Consistent with this, some RNA species and degradation intermediates do not require ATP-dependent RNA nucleotide tagging or helicase activity for efficient TRAMP-dependent decay [14,17,18]. Moreover, recent high-throughput sequencing studies have uncovered oligoadenylated fragments with 3′ ends corresponding to multiple sites across TRAMP-target RNAs [22,23], which may reflect alternating rounds of TRAMP-mediated RNA tailing and 3′-to-5′ degradation.
Thus, the bacterial degradosome and the TRAMP complex of eukaryotic nuclei exemplify complexes that carry out tagging and ATP-dependent remodeling of RNPs to determine RNA fate.
RNP modification by RNA tailing
Observations in recent years suggest that RNA tailing outside of TRAMP-mediated quality control or normal nuclear polyadenylation during mRNA biogenesis may be a widespread modification that alters RNP fate. In the cytoplasm, the extension of mRNA poly(A) tails serves to stabilize and/or activate repressed mRNAs under certain conditions, including oocyte maturation, neuronal stimulation and inflammation [24]. In contrast, the appending of short RNA tails composed of uridine, or both uridine and cytosine, to RNA targets has emerged to be linked to RNA instability or repression. Both poly(A) tail extensions and uridine-rich tails are added by nucleotide-specific rNTrs in the same family as the TRAMP rNTr [25–27]. In mammals, known RNA targets of uridylation-associated repression include mRNA cleavage fragments generated by RNAi targeting [28], histone mRNAs [29], pre-micro (mi)RNAs, and mature miRNAs [30,31]. In addition, the instability of a tRNA-like small RNA has been linked to 3′ extension by the CCA-adding enzyme through a proposed tRNA quality control pathway [32]. However, it is likely that this represents just a small fraction of tailed RNAs in mammalian cells, with many more RNA targets of uridylation or other nucleotide tailing left to uncover. In fact, studies in Saccharomyces pombe and Aspergillus nidulans have reported that apparently normal polyadenylated mRNAs [33–35], as well as targets for the nonsense-mediated mRNA decay (NMD) pathway [35], are subject to uridylation.
While the molecular details of how RNA tailing contributes to RNA repression and decay have not been fully elucidated, closer study of certain uridylated RNAs suggests that RNA tailing may trigger RNA repression in at least a few different ways. Synthetic RNAs bearing 3′ uridine tails stimulate decapping in human leukemia cell extracts, in a manner dependent on Lsm1 [36]. In yeast, Lsm1 is part of a complex that functions as an enhancer of decapping, with strong intrinsic affinity for oligoadenylated and deadenylated mRNAs with a 3′ U tract [37]. In vivo support that 3′ U tails serve as a tag for Lsm1 binding to stimulate decapping and 5′-to-3′ degradation comes from S. pombe, in which strains lacking Lsm1 or bearing a mutant decapping activator accumulate both capped and decapped mRNAs with U-tails to higher levelsthan in the corresponding wild-type strain [33]. It has also been suggested that uridylation-induced Lsm1 binding may stimulate 3′-to-5′ decay as well, by recruiting the 3′-to-5′ exonuclease Eri1 in the decay of replication-dependent histone mRNAs in mouse embryonic fibroblasts [38]. Finally, a very recent study found that S. pombe Dis3l2, a cytoplasmic 3′-to-5′ exonuclease, exhibits an intrinsic preference in vitro for degrading RNA substrates with 3′ U tracts over those lacking 3′ U under competitive conditions [39]. This activity may target mRNA substrates in vivo that have undergone 3′-to-5′ trimming and U tailing, as mutant strains lacking both Dis3l2 and Lsm1 accumulate 3′ truncated mRNAs with oligoU tails of longer length and to higher levels than strains lacking only Lsm1 [39].
mRNA tailing in the cytoplasm may also contribute to translational repression. In the filamentous fungus Aspergillus nidulans, the tailing of mRNAs with a mixture of uridine and cytidine not only contributes to the decay of normal mRNAs, but also plays a role in the liberation of mRNAs targeted by NMD from polyribosomes [34,35]. Translational repression is proposed to precede the RNA tailing-dependent ribosome release step. It remains to be determined whether this activity is mediated by Lsm1, which forms a complex with the translational repressor Pat1 [40,41].
A very recent study implicated Dis3l2 in the degradation of oligouridylated pre-let-7 in mouse embryonic stem cells [44], while it remains unclear how uridylation leads to the destabilization and/or repression of mature small (s)RNAs [30,31,42,43]. However, it is worth noting that monoadenylation was recently found to stabilize mature miRNAs in mammalian cells [45–47]. Thus, RNA tailing appears to be a means for mediating RNP transitions in activity or stability for both mRNAs and functional non-coding RNAs alike.
Collectively, these studies reveal an emerging, yet poorly understood, role for RNA tailing in controlling RNP fate, not only as a mechanism for ridding the cell of aberrant RNAs, but also for regulating the function and levels of normal RNAs.
RNP modification by RNA nucleotide modification
Over a hundred RNA modifications are known to exist, affecting stable non-coding RNAs such as transfer RNAs and ribosomal RNAs, as well as mRNAs and other non-coding RNAs, yet knowledge of their function and potential for influencing RNP fate is limited [48,49]. For example, modification of sRNAs functioning in RNA interference pathways by 2′-O-methylation provides a protective role against sRNA tailing and degradation [31], yet it is unknown if methylation is reversible or otherwise regulated, or simply part of normal biogenesis. A-to-I editing [50] and 5-methyl-cytosine (m5C) modification [51] have been detected in both mRNAs and non-coding RNAs. Though the small fraction of A-to-I editing that occurs in mRNA coding regions can cause protein recoding, the function of the vast majority of A-to-I and m5C modifications is unknown.
Interest in the RNA modification N6-methyl-adenosine (m6A) was revived recently with the discovery of tens of thousands of m6A-modified segments in mRNA and non-coding RNAs in mice and human cultured cells [52–54]. The level of m6A appears to vary by tissue and stage of development in mice, and a subset of modified sites was found to be dynamically modified in response to various stimuli in human cells, indicating that m6A modification is subject to regulation. Further support for m6A regulation comes from studies that have revealed that methylation of a given mRNA species only affects a fraction of its transcripts at a given site [55] and that demethylases with specificity for the m6A tag exist [56,57].
m6A-modified RNA segments appear to be conserved through evolution, suggesting that an important function is served by this modification [52,53]. Proposed functions have included mRNA splicing, nuclear export, stability and translational control [54,58], yet direct involvement of RNA methylation in the biogenesis, function and/or fate of the vast majority of modified RNAs has yet to be demonstrated. Curiously, in the case of modified mRNAs, m6A is enriched in coding and 3′ UTR regions near the translation termination codon [52,53], suggestive of possible roles in translation or translation-dependent processes. Although it is tempting to speculate that this modification alters RNA structure, as suggested by in vitro studies [59], or impacts RNA-protein interactions, perhaps by acting as a tag specifically recognized by binding effector proteins, more research into the function and regulation of m6A in vivo is needed. An important question for future studies is whether this and possibly other RNA nucleotide modifications represent a new layer of gene regulation.
RNP modification at the protein component level
Like RNA modification, there is emerging evidence that modification of proteins in RNPs can also have profound effects on RNP stability and activity. These effects can be mediated indirectly by the creation of binding sites for proteins that act on the RNP, or by direct modulation of protein-protein or protein-RNA interactions within the RNP. As an example of the former, Upf1, the key effector of NMD, undergoes a phosphorylation-dephosphorylation cycle [60,61]. Phosphorylated sites in Upf1 promote Upf1 decay function by serving as tags that are recognized and bound by the endonuclease Smg6 and other proteins that physically associate with decapping and 5′-to-3′ degradation machineries [62,63]. In addition, phosphorylated Upf1 has been reported to repress translation initiation through interaction with initiation factor eIF3 [64].
Phosphorylation can also have an RNA stabilizing function, as seen for decay-promoting AU-rich element (ARE)-binding proteins (AUBPs), which negatively regulate mRNAs containing AREs in their 3′ UTRs. In this case, phosphorylation interferes with mRNA decay by disrupting AUBP RNA binding or preventing the recruitment of degradation enzymes [65,66]. In an intriguing model for the dynamic control of mRNPs by protein modification, it is thought that the transient inactivation of the AUBP TTP through phosphorylation contributes to the initial stabilization and expression of ARE mRNAs encoding proteins important for the inflammatory response upon macrophage stimulation. As TTP phosphorylation prevents its recruitment of deadenylases, rapid revival of TTP-dependent mRNA decay upon dissipation of the inflammatory response trigger may be achieved by TTP dephosphorylation, which would be expected to limit the destructive potential of an unchecked inflammatory response [67,68].
Other types of protein modifications impacting RNPs include lysine acetylation and arginine methylation, both of which impact protein-RNA interactions in known cases. For example, a recent report described developmentally regulated lysine acetylation of MVH, an RNA helicase that localizes in germline-specific RNA granules associated with translational repression [69]. MVH lysine acetylation was found to interfere with RNA-binding in vitro, and during spermatogenesis, MVH lysine acetylation correlates with the selective release of a target mRNA from MVH-RNPs. Loss of MVH-mediated translational repression due to acetylation was inferred from the increase in target mRNA protein expression that followed.
Arginine methylation of the mRNA stabilizing factor HuR appears important to its regulation of SIRT1 deacetylase mRNA [70]. Decreased mRNA stability partly accounts for a decrease in SIRT1 protein levels during differentiation of human embryonic stem cells. This reduction in mRNA stability parallels a decrease in HuR-modifying methyltransferase levels, resulting in loss of HuR methylation and HuR-SIRT1 mRNA association. That HuR methylation stabilizes HuR binding to SIRT1 mRNA is supported by the finding that a methylation-resistant version of HuR is compromised in SIRT1 mRNA binding compared to wild-type HuR. In contrast, phosphorylation of HuR under oxidative stress in cancer cells causes a release of SIRT1 mRNA, resulting in mRNA destabilization [71].
The above examples of regulatory protein modifications in RNPs seem likely to be followed by many more that underlie important RNP transitions in activity and stability. In fact, a recent study of human cytoplasmic poly(A) binding protein PABP1 revealed 14 novel post-translational modifications, including methylation of glutamate, aspartate, lysine and arginine, as well as acetylation of lysine, the latter of which appears to be regulated in an cell cycle-dependent manner [72]. As PABP1 plays central roles in mRNA translation and stability, an important question is whether these modifications make significant contributions to PABP1-dependent gene regulation. In addition, a number of conserved proteins containing arginine-glycine rich motifs (RGG) were found recently to inhibit mRNA translation through interaction with translation initiation factor eIF4G in yeast [73,74]. Notably, RGG motifs are often targeted by methyltransferases for arginine methylation [73,75].
Taken together, the above examples indicate that RNP modification by different means - RNA tailing, attachment of chemical moieties to RNP protein components, and possibly RNA nucleotide modification – contribute to the control of RNP function and stability (Figure 1). In the case of RNA tailing and some protein modifications, it appears that RNP modification creates a tag that is recognized and bound by RNA regulatory proteins that contribute to RNP fate. Borrowing terms established in the chromatin field for histone modifying enzymes and the proteins that bind histone modifications [76], RNP modification enzymes may act as writers, marking RNPs with tags that are recognized by regulatory protein readers. Other modifications may directly cause the remodeling of RNPs, by physically disrupting interactions within the RNP or altering RNA structure.
RNP regulation by ATP-dependent RNP remodeling
Exposure of RNA to regulatory proteins and/or RNP remodeling can also take place without chemical modification of the RNP, but rather through the ATP-dependent activity of members of the RNA helicase/RNA-dependent ATPase family (Figure 2). In fact, Ski2 helicase is considered the cytoplasmic equivalent of the TRAMP helicase component Mtr4, bearing similarity in amino acid sequence and structure, and functioning in conjunction with 3′-to-5′ degradation machinery in cytoplasmic decay pathways [77,78]. However, whether Ski2 functions molecularly in the same manner proposed for Mtr4, through target binding and exposure of a 3′ single-stranded RNA end to 3′-to-5′ nucleases, remains to be determined. In addition, recent studies suggest that RNA helicase/RNA-dependent ATPase family members may impact RNP stability or function through RNP complex remodeling, which may also include release of the RNA helicase itself.
The core NMD factor Upf1 serves as an example of an RNA helicase/RNA-dependent ATPase which may enable 5′-to-3′ RNA degradation through protein displacement [79]. Mutations disrupting Upf1 ATP binding or ATP hydrolysis activities are inhibitory to NMD [79,80]. ATPase-deficient human Upf1 causes the accumulation of 3′ degradation intermediates of NMD substrates bearing a premature termination codon [79]. As NMD factors are also retained on the 3′ RNA fragments under these conditions, it has been suggested that Upf1 ATP hydrolysis is required for the removal of the NMD complex in order to grant degradation machinery access to the RNA and recycle NMD factors. This Upf1-catalyzed RNP transition is likely under tight regulation, with activation triggered only after the NMD complex has been fully assembled and decay enzymes recruited. In support of this model, the NMD complex component Upf2 has been found to stimulate Upf1 ATPase activity in vitro through interaction with and displacement of an autoinhibitory domain of Upf1 [81,82].
Use of ATP hydrolysis for RNP component release and recycling may also be exemplified by the widely conserved RNA-dependent ATPase Dhh1, which functions as a translational repressor [83]. Recent studies in yeast have demonstrated that the translational repression and subsequent 5′-to-3′ decay induced by Dhh1-tethering to mRNAs is retained in a putative ATPase-deficient mutant [84,85]. However, rescue of Dhh1-dependent decay of some endogenous mRNAs by untethered Dhh1 is compromised by the ATPase mutation [84,86]. These seemingly contradictory data may be explained by the finding that Dhh1 mutant expression causes the accumulation of cytoplasmic RNP granules containing decapping machinery in which the Dhh1 mutant itself is trapped [84,86]. Thus, while Dhh1 ATP hydrolysis is not required for its mRNA repression activities, it may be required to release Dhh1 itself and possibly other factors from repressed RNP complexes targeted for decay for reuse in the repression of additional RNPs.
Examination of another broadly conserved RNA helicase, Ded1, suggests that members of this enzyme class may also have the potential to contribute to opposing RNP states, with ATP binding- or hydrolysis-dependent remodeling triggering the switch between states. Ded1 has long been implicated in both translational initiation and repression [87], but only recently was Ded1-mediated initiation, and not repression, found to be dependent on ATP-binding [88]. Although the underlying molecular outcome of Ded1-ATPase activity has yet to be determined, it is tempting to speculate, given the examples of Upf1 and Dhh1, that Ded1 ATP hydrolysis is required for remodeling mRNP complexes from a translationally inactive state into one that is active, perhaps by releasing inhibitory factors that could include Ded1 itself.
The examples above indicate a common, critical role for RNA helicases in ATP-driven remodeling of RNPs to control RNA function and/or stability. Such remodeling could occur by changes in RNA structure and/or the assembly, remodeling or disassembly of protein components of the RNP. An intriguing area for future research is whether RNP tagging is coupled to ATP-dependent RNP remodeling to control RNA fate in the cytoplasm of eukaryotic cells, as has been found for the degradosome of bacteria and for the TRAMP complex of eukaryotic nuclei.
Concluding remarks
The activity and stability of RNPs are significantly affected by covalent modification at both the RNA and protein levels and by ATP-dependent remodeling. In some cases, these RNP alterations have been found to directly impact the accessibility of RNA components to nucleases and the recruitment or binding of other proteins. However, for many alterations, the precise biochemical and molecular changes that ultimately result in changes to RNP activity or stability have yet to be described.
As our understanding of the above pathways grows, an important area for future study is how the enzymes involved are regulated. How are RNP modification and ATP-dependent remodeling enzymes recruited to their RNP targets in the first place? How are the biochemical functions of these enzymes activated or repressed in response to cellular needs? How common is the coupling between RNP modifiers and remodelers as exemplified by the bacterial degradosome and nuclear TRAMP complexes? Will the creation of RNP tags through RNP modification by writer enzymes for recognition by reader proteins emerge as a general theme in RNA regulation as it has in chromatin?
The processes and factors described above likely represent only a small fraction of the activities that influence the activity and stability of RNPs. In principle, RNP modification and remodeling could impact RNPs of any flavor, whether these bear mRNAs, sRNAs, or functional noncoding RNAs. In addition, recently identified mRNP-associated enzymes with potential RNA and protein modifying or remodeling activities represent tantalizing avenues for further research [89,90]. Such studies are predicted to reveal exciting new layers in the regulation of RNP stability and function.
Highlights.
RNAs function in, and are regulated as, ribonucleoprotein complexes (RNPs)
Chemical modification and ATP-dependent remodeling of RNPs regulate RNA fate
RNP modifications can serve as tags for the binding of regulatory proteins
Acknowledgments
The authors wish to thank Marcos Arribas-Layton for thought-provoking discussions on RNP remodeling. Research on mRNP remodeling in the JL-A laboratory is supported by grant R01 GM099717 from the National Institutes of Health. SRL was supported by a fellowship from the Helen Hay Whitney foundation.
Glossary terms
- AUBPs
AU-rich mRNA element-binding proteins
- NMD
Nonsense-mediated decay, a cytoplasmic RNA degradation pathway that targets certain mRNAs, such as those bearing a premature stop codon or a long 3′ untranslated region, for destruction
- RNA helicases/RNA-dependent ATPases
RNA-binding enzymes that couple ATP binding and hydrolysis to the disruption of protein-RNA or RNA-RNA interactions in RNPs
- RNAi
RNA interference
- RNPs
ribonucleoprotein complexes composed of RNA and associated proteins
- rNTRs
ribonucleotidyltranferases, RNA tailing enzymes capable of appending one or more nucleotides to the 3′ end of target RNAs
- TRAMP
A protein complex involved in nuclear RNA quality control consisting of Trf4 (a A-specific rNTR), Mtr4 (an RNA helicase), and either Air1 or 2 (RNA binding proteins)
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glisovic T, et al. RNA-binding proteins and post-transcriptional gene regulation. FEBS Letters. 2008;582:1977–86. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukong KE, et al. RNA-binding proteins in human genetic disease. Trends in Genetics. 2008;24:416–25. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Cooper Ta, et al. RNA and disease. Cell. 2009;136:777–93. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castello A, et al. RNA-binding proteins in Mendelian disease. Trends in Genetics. 2013;29(5):318–27. doi: 10.1016/j.tig.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Mühlemann O, Jensen TH. mRNP quality control goes regulatory. Trends in Genetics. 2012;28:70–7. doi: 10.1016/j.tig.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–8. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Kiledjian M. Regulation of mRNA decapping. Wiley Interdisciplinary Reviews: RNA. 2010;1:253–65. doi: 10.1002/wrna.15. [DOI] [PubMed] [Google Scholar]
- 8.Brook M, Gray NK. The role of mammalian poly(A)-binding proteins in coordinating mRNA turnover. Biochemical Society Transactions. 2012;40:856–64. doi: 10.1042/BST20120100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Górna MW, et al. From conformational chaos to robust regulation: the structure and function of the multi-enzyme RNA degradosome. Quarterly Reviews of Biophysics. 2012;45:105–45. doi: 10.1017/S003358351100014X. [DOI] [PubMed] [Google Scholar]
- 10.Wolin SL, et al. Nuclear noncoding RNA surveillance: is the end in sight? Trends in Genetics. 2012;28:306–13. doi: 10.1016/j.tig.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubas M, et al. Interaction profiling identifies the human nuclear exosome targeting complex. Molecular Cell. 2011;43:624–37. doi: 10.1016/j.molcel.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Vanácová S, et al. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biology. 2005;3:e189. doi: 10.1371/journal.pbio.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaCava J, et al. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713–24. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Wyers F, et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–37. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C, et al. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Molecular Cell. 2007;27:324–31. doi: 10.1016/j.molcel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan KP, Butler JS. TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6. The Journal of Biological Chemistry. 2010;285:3540–7. doi: 10.1074/jbc.M109.058396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San Paolo S, et al. Distinct roles of non-canonical poly(A) polymerases in RNA metabolism. PLoS Genetics. 2009;5:e1000555. doi: 10.1371/journal.pgen.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, et al. Degradation of hypomodified tRNA(iMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA. 2008;14:107–16. doi: 10.1261/rna.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein J, et al. Characterization of the essential activities of Saccharomyces cerevisiae Mtr4p, a 3′->5′ helicase partner of the nuclear exosome. The Journal of Biological Chemistry. 2008;283:4930–42. doi: 10.1074/jbc.M706677200. [DOI] [PubMed] [Google Scholar]
- 20.Weir JR, et al. Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12139–44. doi: 10.1073/pnas.1004953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia H, Wang X. RNA unwinding by the Trf4/Air2/Mtr4 polyadenylation (TRAMP) complex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7292–7297. doi: 10.1073/pnas.1201085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wlotzka W, et al. The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. The EMBO Journal. 2011;30:1790–803. doi: 10.1038/emboj.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider C, et al. Transcriptome-wide Analysis of Exosome Targets. Molecular Cell. 2012;48:422–433. doi: 10.1016/j.molcel.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weill L, et al. Translational control by changes in poly(A) tail length: recycling mRNAs. Nature Structural & Molecular Biology. 2012;19:577–85. doi: 10.1038/nsmb.2311. [DOI] [PubMed] [Google Scholar]
- 25.Morozov IY, Caddick MX. Cytoplasmic mRNA 3′ tagging in eukaryotes: does it spell the end? Biochemical Society Transactions. 2012;40:810–4. doi: 10.1042/BST20120068. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt MJ, Norbury CJ. Polyadenylation and beyond: emerging roles for noncanonical poly(A) polymerases. Wiley Interdisciplinary Reviews: RNA. 2010;1:142–51. doi: 10.1002/wrna.16. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim F, et al. Untemplated oligoadenylation promotes degradation of RISC-cleaved transcripts. Science. 2006;314:1893. doi: 10.1126/science.1135268. [DOI] [PubMed] [Google Scholar]
- 28.Shen B, Goodman HM. Uridine addition after microRNA-directed cleavage. Science. 2004;306:997. doi: 10.1126/science.1103521. [DOI] [PubMed] [Google Scholar]
- 29.Mullen TE, Marzluff WF. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5′ to 3′ and 3′ to 5′. Genes & Development. 2008;22:50–65. doi: 10.1101/gad.1622708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YK, et al. Modifications of small RNAs and their associated proteins. Cell. 2010;143:703–9. doi: 10.1016/j.cell.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Ji L, Chen X. Regulation of small RNA stability: methylation and beyond. Cell Research. 2012;22:624–36. doi: 10.1038/cr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilusz JE, et al. tRNAs marked with CCACCA are targeted for degradation. Science. 2011;334:817–21. doi: 10.1126/science.1213671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rissland OS, Norbury CJ. Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nature Structural & Molecular Biology. 2009;16:616–23. doi: 10.1038/nsmb.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morozov IY, et al. CUCU modification of mRNA promotes decapping and transcript degradation in Aspergillus nidulans. Molecular and Cellular Biology. 2010;30:460–9. doi: 10.1128/MCB.00997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morozov IY, et al. mRNA 3′ tagging is induced by nonsense -mediated decay and promotes ribosome dissociation. Molecular and Cellular Biology. 2012;32:2585–95. doi: 10.1128/MCB.00316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song MG, Kiledjian M. 3′ Terminal oligo U-tract-mediated stimulation of decapping. RNA. 2007;13:2356–65. doi: 10.1261/rna.765807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury A, et al. The decapping activator Lsm1p-7p-Pat1p complex has the intrinsic ability to distinguish between oligoadenylated and polyadenylated RNAs. RNA. 2007;13:998–1016. doi: 10.1261/rna.502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoefig KP, et al. Eri1 degrades the stem-loop of oligouridylated histone mRNAs to induce replication-dependent decay. Nature Structural & Molecular Biology. 2013;20(1):73–81. doi: 10.1038/nsmb.2450. [DOI] [PubMed] [Google Scholar]
- 39.Malecki M, et al. The exoribonuclease Dis3L2 defines a novel eukaryotic RNA degradation pathway. The EMBO Journal. 2013 doi: 10.1038/emboj.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tharun S, et al. Yeast Sm-like proteins function in mRNA decapping and decay. Nature. 2000;404:515–8. doi: 10.1038/35006676. [DOI] [PubMed] [Google Scholar]
- 41.Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–86. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren G, et al. Uridylation of miRNAs by hen1 suppressor1 in Arabidopsis. Current Biology. 2012;22:695–700. doi: 10.1016/j.cub.2012.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, et al. The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Current Biology. 2012;22:689–94. doi: 10.1016/j.cub.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang H-M, et al. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28–let-7 pathway. Nature. 2013 doi: 10.1038/nature12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh T, et al. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes & Development. 2009;23:433–8. doi: 10.1101/gad.1761509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burns DM, et al. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature. 2011;473:105–8. doi: 10.1038/nature09908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Ambrogio A, et al. Specific miRNA Stabilization by Gld2-Catalyzed Monoadenylation. Cell Reports. 2012;2:1537–1545. doi: 10.1016/j.celrep.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantara Wa, et al. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Research. 2011;39:D195–201. doi: 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machnicka Ma, et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Research. 2013;41:D262–7. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annual Review of Biochemistry. 2010;79:321–49. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Squires JE, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Research. 2012;40:5023–33. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 53.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–46. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan T. N6-methyl-adenosine modification in messenger and long non-coding RNA. Trends in Biochemical Sciences. 2013;38(4):204–9. doi: 10.1016/j.tibs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horowitz S, et al. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:5667–71. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng G, et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Molecular Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia G, et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nature Chemical Biology. 2011;7(12):885–7. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, et al. NSun. 2012. [DOI] [Google Scholar]
- 59.Dai Q, et al. Identification of recognition residues for ligation-based detection and quantitation of pseudouridine and N6-methyladenosine. Nucleic Acids Research. 2007;35:6322–9. doi: 10.1093/nar/gkm657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kervestin S, Jacobson A. NMD: a multifaceted response to premature translational termination. Nature reviews Molecular Cell Biology. 2012;13:700–12. doi: 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Durand S, Lykke-Andersen J. SnapShot: Nonsense-mediated mRNA decay. Cell. 2011;145:324–324.e2. doi: 10.1016/j.cell.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 62.Okada-Katsuhata Y, et al. N- and C-terminal Upf1 phosphorylations create binding platforms for SMG-6 and SMG-5:SMG-7 during NMD. Nucleic Acids Research. 2012;40:1251–66. doi: 10.1093/nar/gkr791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho H, et al. Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Molecular Cell. 2009;33:75–86. doi: 10.1016/j.molcel.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 64.Isken O, et al. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell. 2008;133:314–27. doi: 10.1016/j.cell.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu X, Brewer G. The regulation of mRNA stability in mammalian cells: 2.0. Gene. 2012;500:10–21. doi: 10.1016/j.gene.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beisang D, Bohjanen PR. Perspectives on the ARE as it turns 25 years old. Wiley Interdisciplinary Reviews: RNA. 2012;3:719–31. doi: 10.1002/wrna.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clement SL, et al. Phosphorylation of tristetraprolin by MK2 impairs AU-rich element mRNA decay by preventing deadenylase recruitment. Molecular and Cellular Biology. 2011;31:256–66. doi: 10.1128/MCB.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marchese FP, et al. MAPKAP kinase 2 blocks tristetraprolin-directed mRNA decay by inhibiting CAF1 deadenylase recruitment. The Journal of Biological Chemistry. 2010;285:27590–600. doi: 10.1074/jbc.M110.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagamori I, et al. Regulation of an RNA granule during spermatogenesis: acetylation of MVH in the chromatoid body of germ cells. Journal of Cell Science. 2011;124:4346–55. doi: 10.1242/jcs.096461. [DOI] [PubMed] [Google Scholar]
- 70.Calvanese V. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(31):13736–41. doi: 10.1073/pnas.1001399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujiwara T, et al. CARM1 Regulates Proliferation of PC12 Cells by Methylating HuD CARM1 Regulates Proliferation of PC12 Cells by Methylating HuD. Molecular and Cellular Biology. 2006;26(6):2273–85. doi: 10.1128/MCB.26.6.2273-2285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brook M, et al. The multifunctional poly(A)-binding protein (PABP) 1 is subject to extensive dynamic post-translational modification, which molecular modelling suggests plays an important role in co-ordinating its activities. The Biochemical Journal. 2012;441:803–12. doi: 10.1042/BJ20111474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajyaguru P, Parker R. RGG motif proteins: Modulators of mRNA functional states. Cell Cycle. 2012;11:2594–2599. doi: 10.4161/cc.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajyaguru P, et al. Scd6 targets eIF4G to repress translation: RGG motif proteins as a class of eIF4G-binding proteins. Molecular Cell. 2012;45:244–54. doi: 10.1016/j.molcel.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blackwell E, Ceman S. Arginine methylation of RNA-binding proteins regulates cell function and differentiation. Molecular Reproduction and Development. 2012;79:163–75. doi: 10.1002/mrd.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruthenburg AJ, et al. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Molecular Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Schaeffer D, Hoof A Van. Different nuclease requirements for exosome-mediated degradation of normal and nonstop mRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(6):2366–71. doi: 10.1073/pnas.1013180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halbach F, et al. The crystal structure of S. cerevisiae Ski2, a DExH helicase associated with the cytoplasmic functions of the exosome. RNA. 2012;18:124–34. doi: 10.1261/rna.029553.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franks T, et al. Upf1 ATPase-dependent mRNP disassembly is required for completion of nonsense-mediated mRNA decay. Cell. 2010;143:938–950. doi: 10.1016/j.cell.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weng Y, et al. Genetic and biochemical characterization of mutations in the ATPase and helicase regions of the Upf1 protein. Molecular and Cellular Biology. 1996;16(10):5477–90. doi: 10.1128/mcb.16.10.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakrabarti S, et al. Molecular mechanisms for the RNA-dependent ATPase activity of Upf1 and its regulation by Upf2. Molecular Cell. 2011;41:693–703. doi: 10.1016/j.molcel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 82.Chamieh H, et al. NMD factors UPF2 and UPF3 bridge UPF1 to the exon junction complex and stimulate its RNA helicase activity. Nature Structural & Molecular Biology. 2008;15:85–93. doi: 10.1038/nsmb1330. [DOI] [PubMed] [Google Scholar]
- 83.Weston A, Sommerville J. Xp54 and related (DDX6-like) RNA helicases: roles in messenger RNP assembly, translation regulation and RNA degradation. Nucleic Acids Research. 2006;34:3082–94. doi: 10.1093/nar/gkl409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carroll JS, et al. The DExD/H box ATPase Dhh1 functions in translational repression, mRNA decay, and processing body dynamics. The Journal of Cell Biology. 2011;194:527–37. doi: 10.1083/jcb.201007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sweet T, et al. The DEAD-box protein Dhh1 promotes decapping by slowing ribosome movement. PLoS Biology. 2012;10:e1001342. doi: 10.1371/journal.pbio.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dutta A, et al. Intermolecular interactions within the abundant DEAD-box protein Dhh1 regulate its activity in vivo. The Journal of Biological Chemistry. 2011;286:27454–70. doi: 10.1074/jbc.M111.220251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarn W, Chang T. The current understanding of Ded1p/DDX3 homologs from yeast to human. RNA Biology. 2009;6(1):17–20. doi: 10.4161/rna.6.1.7440. [DOI] [PubMed] [Google Scholar]
- 88.Hilliker A, et al. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Molecular Cell. 2011;43:962–72. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castello A, et al. Insights into RNA Biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell SF, et al. Global analysis of yeast mRNPs. Nature Structural & Molecular Biology. 2012;20(1):127–33. doi: 10.1038/nsmb.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]