Abstract
A new era in medical science has dawned with the realization of the critical role of the “forgotten organ,” the gut micro-biota, in health and disease. Central to this beneficial interaction between the microbiota and host is the manner in which bacteria and most likely other microorganisms contained within the gut communicate with the host’s immune system and participate in a variety of metabolic processes of mutual benefit to the host and the microbe. The advent of high-throughput methodologies and the elaboration of sophisticated analytic systems have facilitated the detailed description of the composition of the microbial constituents of the human gut, as never before, and are now enabling comparisons to be made between health and various disease states. Although the latter approach is still in its infancy, some important insights have already been gained about how the microbiota might influence a number of disease processes both within and distant from the gut. These discoveries also lay the groundwork for the development of therapeutic strategies that might modify the microbiota (eg, through the use of probiot-ics). Although this area holds much promise, more high-quality trials of probiotics, prebiotics, and other microbiota-modifying approaches in digestive disorders are needed, as well as laboratory investigations of their mechanisms of action.
Keywords: Gut flora, microbiota, probiotic, gut bacteria, microbial metabolism, mucosal immunology
Due largely to rapidly evolving advances in analytic techniques in microbiology, molecular biology, and bioinfor-matics, the true diversity of microorganisms that inhabit the gastrointestinal tract of humans (collectively referred to as the human gut microbiota) is being revealed and its contributions to homeostasis in health and to the pathogenesis of disease appreciated (Table 1). As a consequence, the study of gut ecology has emerged as one of the most active and exciting fields in biology and medicine. It is in this context that maneuvers to alter or modify the microbiota, either through dietary modifications or by the administration of antibiotics, probiotics, or prebiotics, must now be viewed.
Table 1.
Important Homeostatic Functions of the Gut Microbiota
1. Metabolic role
|
| 2. Deconjugation of bile acids |
| 3. Prevention of colonization by pathogens |
4. Immunologic effects
|
The Normal Gut Microbiota: An Essential Factor in Health
Basic Definitions and Development of the Microbiota
The term microbiota is to be preferred to the older term flora, as the latter fails to account for the many nonbacte-rial elements (eg, archea, viruses, and fungi) that are now known to be normal inhabitants of the gut. Given the relatively greater understanding that currently exists of the role of bacteria, in comparison with the other constituents of the microbiota in health and disease, gut bacteria will be the primary focus of this review. Within the human gastrointestinal microbiota exists a complex ecosystem of approximately 300 to 500 bacterial species, comprising nearly 2 million genes (the microbiome).1 Indeed, the number of bacteria within the gut is approximately 10 times that of all of the cells in the human body, and the collective bacterial genome is vastly greater than the human genome.
At birth, the entire intestinal tract is sterile; the infant’s gut is first colonized by maternal and environmental bacteria during birth and continues to be populated through feeding and other contacts.2 Factors known to influence colonization include gestational age, mode of delivery (vaginal birth vs assisted delivery), diet (breast milk vs formula), level of sanitation, and exposure to antibiotics.3,4 The intestinal microbiota of newborns is characterized by low diversity and a relative dominance of the phyla Proteobacteria and Actinobacteria; thereafter, the microbiota becomes more diverse with the emergence of the dominance of Firmicutes and Bacteroidetes, which characterizes the adult microbiota.5–7 By the end of the first year of life, the microbial profile is distinct for each infant; by the age of 2.5 years, the microbiota fully resembles the microbiota of an adult in terms of composition.8,9 This period of maturation of the microbiota may be critical; there is accumulating evidence from a number of sources that disruption of the microbiota in early infancy may be a critical determinant of disease expression in later life. It follows that interventions directed at the microbiota later in life may, quite literally, be too late and potentially doomed to failure.
Following infancy, the composition of the intestinal microflora remains relatively constant until later life. Although it has been claimed that the composition of each individual’s flora is so distinctive that it could be used as an alternative to fingerprinting, more recently, 3 differ-ent enterotypes have been described in the adult human microbiome.10 These distinct enterotypes are dominated by Prevotella, Ruminococcus, and Bacteroides, respectively, and their appearance seems to be independent of sex, age, nationality, and body mass index. The microbiota is thought to remain stable until old age when changes are seen, possibly related to alterations in digestive physiology and diet.11–13 Indeed, Claesson and colleagues were able to identify clear correlations in elderly individuals, not only between the composition of the gut microbiota and diet, but also in relation to health status.14
Regulation of the Microbiota
Because of the normal motility of the intestine (peristalsis and the migrating motor complex) and the antimicrobial effects of gastric acid, bile, and pancreatic and intestinal secretions, the stomach and proximal small intestine, although certainly not sterile, contain relatively small numbers of bacteria in healthy subjects.15 Interestingly, commensal organisms with probiotic properties have recently been isolated from the human stomach.16 The microbiology of the terminal ileum represents a transition zone between the jejunum, containing predominantly aerobic species, and the dense population of anaerobes found in the colon. Bacterial colony counts may be as high as 109 colony-forming units (CFU)/mL in the terminal ileum immediately proximal to the ileocecal valve, with a predominance of gram-negative organisms and anaerobes. On crossing into the colon, the bacterial concentration and variety of the enteric flora change dramatically. Concentrations of 1012 CFU/mL or greater may be found and are comprised mainly of anaerobes such as Bacteroides, Porphyromonas, Bifidobacterium, Lactobacillus, and Clos-tridium, with anaerobic bacteria outnumbering aerobic bacteria by a factor of 100 to 1000:1. The predominance of anaerobes in the colon reflects the fact that oxygen concentrations in the colon are very low; the flora has simply adapted to survive in this hostile environment.
At any given level of the gut, the composition of the flora also demonstrates variation along its diameter, with certain bacteria tending to be adherent to the mucosal surface, while others predominate in the lumen. It stands to reason that bacterial species residing at the mucosal surface or within the mucus layer are those most likely to participate in interactions with the host immune system, whereas those that populate the lumen may be more relevant to metabolic interactions with food or the products of digestion. It is now evident that different bacterial populations may inhabit these distinct domains. Their relative contributions to health and disease have been explored to a limited extent, though, because of the relative inaccessibility of the juxtamucosal populations in the colon and, especially, in the small intestine. However, most studies of the human gut microbiota have been based on analyses of fecal samples, therefore representing a major limitation. Indeed, a number of studies have already shown differ-ences between luminal (fecal) and juxtamucosal populations in disorders such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS).17,18
In humans, the composition of the flora is influenced not only by age but also by diet and socioeconomic conditions. In a recent study of elderly individuals, the interaction of diet and age was demonstrated, firstly, by a close relationship between diet and microbiota composition in the subjects and, secondly, by interactions between diet, the microbiota, and health status.14 It must also be remembered that nondigestible or undigested components of the diet may contribute substantially to bacterial metabolism; for example, much of the increase in stool volume resulting from the ingestion of dietary fiber is based on an augmentation of bacterial mass. The subtleties of interaction between other components of diet and the microbiota are now being explored and will, undoubtedly, yield important information. For example, data indicating a potential role of certain products of bacterial metabolism in colon carcinogenesis have already provided strong hints of the relevance of diet-microbiota interactions to disease. Antibiotics, whether prescribed or in the food chain as a result of their administration to animals, have the potential to profoundly impact the microbiota.19 In the past, it was thought that these effects were relatively transient, with complete recovery of the microbiota occurring very soon after the course of antibiotic therapy was complete. However, while recent studies have confirmed that recovery is fairly rapid for many species, some species and strains show more sustained effects.20
Host-Microbiota Interactions
Gut-commensal microbiota interactions play a fundamental role in promoting homeostatic functions such as immunomodulation, upregulation of cytoprotec-tive genes, prevention and regulation of apoptosis, and maintenance of barrier function.21 The critical role of the microbiota on the development of gut function is amply demonstrated by the fate of the germ-free animal.22,23 Not only are virtually all components of the gut-associated and systemic immune systems affected in these animals, but the development of the epithelium, vasculature, neu-romuscular apparatus, and gut endocrine system also is impaired. The subtleties of the interactions between the microbiota and the host are exemplified by studies that demonstrate the ability of a polysaccharide elaborated by the bacterium Bacteroides fragilis to correct T-cell deficien-cies and Th1/Th2 imbalances and direct the development of lymphoid organs in the germ-free animal.24 Intestinal dendritic cells appear to play a central role in these critical immunologic interactions.24,25
How does the gut immune system differentiate between friend and foe when it comes to the bacteria it encounters?26 At the epithelial level, for example, a number of factors may allow the epithelium to tolerate commensal (and thus probiotic) organisms. These include the masking or modification of microbial-associated molecular patterns that are usually recognized by pattern recognition receptors, such as Toll-like receptors,27 and the inhibition of the NFκB inflammatory pathway.28 Responses to commensals and pathogens also may be distinctly different within the mucosal and systemic immune systems. For example, commensals such as Bifidobacterium infantis and Faecalibacterium prausnitzii have been shown to differentially induce regulatory T cells and result in the production of the anti-inflammatory cytokine interleukin (IL)-10.29 Other commensals may promote the development of T-helper cells, including TH17 cells, and result in a controlled inflammatory response that is protective against pathogens in part, at least, through the production of IL-17.30 The induction of a low-grade inflammatory response (physiologic inflammation) by commensals could be seen to prime the host’s immune system to deal more aggressively with the arrival of a pathogen.31
Through these and other mechanisms, the microbiota can be seen to play a critical role in protecting the host from colonization by pathogenic species.32 Some intestinal bacteria produce a variety of substances, ranging from relatively nonspecifc fatty acids and peroxides to highly specific bacteriocins,33,34 which can inhibit or kill other potentially pathogenic bacteria,35 while certain strains produce proteases capable of denaturing bacterial toxins.36
The Microbiota and Metabolism
Although the immunologic interactions between the microbiota and the host have been studied in great detail for some time, it has been only recently that the true extent of the metabolic potential of the microbiota has begun to be grasped. Some of these metabolic functions were well known, such as the ability of bacterial disac-charidases to salvage unabsorbed dietary sugars, such as lactose, and alcohols and convert them into short-chain fatty acids (SCFAs) that are then used as an energy source by the colonic mucosa. SCFAs promote the growth of intestinal epithelial cells and control their proliferation and differentiation. It has also been known for some time that enteric bacteria can produce nutrients and vitamins, such as folate and vitamin K, deconjugate bile salts,37 and metabolize some medications (such as sul-fasalazine) within the intestinal lumen, thereby releasing their active moieties. However, it is only recently that the full metabolic potential of the microbiome has come to be recognized and the potential contributions of the microbiota to the metabolic status of the host in health and in relation to obesity and related disorders have been appreciated. The application of genomics, metabolomics, and transcriptomics can now reveal, in immense detail, the metabolic potential of a given organism.38–41
It is now also known that certain commensal organisms also produce other chemicals, including neurotrans-mitters and neuromodulators, which can modify other gut functions, such as motility or sensation.42–44 Most recently and perhaps most surprisingly, it has been proposed that the microbiota can influence the development45 and func-tion46 of the central nervous system, thereby leading to the concept of the microbiota-gut-brain axis.47–49
The Gut Microbiota in Disease
Just as we are only now beginning to understand the key role of the flora in health, it has only been in very recent years that the true extent of the consequences of disturbances in the flora, or in the interaction between the flora and the host, has been recognized. Some of these consequences are relatively obvious. For example, when many components of the normal flora are eliminated or suppressed by a course of broad-spectrum antibiotics, the stage is set for other organisms that may be pathogenic to step in and cause disease.1,2,32 The classic example of this is antibiotic-associated diarrhea and its deadliest manifestation, Clostridium difficile colitis. Similar perturbations in the flora are thought to be involved in a devastating form of intestinal inflammation that may occur in newborns and especially premature infants: necrotizing enteroco-litis. In other situations, bacteria may simply be where they should not be. If motility of the bowel is impaired and/or acid secretion from the stomach is drastically reduced, an environment conducive to the proliferation of organisms in the small intestine that are normally con-fined to the colon results; the consequence is the syndrome of small bowel bacterial overgrowth. In other situations, the immunologic interaction between the flora and the host is disturbed, and the host may, for example, begin to recognize the constituents of the normal flora not as friend but as foe and may mount an inappropriate inflammatory response, which, some believe, may ultimately lead to conditions such as IBD.1,2,32 In other situations, damage to the intestinal epithelium renders the gut wall leaky and permits bacteria (in whole or in part) from the gut to gain access to the submucosal compartments or even to the systemic circulation, with the associated potential to cause catastrophic sepsis. This mechanism is thought to account for many of the infections that occur in critically ill patients in the intensive care unit, for example.
Most recently, qualitative changes in the microbiota have been invoked in the pathogenesis of a global epidemic: obesity.41 It has been postulated that a shift in the composition of the flora toward a population dominated by bacteria that are more avid extractors of absorbable nutrients—which are then available for assimilation by the host—could play a major role in obesity.41 Such studies rely on the application of modern technologies (genomics, metagenomics, and metabolomics) to the study of the colonic flora and have the potential to expose the true diversity and metabolic profile of the microbiota and the real extent of changes in disease. Rather than provide an exhausting survey of all the disease states that might be influenced by the microbiota, a brief overview of current information on the role of the microbiota in a few common diseases/disorders will be provided below.
Inflammatory Bowel Disease
There is a considerable body of evidence to support the hypothesis that the endogenous intestinal microflora plays a crucial role in the pathogenesis of IBD and its variants and related disorders.50,51 Some of this evidence is time-honored, such as the predilection of IBD for areas of high bacterial numbers and the role of contact with the fecal stream in sustaining inflammation. Other evidence is more recent and includes studies described above that illustrate the key roles of the microbiota in host immune responses and the generation of inflammatory responses. This evidence is supplemented by experimental observations on the ability of strategies that modify the microbiota (eg, the administration of probiotics) to modulate the inflamma-tory response in experimental models of IBD.52–58 Studies of the gut microbiota in IBD have revealed quantitative and qualitative changes,59 including the intriguing finding in some studies60 that a bacterium with anti-inflammatory properties, F prausnitzii, is less abundant in patients with IBD than in healthy individuals. The importance of microbiota-host interactions in IBD is further supported by the many studies of IBD genetics that have identified a host of changes in genes that code for molecules involved in bacterial recognition, host-bacteria engagement, and the resultant inflammatory cascade.61 On a more clinical level, the role of the microbiota is supported by the efficacy, albeit variable, of antibiotics in IBD62 and the suggestion, not always supported by high-quality clinical trials, that a number of probiotic organisms, including nonpathogenic Escherichia coli, Saccharomyces boulardii, and a Bifidobacte-rium, have efficacy in maintaining remission and in treating mild to moderate flare-ups in ulcerative colitis.63–70 There are some preliminary data to suggest that fecal transplan-tation,71 a strategy used with considerable success in the treatment of resistant and recurrent C difficile infection,72 may be effective in ulcerative colitis.73,74
A more convincing clinical illustration of the impact of modulation of the microbiota is provided by the example of pouchitis, an IBD variant that occurs in the neorectum in patients with ulcerative colitis who have undergone a total colectomy and ileo-anal pouch procedure. Here, VSL#3 (Sigma Tau Pharmaceuticals), a probiotic cocktail containing 8 different strains of lactic acid bacteria, has proven to be effective in the primary prevention and maintenance of remission of patients with pouchitis. In one study, remission was maintained in 85% of patients on VSL#3 compared with 6% of patients receiving placebo.75
Irritable Bowel Syndrome
A variety of strands of evidence suggest a role for the gut microbiota in IBS76 (Table 2). First and foremost among these is the clinical observation that IBS can develop in individuals de novo following exposure to enteric infections and infestations (ie, postinfectious IBS).77 More contentious has been the suggestion that patients with IBS may harbor small intestinal bacterial overgrowth (SIBO).78 More indirect evidence of a role for the micro-biota can be gleaned from some of the metabolic functions of the components of the microbiota. Thus, given the effects of bile salts on colonic secretion, changes in bile salt deconjugation could lead to changes in stool volume and consistency. Similarly, changes in bacterial fermentation could result in alterations in gas volume and/or composition. Further evidence comes from the clinical impact of therapeutic interventions, such as antibiotics, prebiotics, or probiotics, which can alter or modify the microbiota. Thus, the poorly absorbed antibiotic rifaximin (Xifaxan, Salix) has been shown to alleviate symptoms in diarrhea-predominant IBS,79 and some probiotics (B infantis 35624 [Align][Procter & Gamble] in particular80) have been shown to exert substantial clinical responses. The latter is of interest, given its demonstrated ability to modulate the systemic immune response in humans.25,81 Also gaining currency is the suggestion that the colonic microbiota may demonstrate qualitative and/or quantitative changes in IBS.82
Table 2.
Evidence for a Role for the Gut Flora in Irritable Bowel Syndrome
1. Direct evidence of an altered gut microbiota
|
2. Evidence of physiologic effects of an altered microbiota
|
| 3. Mediator of a proinflammatory state |
4. Therapeutic impact of altering the microbiota
|
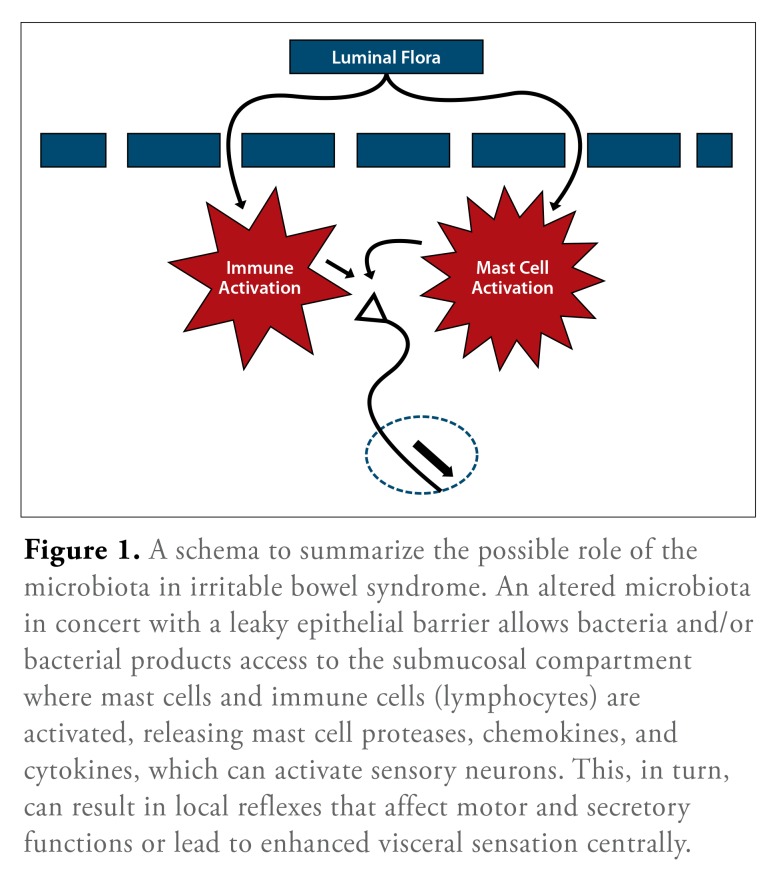
Modern molecular microbiologic methods are now being applied to this complex issue and have, indeed, confirmed that patients with IBS, regardless of subtype, do exhibit a fecal flora that is clearly different from that of control subjects.83 Studies by my colleagues and I have demonstrated, firstly, a reduced microbial diversity in IBS84 and, secondly, using high-throughput pyrosequencing, the existence of different IBS subgroups based on a detailed examination of the microbiota.85 At the phylum level, 1 of these subgroups resembled control subjects, whereas another demonstrated a shift in the relative proportion of the 2 major phyla, Firmicutes and Bacteroidetes, as well as significant changes at species and strain levels.82,84 The primacy of these microbial shifts and their potential to disturb mucosal or myoneural function in the gut wall, impact the brain-gut axis, or induce local or systemic immune responses remains to be defined (Figure 1). Most intriguing has been the suggestion, from animal studies, that the gut microbiota can influence brain function and morphology.49
Figure 1.
A schema to summarize the possible role of the microbiota in irritable bowel syndrome. An altered microbiota in concert with a leaky epithelial barrier allows bacteria and/or bacterial products access to the submucosal compartment where mast cells and immune cells (lymphocytes) are activated, releasing mast cell proteases, chemokines, and cytokines, which can activate sensory neurons. This, in turn, can result in local reflexes that affect motor and secretory functions or lead to enhanced visceral sensation centrally.
Several experimental observations provide a sci-entifc basis for the use of therapies that might modify the microbiota in IBS.85–87 Thus, oral administration of B infantis 35624 has been shown to attenuate interferon γ, tumor necrosis factor α (TNF-α), and IL-6 responses following mitogen stimulation, increase plasma levels of tryptophan and kynurenic acid, and, most strikingly, reduce concentrations of 5-hydroxyindoleacetic acid and dihydroxyphenylacetic acid concentrations in the frontal cortex and amygdala, respectively.88
These observations were taken one step further by the same research group by demonstrating normalization of immune responses, reversal of behavioral deficits, and restoration of basal norepinephrine concentrations in the brainstem in an animal model of depression (the maternally separated rat).89 While these latter observations could address some of the proposed pathophysiologic mechanisms associated with symptom development in IBS, namely, immune activation and disturbances in the brain-gut axis, other studies suggest that the same strain can also modify peripheral mechanisms linked with IBS, such as visceral hypersensitivity.90
Addressing another gut abnormality identified in IBS, Zeng and colleagues partially reversed changes in small intestinal permeability with a probiotic cocktail.91 Another organism, Lactobacillus acidophilus, has been shown to produce visceral analgesic effects through the induction of μ-opioid and cannabinoid receptors,92 and Lactobacillus paracasei has been shown to attenuate gut muscle hypercontractility in an animal model of post-infectious IBS.93 Again, this effect was strain-dependent and appeared to be mediated, in part, through a modulation of the immunologic response to the initial infection and, in part, through the direct effects of the organism, or a metabolite thereof, on gut muscle. In other experiments, this same organism was capable of attenuating antibiotic-induced visceral hypersensitivity in mice.94
Lactobacillus reuteri also has been shown to inhibit visceral pain induced by colorectal distension in the rat.95 Of clinical relevance, this same probiotic organism has been shown to readily colonize and induce an immune response in the small intestine in humans.96 Interestingly, in view of the relevance of techoic acid biosynthesis in the immunologic responses to certain lactobacilli, it has been shown by Duncker and colleagues that a Lactobacil-lus mutant (leading to D-alanine depletion of lipotechoic acid) also significantly inhibited visceral pain perception in healthy noninflamed rats.97
Functional and morphologic changes in the enteric neuromuscular apparatus develop in mice infected with Trichinella spiralis long after the worms have been expelled and the related inflammatory response has subsided, thus providing an animal model of postinfectious IBS.98,99 L paracasei, but not other strains, has been shown to attenuate gut muscle hypercontractility, reduce immune activation,93 and normalize the metabolic profile of mice in this model.100
These experimental observations are now supported by clinical studies with probiotics in IBS in humans. Results in IBS continue to be variable with a number of organisms, such as Lactobacillus GG, Lactobacillus plantarum, L acidophilus, Lactobacillus casei, the probiotic cocktail VSL#3, and Bifidobacterium animalis,101,102 alleviating individual IBS symptoms (eg, bloating, flatulence, and constipation) and only a few products affecting pain and global symptoms.80,103–105 Other products have shown no benefit.106,107
Obesity, Metabolic Syndrome, Nonalcoholic Fatty Liver Disease, and Nonalcoholic Steatohepatitis
Several mechanisms involving the microbiota in the patho-genesis of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) have been identified. In particular, a role for the microbiota in relation to diet in the pathogenesis of obesity per se has been extensively investigated.108,109 Pertinent findings include the ability of gram-negative anaerobes, such as Bacteriodes thetaiotami-cron, to cleave most glycosidic linkages and degrade plant polysaccharides, thereby supplying the host with 10% to 15% of its calorific requirement.108–111 The microbiota of obese individuals, as well as the cecal microbiota of ob/ob mice, is more efficient at the extraction of energy from the diet and in the production of SCFAs.110,112 Furthermore, the microbiota has been shown to stimulate hepatic triglyceride production through suppression of the lipoprotein lipase (LPL) inhibitor, fasting-induced adipose factor (also known as angiopoietin-like 4), thereby leading to continued expression of LPL, a key regulator of fatty acid release from triglycerides in the liver.113 The gut microbiota also can modulate systemic lipid metabolism through modification of bile acid metabolic patterns, also impacting directly on the emulsification and absorption properties of bile acids and, thus, indirectly on the storage of fatty acids in the liver. The microbiota also has been implicated in the development of insulin resistance,113 a fundamental abnormality in metabolic syndrome, by affecting energy balance, glucose metabolism, and the low-grade inflammatory state that has been associated with obesity and related metabolic disorders. Its role in choline metabolism,114–116 as well as inactivation of pro-inflammatory cytokines (eg, TNF-α), appears relevant to the development of NAFLD and progression to NASH. Most recently, studies in experimental models have shown that defective/deficient inflammasome sensing and related dysbiosis result in an abnormal accumulation of bacterial products in the portal circulation and promote progression of NAFLD/NASH.117
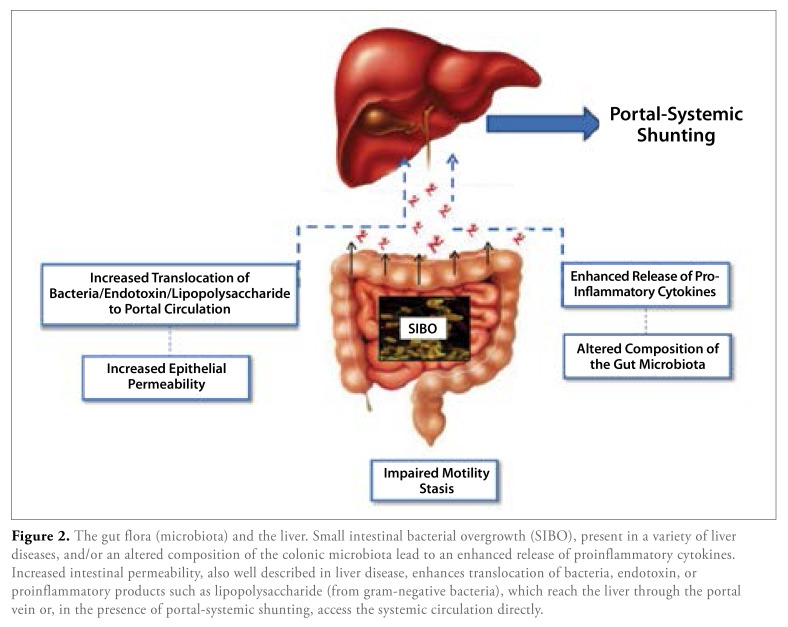
A more fundamental role for SIBO has been proposed in NAFLD by promoting both steatosis and inflammation118,119 (Figure 2). The potential of microbes of enteric origin to induce a progressive and even fatal steatohepatitis had been recognized several years ago in relation to the liver injury that complicated jejuno-ileal bypass operations for morbid obesity; indeed, that procedure has provided a valuable experimental model for exploring the impact of the microbiota in liver disease.
Figure 2.
The gut flora (microbiota) and the liver. Small intestinal bacterial overgrowth (SIBO), present in a variety of liver diseases, and/or an altered composition of the colonic microbiota lead to an enhanced release of proinflammatory cytokines. Increased intestinal permeability, also well described in liver disease, enhances translocation of bacteria, endotoxin, or proinflammatory products such as lipopolysaccharide (from gram-negative bacteria), which reach the liver through the portal vein or, in the presence of portal-systemic shunting, access the systemic circulation directly.
Summary
The true diversity and function of the human gut micro-biota as well as the extent and nature of its interactions with the host continue to be revealed; although much progress has been made in a very short time, the story is by no means complete, and the impact of a number of host, bacterial, and environmental factors on the composition and function of the microbiota is just beginning to be recognized. These factors must be taken into account when interpreting changes in the microbiota reported in disease states, and caution should be exercised in attributing a causative role to microbial changes seen in any disease state until much more is known of the primacy of these changes in that disorder. Some of the observed deviations in microbiota composition observed in a disease may be no more than epiphenomena. Nevertheless, as the critical role of the heretofore “ignored organ”—the gut microbiota—in health and disease has come to be recognized, so has the possibility that modifying the flora might be of therapeutic benefit.
Footnotes
Dr Quigley is a nonexecutive director of a multidepart-mental university campus company (Alimentary Health), which investigates host-flora interactions and the therapeutic manipulation of these interactions in various human and animal disorders and holds patents in these areas. He is also a consultant to Almirall, Forest, Ironwood, Rhythm, Salix, Shire-Movetis, and Tioga and has received honoraria for speaking engagements from Danone, Korea Yakult, Procter & Gamble, and Yakult and research support from GlaxoSmithKline, Norgine, and Procter & Gamble.
References
- 1.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 2.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 3.Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol. 2010;21(2):149–156. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes. 2012;3(3):203–220. doi: 10.4161/gmic.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58(suppl 2):44–52. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- 7.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut micro-biome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut micro-biota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariat D, Firmesse O, Levenez F, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Toole PW, Claesson MJ. Gut microbiota: changes throughout the lifespan from infancy to elderly. Int Dairy J. 2010;20(4):281–291. [Google Scholar]
- 14.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 15.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan KA, Jayaraman T, Daly P, et al. Isolation of lactobacilli with probiotic properties from the human stomach. Lett Appl Microbiol. 2008;47(4):269–274. doi: 10.1111/j.1472-765x.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 17.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal-and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G799–G807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43(7):3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6(10):776–778. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1(4):279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel RM, Lin PW. Developmental biology of gut-probiotic interaction. Gut Microbes. 2010;1(3):186–195. doi: 10.4161/gmic.1.3.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know about gnotobiology. Microbiol Mol Biol Rev. 1998;62(4):1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterol-ogy. 1994;107(5):1259–1269. doi: 10.1016/0016-5085(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 24.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(5):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Konieczna P, Groeger D, Ziegler M, et al. Bifidobacterium infantis 35624 administration induces Foxp3+ 1 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61(3):354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;452(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 27.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8(3):171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 28.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science. 2000;289(5484):1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 29.O’Mahony C, Scully P, O’Mahony D, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4(8):e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probi-otics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107(1):454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanahan F. The host-microbe interface within the gut. Best Pract Res Clin Gastroenterol. 2002;16(6):915–931. doi: 10.1053/bega.2002.0342. [DOI] [PubMed] [Google Scholar]
- 33.Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG, et al. Bacteriocin production as a mechanism for the anti-infective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104(18):7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rea MC, Sit CS, Clayton E, et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A. 2010;107(20):9352–9357. doi: 10.1073/pnas.0913554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Hara AM, Shanahan F. Gut microbiota: mining for therapeutic potential. Clin Gastroenterol Hepatol. 2007;5(3):274–284. doi: 10.1016/j.cgh.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharo-myces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67(1):302–307. doi: 10.1128/iai.67.1.302-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A. 2008;105(36):13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claesson MJ, O’Toole PW. Evaluating the latest high-throughput molecular techniques for the exploration of microbial gut communities. Gut Microbes. 2010;1(4):277–278. doi: 10.4161/gmic.1.4.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraher MH, O’Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol. 2012;9(6):312–322. doi: 10.1038/nrgastro.2012.44. [DOI] [PubMed] [Google Scholar]
- 40.Saulnier DM, Santos F, Roos S, et al. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One. 2011;6(4):e18783. doi: 10.1371/journal.pone.0018783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 42.Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents—physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22(6):495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 43.Lesniewska V, Rowland I, Laerke HN, Grant G, Naughton PJ. Relationship between dietary-induced changes in intestinal commensal microflora and duode-nojejunal myoelectric activity monitored by radiotelemetry in the rat in vivo. Exp Physiol. 2006;91(1):229–237. doi: 10.1113/expphysiol.2005.031708. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Mao Y-K, Diorio C, et al. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB J. 2010;24(10):4078–4088. doi: 10.1096/fj.09-153841. [DOI] [PubMed] [Google Scholar]
- 45.Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 47.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 48.Fleshner M. The gut microbiota: a new player in the innate immune stress response? Brain Behav Immun. 2011;25(3):395–396. doi: 10.1016/j.bbi.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9(6):497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 51.Shanahan F. The microbiota in inflammatory bowel disease: friend, bystander, and sometime-villain. Nutr Rev. 2012;70(suppl 1):S31–S37. doi: 10.1111/j.1753-4887.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 52.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53(11):1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jijon H, Backer J, Diaz H, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126(5):1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 2010;1(3):148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isolauri E, Salminen S. Probiotics, gut inflammation and barrier function. Gastroenterol Clin North Am. 2005;34(3):437–450. doi: 10.1016/j.gtc.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy J, O’Mahony L, O’Callaghan L, et al. Double-blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokines. Gut. 2003;52(7):975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Sheil B, McCarthy J, O’Mahony L, et al. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut. 2004;53(5):694–700. doi: 10.1136/gut.2003.027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. 2012;3(6):544–555. doi: 10.4161/gmic.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 61.Van Limbergen J, Philpott D, Griffiths AM. Genetic profiling in inflammatory bowel disease: from association to bedside. Gastroenterology. 2011;141(5):1566–1571. doi: 10.1053/j.gastro.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Ewaschuk JB, Tejpar QZ, Soo I, Madsen K, Fedorak RN. The role of antibiotic and probiotic therapies in current and future management of inflammatory bowel disease. Curr Gastroenterol Rep. 2006;8(6):486–498. doi: 10.1007/s11894-006-0039-z. [DOI] [PubMed] [Google Scholar]
- 63.Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11(5):853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 64.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354(9179):635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 65.Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22(1):56–63. doi: 10.1080/07315724.2003.10719276. [DOI] [PubMed] [Google Scholar]
- 66.Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zocco MA, dal Verme LZ, Cremonini F, et al. Efficacy of Lactobacil-lus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23(11):1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 68.Kato K, Mizuno S, Umesaki Y, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20(10):1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 69.Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol. 2003;15(6):697–698. doi: 10.1097/00042737-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 70.Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100(7):1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 71.Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44(8):551–561. doi: 10.1097/MCG.0b013e3181e5d06b. [DOI] [PubMed] [Google Scholar]
- 72.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman LA. Systematic review: faecal transplantation for the treatment of Clostridium difficile-associated disease. Aliment Pharmacol Ther. 2012;35(8):865–875. doi: 10.1111/j.1365-2036.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 73.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37(1):42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 74.Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107(10):1452–1459. doi: 10.1038/ajg.2012.93. [DOI] [PubMed] [Google Scholar]
- 75.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53(1):108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghoshal UC, Shukla R, Ghoshal U, et al. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflam. 2012 doi: 10.1155/2012/151085. 2012:151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastro-enterol Motil. 2012;18(3):258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quigley EM. A 51-year-old with IBS: test or treat for bacterial overgrowth? Clin Gastroenterol Hepatol. 2007;5(10):1140–1143. doi: 10.1016/j.cgh.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 79.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 80.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine pro-files. Gastroenterology. 2005;128(3):541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 81.Konieczna P, Akdis CA, Quigley EM, Shanahan F, O’Mahony L. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes. 2012;3(3):261–266. doi: 10.4161/gmic.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeffery IB, Quigley EM, Öhman L, Simrén M, O’Toole P W. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes. 2012;3(6):572–576. doi: 10.4161/gmic.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Codling C, O’Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55(2):392–397. doi: 10.1007/s10620-009-0934-x. [DOI] [PubMed] [Google Scholar]
- 84.Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 85.Quigley EM. Bacteria: a new player in gastrointestinal motility disorders— infections, bacterial overgrowth, and probiotics. Gastroenterol Clin North Am. 2007;36(3):735–748. doi: 10.1016/j.gtc.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Quigley EM, Flourie B. Probiotics in irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil. 2007;19(3):166–172. doi: 10.1111/j.1365-2982.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 87.Barbara G, Stanghellini V, Brandi G, et al. Interactions between commensal gut flora and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100(11):2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 88.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacterium infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 89.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 90.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacte-rium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastro-enterol Motil. 2010;22(9):1029–1035. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 91.Zeng J, Li Y-Q, Zuo X-L, Zhen Y-B, Yang J, Liu C-H. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28(8):994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 92.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 93.Verdu EF, Bercik P, Bergonzelli GE, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127(3):826–837. doi: 10.1053/j.gastro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamiya T, Eang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol. 2004;70(2):1176–1181. doi: 10.1128/AEM.70.2.1176-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duncker SC, Wang L, Hols P, Bienenstock J. The D-alanine content of lipote-choic acid is crucial for Lactobacillus plantarum-mediated protection from visceral pain perception in a rat colorectal distension model. Neurogastroenterol Motil. 2008;20(7):843–850. doi: 10.1111/j.1365-2982.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 98.Collins SM. The immunomodulation of enteric neuromuscular func tion: implications for motility and inflammatory disorders. Gastroenterology. 1996;111(6):1683–1699. doi: 10.1016/s0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- 99.Khan WI, Collins SM. Gut motor inflammation: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2005;143(3):389–397. doi: 10.1111/j.1365-2249.2005.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin F-PJ, Verdu EF, Yang Y, et al. Transgenomic metabolic interactions in a mouse model: interaction of Trichinella spiralis infection with dietary Lactobacil-lus paracasei supplementation. J Proteome Res. 2006;5(9):2185–2193. doi: 10.1021/pr060157b. [DOI] [PubMed] [Google Scholar]
- 101.Williams EA, Stimpson J, Wang D, et al. Clinical trial: a multistrain probi-otic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled trial. Aliment Pharmcol Ther. 2009;29(1):97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 102.Sinn DH, Song JH, Kim HJ, et al. Therapeutic effect of Lactobacillus aci-dophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53(10):2714–2718. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 103.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of pro-biotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104(4):1033–1049. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 104.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the therapy of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 105.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):326–333. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 106.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P, et al. A double-blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32(2):147–152. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Simrén M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome—a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31(2):218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 108.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 109.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 111.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 112.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 113.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidyl-choline promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rak K, Rader DJ. Cardiovascular disease: the diet-microbe morbid union. Nature. 2011;472(7341):40–41. doi: 10.1038/472040a. [DOI] [PubMed] [Google Scholar]
- 117.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(12):691701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 119.Abu-Shanab A, Scully P, Crosbie O, et al. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56(5):1524–1534. doi: 10.1007/s10620-010-1447-3. [DOI] [PubMed] [Google Scholar]