Abstract
IMPORTANCE
It is not known whether hospital and surgeon volumes have an association with readmission among patients undergoing pancreatoduodenectomy.
OBJECTIVE
To evaluate patient-, surgeon-, and hospital-level factors associated with readmission.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER)–Medicare data with cases diagnosed from January 1, 1998, to December 31, 2005, and followed up until December 2007. Population-based cancer registry data were linked to Medicare data for the corresponding patients. A total of 1488 unique individuals who underwent a pancreatoduodenectomy were identified.
INTERVENTIONS
Undergoing pancreatoduodenectomy at hospitals classified by volume of pancreatoduodenectomy procedures performed at the facility were either very-low, low, medium, or high volume. Undergoing pancreatoduodenectomy by surgeons classified by volume of pancreatoduodenectomy procedures performed by the surgeon were either very-low, low, medium, or high volume.
MAIN OUTCOMES AND MEASURES
In-hospital morbidity, mortality, and 30-day readmission were examined.
RESULTS
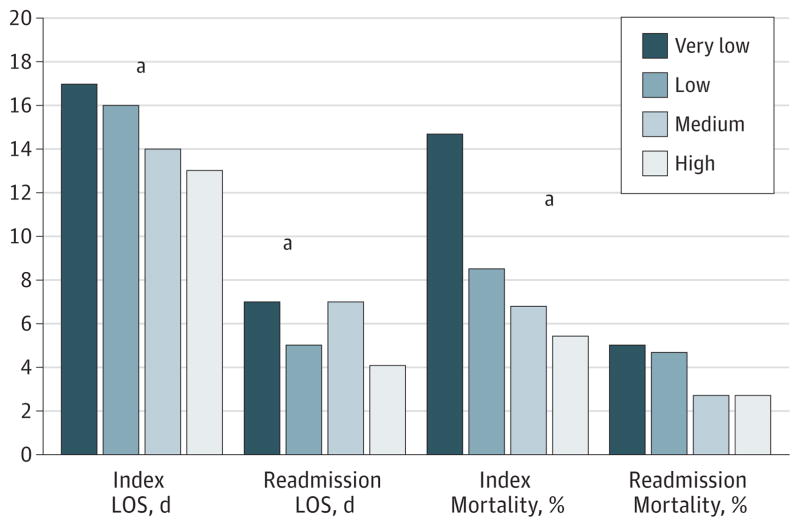
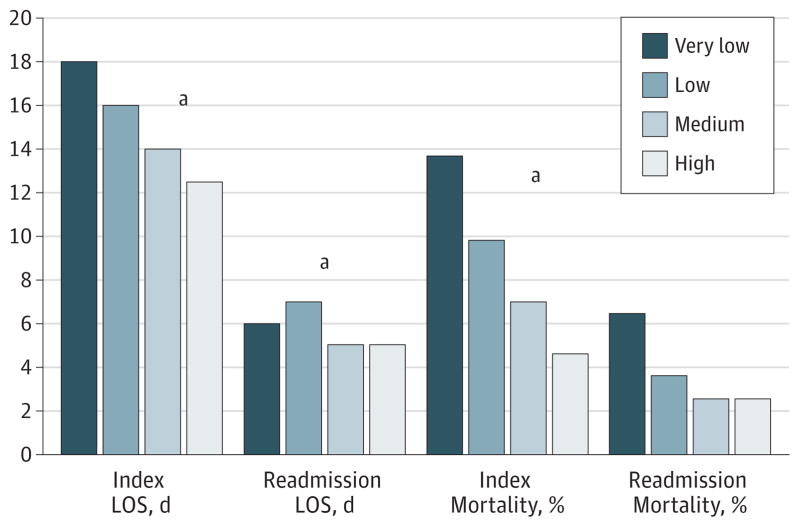
The median age was 74 years, and 1436 patients (96.5%) had a least 1 medical comorbidity. Patients were treated by 575 distinct surgeons at 298 distinct hospitals. Length of stay was longest (median, 17 days) and 90-day mortality highest (17.2%) at very-low-volume hospitals (P < .001). Among all pancreatoduodenectomy patients, 292 (21.3%) were readmitted within 30 days of discharge. There was no effect of surgeon volume and a modest effect of hospital volume (odds ratio for highest- vs lowest-volume quartiles, 1.85; 95% CI, 1.22–2.80; P = .02). The presence of significant preoperative medical comorbidities was associated with an increased risk for hospital readmission after pancreatoduodenectomy. A comorbidity score greater than 13 had a pronounced effect on the chance of readmission following pancreatoduodenectomy (odds ratio, 2.06; 95% CI, 1.56–2.71; P < .001). The source of variation in readmission was primarily attributable to patient-related factors (95.4%), while hospital factors accounted for 4.3% of the variability and physician factors for only 0.3%.
CONCLUSIONS AND RELEVANCE
Nearly 1 in 5 patients are readmitted following pancreatoduodenectomy. While variation in readmission is, in part, attributable to differences among hospitals, the largest share of variation was found at the patient level.
In 2010, roughly 20% of 11 855 702 Medicare beneficiaries required readmission within 30 days of hospital discharge, resulting in an estimated cost of $17 billion.1 In fact, some groups have estimated that readmissions in the United States may cost more than $40 billion annually.1,2 In addition to health care costs, readmission has been linked to worse outcomes including increased patient morbidity and mortality.1 However, the Medicare Payment Advisory Commission estimates that up to 75% of readmissions are avoidable. Therefore, readmission has been proposed as a quality measure for hospitals, surgeons, and health care delivery systems.3
Readmission following surgical procedures may have particular significance. Several groups, including our own, have reported that readmission following hepato-pancreato-biliary or colorectal surgery was associated with increased 90-day mortality, as well as worse long-term survival.4–8 The incidence of readmission, especially after major procedures, is not inconsequential. A recent study9 of pancreatoduodenectomy (PD) procedures from 6 high-volume institutions noted a 30-day readmission of 15%. In a separate population-based study using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, our group noted an incidence of readmission of 18% following PD.8
In an attempt to understand and, in turn, mitigate the likelihood of readmission, investigators have sought to identify factors most associated with readmission.4,6–8 To our knowledge, to date, studies on readmission following surgery have almost exclusively focused on patient- and disease-specific factors. For example, increased risk for readmission has been associated with patient age and comorbidities, as well as disease stage and history of postoperative complications.4,8–10 However, the incidence and risk for readmission may be owing to other factors not related to the patient or tumor. Donabedian11 proposed a model for assessing health care outcomes based on categorizing different factors into structures, processes, and outcomes. The Donabedian model is commonly used to describe how different variables relate and how each can act independently to influence outcomes.12,13 In light of the Donabedian model, variation in readmission may be influenced by factors not only at the patient level, but also at the health care provider and hospital levels. In addition to identifying individual variables that predict readmission, a potentially useful approach is to quantify the relative proportions of the variation in readmission occurring at the patient, surgeon, and hospital levels. Such an approach may allow focusing of quality improvement efforts at the appropriate level even when the relevant variables affecting care are not explicitly known.14 To our knowledge, no previous study has explicitly assessed readmission variability at all of these levels. Therefore, the objective of the current study was to evaluate and quantify patient-, surgeon-, and hospital-level factors associated with readmission using data from the SEER-Medicare database. Specifically, we sought to quantitatively assess the relative contributions of patient-, surgeon-, and hospital-level factors on readmission following PD.
Methods
A retrospective cohort study was performed using the SEER-Medicare–linked database from 1998–2005. Johns Hopkins Hospital’s institutional review board approved this study; individual patient consent was not required. The characteristics and representativeness of this database have been previously reported.15 Patients aged 66 years and older with at least 1 year of continuous health maintenance organization Medicare Part A and Part B enrollment prior to diagnosis were identified; patients who underwent PD for pancreatic cancer, as noted in the Medicare records, were selected and comprised the study cohort. Readmission within 30 days of discharge from index admission, length of index admission, and postoperative complications were determined using the Medicare inpatient records. In this study, a readmitted patient was defined as a person who was discharged from an acute care hospital and was subsequently admitted to an acute care hospital within 30 days of the index discharge.10
Patient demographic and tumor variables were ascertained from SEER data. Median income was used as a surrogate for socioeconomic status, with a preference for census tract median income if available, followed by zip code median income, census tract per capita income, and zip code per capita income.16 Using Medicare data, patient records were linked to hospital data and hospital procedure volumes for PD. Similarly, physician procedure volume was determined using the physician billing records linked to the SEER database. Treating surgeons and hospitals were identified using encoded unique physician identification numbers from Medicare claims. Mean annual PD volumes were calculated for each provider and hospital. For descriptive and analytical purposes, providers and hospitals were grouped into quartiles and classified as very low, low, medium, and high.17
Patient comorbidities were ascertained using both inpatient and outpatient Medicare claims along with physician billing records18 for a window up to 1 year before the date of PD; comorbidities were defined using the comorbidity index proposed by Elixhauser et al19 and the composite Elixhauser et al comorbidity score proposed by van Walraven et al.20
Demographic and Clinical Characteristics
The cohort consisted of 1488 patients who underwent PD and for whom physician- and hospital-specific data could be defined. Table 1 presents patient characteristics of the entire cohort stratified by surgeon- and physician-specific volume. The median age was 74 years and one-half of patients were female (n = 753; 50.6%); most patients were white (n = 1280; 86.0%). Among patients who underwent PD, 1436 (96.5%) had at least 1 medical comorbidity, while 1152 (77.4%) had 3 or more preoperative medical comorbidities. The median Elixhauser comorbidity score was 13. According to the SEER historic staging, most patients had regional disease (n = 975; 65.5%); among the 1287 patients for whom information was available, 599 (46.5%) had lymph node metastasis.
Table 1.
Patient Characteristics for the Whole Cohort (N=1488) and by Volume Groups
| Characteristic | Total, No. (%) | Patients Treated, No. (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospital Volume Quartile
|
Surgeon Volume Quartile
|
||||||||
| Very Low (1–4) | Low (5–12) | Medium (13–24) | High (25–53) | Very Low (1–2) | Low (3–6) | Medium (7–20) | High (21–84) | ||
| Age, y | |||||||||
|
| |||||||||
| 65–69 | 392 (26.3) | 114 (27.5) | 96 (27.0) | 93 (25.3) | 89 (25.3) | 132 (30.2) | 93 (26.1) | 76 (22.2) | 91 (25.6) |
|
| |||||||||
| 70–74 | 444 (29.8) | 111 (28.8) | 110 (31.0) | 110 (30.0) | 113 (32.1) | 120 (27.5) | 99 (27.8) | 110 (32.1) | 115 (32.7) |
|
| |||||||||
| 75–79 | 399 (26.8) | 109 (26.3) | 81 (22.8) | 106 (28.9) | 103 (29.3) | 110 (25.2) | 95 (26.7) | 104 (30.3) | 90 (25.6) |
|
| |||||||||
| ≥80 | 253 (17.0) | 80 (19.3) | 68 (19.2) | 58 (15.8) | 47 (13.4) | 75 (17.2) | 69 (19.4) | 53 (16.5) | 56 (15.9) |
|
| |||||||||
| Sex | |||||||||
|
| |||||||||
| Male | 735 (49.4) | 202 (48.8) | 176 (49.6) | 184 (50.1) | 173 (49.2) | 219 (50.1) | 179 (50.3) | 161 (46.9) | 176 (50.0) |
|
| |||||||||
| Female | 753 (50.6) | 212 (51.2) | 179 (50.4) | 183 (49.9) | 179 (50.9) | 218 (49.0) | 177 (49.7) | 182 (53.1) | 176 (50.0) |
|
| |||||||||
| Race/ethnicity | |||||||||
|
| |||||||||
| White | 1280 (86.0) | 339 (81.9) | 308 (86.8) | 322 (87.7) | 311 (88.4) | 354 (81.0) | 300 (84.3) | 309 (90.1) | 317 (90.1) |
|
| |||||||||
| Black | 92 (6.2) | 30 (7.3) | 20 (5.6) | 22 (6.0) | 20 (5.7) | 40 (9.2) | 26 (7.3) | <11 | 17 (4.8) |
|
| |||||||||
| Other | 116 (7.8) | 45 (10.9) | 27 (7.6) | 23 (6.3) | 21 (6.0) | 43 (9.8) | 30 (8.4) | 25 (7.3) | 18 (5.1) |
|
| |||||||||
| Zip code income quartile | |||||||||
|
| |||||||||
| Lowest | 327 (22.1) | 126 (30.4) | 70 (19.7) | 79 (21.5) | 52 (14.8) | 79 (18.1) | 68 (19.1) | 108 (31.5) | 92 (26.1) |
|
| |||||||||
| 2nd | 363 (24.4) | 96 (23.2) | 98 (27.6) | 91 (24.8) | 78 (22.2) | 183 (41.9) | 150 (42.1) | 118 (34.4) | 168 (47.7) |
|
| |||||||||
| 3rd | 362 (24.2) | 95 (23.0) | 80 (22.5) | 100 (27.3) | 87 (24.7) | 83 (19.0) | 88 (24.7) | 63 (18.4) | 73 (20.7) |
|
| |||||||||
| Top | 436 (29.3) | 97 (23.4) | 107 (30.1) | 97 (26.4) | 135 (38.4) | 92 (21.1) | 50 (14.0) | 54 (15.7) | 19 (5.4) |
|
| |||||||||
| Geographic location | |||||||||
|
| |||||||||
| Northeast | 347 (23.3) | 85 (20.5) | 84 (23.7) | 61 (16.6) | 117 (33.2) | 79 (18.1) | 68 (19.1) | 108 (31.5) | 92 (26.1) |
|
| |||||||||
| West | 619 (41.6) | 201 (48.6) | 133 (37.5) | 142 (38.7) | 143 (40.6) | 183 (41.9) | 150 (42.1) | 118 (34.4) | 168 (47.7) |
|
| |||||||||
| Midwest | 307 (20.6) | 46 (11.1) | 47 (13.2) | 124 (33.8) | 90 (25.6) | 83 (19.0) | 88 (24.7) | 63 (18.4) | 73 (20.7) |
|
| |||||||||
| South | 215 (14.5) | 82 (19.8) | 91 (25.6) | 40 (10.9) | <11 | 92 (21.1) | 50 (14.0) | 54 (15.7) | 19 (5.4) |
|
| |||||||||
| Comorbidity score | |||||||||
|
| |||||||||
| ≤13 | 790 (53.1) | 226 (54.6) | 194 (54.7) | 180 (49.1) | 190 (54.0) | 225 (51.5) | 203 (57.0) | 175 (51.0) | 187 (53.1) |
|
| |||||||||
| >13 | 698 (46.9) | 188 (45.4) | 161 (45.4) | 187 (51.0) | 162 (46.0) | 212 (48.5) | 153 (43.0) | 168 (49.0) | 165 (46.9) |
|
| |||||||||
| Period of diagnosis | |||||||||
|
| |||||||||
| 1998–2001 | 1021 (68.6) | 293 (70.8) | 234 (65.9) | 237 (64.6) | 257 (73.0) | 327 (74.8) | 253 (71.1) | 217 (63.3) | 224 (63.6) |
|
| |||||||||
| 2002–2005 | 467 (31.4) | 121 (29.2) | 121 (34.1) | 130 (35.4) | 95 (27.0) | 110 (25.2) | 103 (28.9) | 126 (36.7) | 128 (36.4) |
|
| |||||||||
| SEER historic stage | |||||||||
|
| |||||||||
| In situ or localized | 350 (23.5) | 94 (22.7) | 76 (21.4) | 88 (24.0) | 92 (26.1) | 103 (23.6) | 78 (21.9) | 75 (21.9) | 94 (26.7) |
|
| |||||||||
| Regional | 975 (65.5) | 261 (63.0) | 249 (70.1) | 236 (64.3) | 229 (65.1) | 273 (62.5) | 251 (70.5) | 231 (67.4) | 220 (62.5) |
|
| |||||||||
| Distant | 60 (4.0) | 23 (5.6) | 11 (3.1) | 16 (4.4) | <11 | 25 (5.7) | <11 | 15 (4.4) | 13 (3.7) |
|
| |||||||||
| Unstaged | 103 (6.9) | 36 (8.7) | 19 (5.4) | 27 (7.4) | 21 (6.0) | 36 (8.2) | 20 (5.6) | 22 (6.4) | 25 (7.1) |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Hospital and Surgeon Characteristics
The 1488 patients in the study were treated by 575 distinct surgeons at 298 distinct hospitals. Most PD procedures were performed by general surgeons (n = 1214, 81.6%). Surgeons were classified into 4 volume quartiles: very-low volume (1–2 procedures annually, 56.0%), low volume (3–6 procedures annually, 28.7%), medium volume (7–20 procedures annually, 12.4%), and high volume (21–84 procedures annually, 3.0%). Similar to surgeon volume, hospitals were classified into the following quartiles based on the volume of procedures per year: very-low volume (1–4 procedures annually, 74.2%), low volume (5–12 procedures annually, 17.1%), medium volume (13–24 procedures annually, 5.7%), and high volume (25–53 procedures annually, 3.0%) (Table 1). With respect to National Cancer Institute (NCI) designation, 245 patients (18.5%) were operated on at a NCI-designated comprehensive cancer center, 37 (2.8%) at a NCI-designated clinical center, and 1041 (78.7%) at a non-NCI designated facility.
Statistical Analysis
Numerical variables were described as medians and interquartile ranges. Categorical variables were described as totals and frequencies. Cells with fewer than 11 patients per variable were relabeled as fewer than 11 (<0.4%) in compliance with the NCI’s regulations for the reporting of SEER-Medicare data. Bivariate comparisons were assessed using analysis of variance, Krus-kal-Wallis test, or χ2 test, as appropriate. Surgeon and hospital volumes were modeled both as random effects in multilevel models and as fixed effects (as quartiles of volumes) to explore their association with readmission. Models were adjusted for only physician and hospital volumes, as well as physician and hospital volumes and case mix. All reported P values were 2-tailed; for all tests, P < .05 was considered statistically significant. All statistical analyses were performed using SAS version 9.3 (SAS Inc).
Results
Following PD, the median length of stay (LOS) during index admission was 16 days. Length of stay was longest for patients who underwent PD at very-low-volume hospitals (median, 17 days) and shortest in the highest-volume hospitals (median, 13 days) (P < .001) (Figure 1). A similar pattern was noted for physician volume (Figure 2). Patients operated on by the lowest-volume surgeons had the longest median LOS (median, 18 days) vs patients who had PD performed by the highest-volume surgeons (median, 12.5 days) (P < .001). Within 90 days of surgery, 181 patients (13.8%) died. Ninety-day mortality following PD among low-volume hospitals (17.2%) and lowest-volume surgeons (16.7%) was considerably higher compared with high-volume hospitals (8.0%) and high-volume surgeons (7.7%) (all P < .05) (Table 2). Ninety-day mortality was also considerably higher among hospitals that were non-NCI designated centers (14.4%) compared with hospitals that were NCI-designated comprehensive cancer centers (4.9%) (P < .001).
Figure 1. Length of Stay (LOS) and Mortality Associated With the Index Admission and the Readmission Stratified by Pancreatoduodenectomy Procedural Hospital Volume.
aP value for comparison <.05.
Figure 2. Length of Stay (LOS) and Mortality Associated With the Index Admission and the Readmission Stratified by Pancreatoduodenectomy Procedural Surgeon Volume.
aP value for comparison <.05.
Table 2.
Patient Outcomes by Volume Groups and Hospital Type
| Variable | Total, No. (%) | Patients Treated, No. (%)
|
NCI Designationa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital Volume Quartile
|
Surgeon Volume Quartile
|
|||||||||||
| Very Low | Low | Medium | High | Very Low | Low | Medium | High | None | Clinical Center | Comprehensive Center | ||
| Patients, No. | 1488 (100) | 414 | 355 | 367 | 352 | 437 | 356 | 343 | 352 | 1041 | 37 | 245 |
|
| ||||||||||||
| Index in-hospital mortalityb | 118 (7.9) | 54 (13.0) | 27 (7.6) | 21 (5.7) | 16 (4.6) | 55 (12.6) | 29 (8.2) | 20 (5.8) | 14 (4.0) | 9.6 | 2.7 | 2.5 |
|
| ||||||||||||
| 90-d mortalityb | 181 (13.8) | 71 (17.2) | 50 (14.1) | 32 (8.7) | 28 (8.0) | 73 (16.7) | 43 (12.1) | 38 (11.1) | 27 (7.7) | 150 (14.4) | <11 | 12 (4.9) |
|
| ||||||||||||
| Index admission LOS, medianb | 16 | 17 | 16 | 14 | 13 | 18 | 16 | 14 | 12.5 | 16 | 14 | 11 |
|
| ||||||||||||
| Readmission to same hospital, %b | 292 (76.0) | 76.7 | 84.7 | 83.6 | 58.1 | 76.9 | 85.5 | 85.0 | 59.4 | 81.1 | 83.3 | 53.7 |
|
| ||||||||||||
| Readmission LOS, medianc | 6 | 7 | 5 | 7 | 4 | 6 | 7 | 5 | 5 | 5 | 5 | 4.5 |
|
| ||||||||||||
| Readmission mortality, %d | 11 (3.9) | 5.0 | 4.7 | 2.7 | 2.7 | 6.4 | 3.6 | 2.5 | 2.5 | 4.0 | 0.0 | 6.3 |
Abbreviations: LOS, length of stay; NCI, National Cancer Institute.
NCI designation data available for 1323 of 1488 patients for index admission and 263 of 292 patients for readmission.
P < .001 for hospital and surgeon volume quartiles and NCI designation.
P < .001 for hospital and surgeon volume quartiles and P = .98 for NCI designation.
P = .82 for hospital volume quartile, P = .53 for surgeon volume quartile, and P = .75 for NCI designation.
Overall, among the 1370 patients who were discharged from the index admission following PD, 292 (21.3%) were readmitted within 30 days of discharge. The median time to readmission for all readmitted patients was 9 days (interquartile range, 4.0–15.5 days). The most frequent indication for readmission was dehydration (n = 87; 29.8%), gastric outlet obstruction (n = 34; 11.6%), cholangitis (n = 14; 4.8%), venous thromboembolism (n = 12; 4.1%), and pneumonia (n = 12; 4.1%).
Rates of readmission stratified by patient, tumor, and provider and hospital characteristics are presented in Table 3. There was a noted difference in readmission rates when comparing patients with a comorbidity score of 13 or less (15.7%) vs patients who had a comorbidity score of greater than 13 (27.4%)(P < .001). Moreover, hospital volume was also associated with the risk for readmission. Paradoxically, very-low-volume hospitals had the lowest rate of 30-day readmission (16.7%); medium-volume hospitals had the highest rate of readmission (25.9%), while readmission at high- and very-high-volume hospitals was comparable (21.1% and 22.2%, respectively). Physician volume was not associated with the incidence of readmission on univariate analysis, although higher-volume surgeons tended to have a higher readmission rate (P = .06).
Table 3.
30-Day Readmission Rate by Multiple Factors Among the 1370 Patients Discharged Alive
| Characteristic | Patients Readmitted, No. (%) | P Value |
|---|---|---|
| SEER historic stage | ||
| In situ or localized | 71 (21.6) | .08 |
| Regional | 16 (30.2) | |
| Distant | 27 (28.7) | |
| Unstaged | 178 (19.9) | |
| Age, y | ||
| 65–69 | 79 (21.0) | .96 |
| 70–74 | 87 (21.0) | |
| 75–79 | 76 (21.2) | |
| ≥80 | 50 (22.7) | |
| Sex | ||
| Male | 156 (23.2) | .10 |
| Female | 136 (19.5) | |
| Race/ethnicity | ||
| White | 248 (21.0) | .82 |
| Black | 19 (22.6) | |
| Other | 25 (23.4) | |
| Comorbidity score | ||
| ≤13 | 112 (15.7) | <.001 |
| >13 | 180 (27.4) | |
| Complications | ||
| None | 149 (19.7) | .10 |
| ≥1 | 143 (23.3) | |
| Income quartile | ||
| Lowest | 63 (21.4) | .97 |
| 2nd | 74 (22.2) | |
| 3rd | 69 (20.8) | |
| Top | 86 (21.0) | |
| Geographic area | ||
| Northeast | 68 (21.5) | .92 |
| West | 127 (22.0) | |
| Midwest | 59 (20.6) | |
| South | 38 (20.0) | |
| Volume quartile | ||
| Physician | ||
| Very low | 78 (20.4) | .06 |
| Low | 55 (16.8) | |
| Medium | 80 (24.8) | |
| High | 79 (23.4) | |
| Hospital | ||
| Very low | 60 (16.7) | .03 |
| Low | 85 (25.9) | |
| Medium | 73 (21.1) | |
| High | 74 (22.0) | |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
The effects of these characteristics were assessed using a multivariable multilevel logistic regression model that explicitly accounted for correlations in readmission among patients treated by the same surgeon or hospital. Demographic characteristics, such as age, sex, and race/ethnicity, did not demonstrate significant effects on readmission. There was no effect of surgeon volume and a modest effect of hospital volume (odds ratio for highest- vs lowest-volume quartile: 1.85; 95% CI, 1.22–2.80; P = .02; Table 4). The presence of significant preoperative medical comorbidities was also associated with an increased risk for hospital readmission after PD. Specifically, a comorbidity score greater than 13 (odds ratio, 2.06; 95% CI, 1.56–2.71; P < .001) had a pronounced effect on the chance of readmission following PD (Table 4). After accounting for all explanatory variables in the full model, the source of variation in patient readmission was primarily patient-related factors (95.4%), while hospital factors accounted for 4.3% of the variability and physician factors for 0.3%.
Table 4.
Multivariate Analysis Adjusted for Case Mix, Hospital Volume, and Physician Volume
| Characteristic | Odds Ratio (95% CI) | P Value |
|---|---|---|
| SEER historic stage | ||
| In situ or localized | 1.10 (0.80–1.51) | .16 |
| Regional | 1.0 [Reference] | |
| Distant | 1.69 (0.90–3.17) | |
| Unstaged | 1.55 (0.95–2.54) | |
| Age, y | ||
| 65–69 | 1.05 (0.70–1.59) | .99 |
| 70–74 | 1.02 (0.68–1.53) | |
| 75–79 | 0.99 (0.65–1.50) | |
| ≥80 | 1.0 [Reference] | |
| Male | 1.13 (0.87–1.48) | .36 |
| Race/ethnicity | ||
| White | 1.0 [Reference] | .55 |
| Black | 1.05 (0.60–1.84) | |
| Other | 1.31 (0.80–2.13) | |
| Comorbidity score >13 | 2.06 (1.56–2.71) | <.001 |
| Any complication | 1.03 (0.78–1.38) | .82 |
| Length of stay (per-day increase) | 1.00 (0.99–1.02) | .68 |
| Period of diagnosis before 2002 | 0.89 (0.66–1.20) | .45 |
| Income quartile | ||
| Lowest | 0.98 (0.65–1.47) | .91 |
| 2nd | 1.10 (0.76–1.59) | |
| 3rd | 0.98 (0.68–1.42) | |
| Top | 1.0 [Reference] | |
| Geographic area | ||
| Northeast | 1.21 (0.74–1.96) | .63 |
| West | 1.31 (0.84–2.03) | |
| Midwest | 1.10 (0.67–1.79) | |
| South | 1.0 [Reference] | |
| Volume quartile | ||
| Physician | ||
| Very low | 1.0 [Reference] | .20 |
| Low | 1.28 (0.82–2.00) | |
| Medium | 1.23 (0.82–1.84) | |
| High | 0.83 (0.56–1.23) | |
| Hospital | ||
| Very low | 1.0 [Reference] | .02 |
| Low | 1.33 (0.82–2.14) | |
| Medium | 1.30 (0.83–2.03) | |
| High | 1.85 (1.22–2.80) | |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Discussion
Reducing the incidence of readmission is an important goal as identified by the Centers for Medicare and Medicaid Services.2 In fact, not only will reimbursement be tied to standardized readmission rates, but the rate of readmission for any given provider or hospital may serve as a publically available quality metric in the future.10 Intuitively, one might expect to find factors associated with readmission on multiple levels of the health care delivery system. For example, the risk for readmission may be related not only to patient-specific factors, but also to individual surgeon practice variation, as well as hospital-level dynamics. To date, data on readmission following complex operations, such as PD, have been scarce,4–8 and all previous reports have focused almost exclusively on patient-level factors associated with readmission. To our knowledge, no previous study has quantified readmission variation at the patient, provider, and hospital levels simultaneously. The current study is important because it quantitatively assessed the relative contributions of the patient, surgeon, and hospital. When examining relative contributions, patient-level variation was the largest source of variation in readmission (95.4%). In comparison, the hospital-level contribution to the variation in readmission was about 5% and physician-level factors were responsible for only a negligible portion of the variability in readmission seen after PD (0.3%). In fact, on multivariate analyses, patient comorbidity level and hospital-level procedure volumes were the 2 factors most strongly associated with a higher risk for readmission. While we noted a minimal trend in the association of physician volume and the incidence of readmission, hospital-level variation was much larger. These results suggest that provider-related variation in readmission is largely a hospital-level phenomenon.
The Donabedian quality of care framework conceptualizes 3 qualities of care dimensions: structure, process, and outcome. Structural elements include the community, institution, provider, and patient, while process elements involve services rendered and types of treatment delivered. The Donabedian model attempts to make more explicit the complex relationship between structural elements (hospital volume or surgeon volume), process elements (surgery or clinical care pathway), and outcome (readmission). One problem in applying this multilevel model to studies on readmission is that detailed provider-specific data are typically not available in population-based data sets. In the current study, however, we were able to use unique physician identification numbers from Medicare claims to identify the treating surgeons so we could examine the impact of this provider-level variation on readmission. Population-based data also do not allow for the ascertainment of certain hospital wide processes such as whether a specific hospital had formally adopted an institution wide pathway to reduce readmission. Similar to our previous work, we addressed this issue by using a multilevel regression model that allowed us to characterize variation at each level (patient, hospital, or surgeon) without explicitly accounting for all the variables at that level.14
Previous studies4–9 have reported readmission rates after PD ranging from 16% to 50%. However, previous data published on readmission after PD are difficult to interpret owing to varying definitions of readmission (eg, within 30-day, 90-day, and 1-year periods).4,5,21 In the current study, we defined readmission as readmission to an acute care facility within 30 days, while excluding those patients admitted to a skilled nursing facility or other nonacute inpatient facility. Prior studies may also have underestimated readmission owing to the inability to capture data from secondary hospitals. Yermilov et al4 found that nearly half of readmissions occurred at a hospital other than the one where the surgery took place. A benefit of the SEER-linked Medicare data is that it covers a large population, making it more likely to capture all readmissions, even to secondary hospitals. In the current study, the incidence of readmission was 21.3%. We paradoxically found that low-volume surgeons and hospitals had a slightly lower risk for readmission compared with high-volume surgeons and hospitals (Tables 3 and 4). Multiple previous studies have demonstrated that increased procedure volume leads to a dramatic decrease in perioperative mortality.22–26 Specifically, Kennedy et al22 found that high-volume PD surgeons have better outcomes including complication rates and overall mortality. In the current study, we also noted that in-hospital and 90-day mortality were higher among lower-volume hospitals and surgeons. Interestingly, our data showed that while the volume-outcome relationship was associated with better perioperative morbidity and mortality, a similar relationship was not found for the risk for readmission. In fact, patients undergoing operations at the lowest-volume hospitals had lower rates of readmission. The reason for the higher readmission among higher-volume hospitals and surgeons is unclear and undoubtedly multifactorial. The lower readmission at low-volume hospitals did come at the expense of a longer LOS and a higher perioperative mortality. However, the effect of hospital volume on readmission was only modest; the major determinant of readmission was the presence of a higher comorbidity burden at the time of index admission.
Despite the effect of hospital-level factors on readmission, data from the current study demonstrated that the overwhelming source of variation with hospital readmissions occurred at the patient level. While age and race/ethnicity did not play important roles in predicting readmission, the presence of significant preoperative medical comorbidities was significantly associated with an increased risk for hospital readmission after PD, as patients with a comorbidity score greater than 13 were 2-fold more likely to be readmitted. The finding that preoperative comorbidities increased the risk for readmission emphasized the importance of preemptively managing certain modifiable comorbidities prior to surgery in an attempt to decrease readmission following PD. Future studies will need to better examine the impact of preoperative management of medical comorbidities to prevent unplanned readmission and by extension improve outcomes for patients undergoing PD.
The current study has several limitations. First, similar to all studies using Medicare data, the study cohort included only patients aged 66 years and older. Given that readmission was strongly associated with the presence of comorbidities, our conclusions may not be generalizable to a young cohort of patients with markedly fewer medical comorbidities.
Second, in assessing provider-level variables, we were limited to those available in the SEER-Medicare data, and we largely focused on provider-specific PD volume. In our analyses, we used a modeling approach that allowed quantification of variability without needing to explicitly identify all important variables at each level.14 Finally, SEER data may not represent a true representative sampling of hospitals and physicians with respect to the provider-level characteristics. As such, other sources of data will need to be used to corroborate our findings.
In conclusion, readmission following complex surgical procedures, such as PD, remains a problem, with nearly 1 in 5 patients being readmitted. While variation in readmission can, in part, be attributed to differences among hospitals, the largest share of variation was found to be at the patient level. Importantly, the finding that patient-to-patient variation was much greater than that attributable to health care providers may have important implications for policy makers. Specifically, our data call into question the use of any arbitrary readmission threshold as a de facto quality or performance metric. Rather, our data strongly suggest that if the readmission is to be used as a hospital quality measure, any guideline will need to extensively account for differences in patient case mix, as well as balance readmission data against other quality metrics such as LOS and perioperative mortality.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Pawlik had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hyder, Nathan, Schneider, Hirose, Herman, Pawlik.
Acquisition of data: Hyder, Nathan, Weiss, Choti, Pawlik.
Analysis and interpretation of data: Hyder, Dodson, Schneider, Weiss, Cameron, Choti, Makary, Wolfgang, Pawlik.
Drafting of the manuscript: Hyder, Dodson, Hirose, Pawlik.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Hyder, Schneider, Pawlik.
Administrative, technical, or material support: Dodson, Nathan, Cameron, Herman, Pawlik.
Study supervision: Schneider, Weiss, Choti, Makary, Wolfgang, Pawlik.
References
- 1.Jencks SF. Defragmenting care. Ann Intern Med. 2010;153(11):757–758. doi: 10.7326/0003-4819-153-11-201012070-00010. [DOI] [PubMed] [Google Scholar]
- 2.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 3.Martin RC, Brown R, Puffer L, et al. Readmission rates after abdominal surgery: the role of surgeon, primary caregiver, home health, and subacute rehab. Ann Surg. 2011;254(4):591–597. doi: 10.1097/sla.0b013e3182300a38. [DOI] [PubMed] [Google Scholar]
- 4.Yermilov I, Bentrem D, Sekeris E, et al. Readmissions following pancreaticoduodenectomy for pancreas cancer: a population-based appraisal. Ann Surg Oncol. 2009;16(3):554–561. doi: 10.1245/s10434-008-0178-6. [DOI] [PubMed] [Google Scholar]
- 5.Reddy DM, Townsend CM, Jr, Kuo YF, Freeman JL, Goodwin JS, Riall TS. Readmission after pancreatectomy for pancreatic cancer in Medicare patients. J Gastrointest Surg. 2009;13(11):1963–1974. doi: 10.1007/s11605-009-1006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu ZY, He JK, Wang YF, et al. Multivariable analysis of factors associated with hospital readmission following pancreaticoduodenectomy for malignant diseases. Chin Med J (Engl) 2011;124(7):1022–1025. [PubMed] [Google Scholar]
- 7.Kent TS, Sachs TE, Callery MP, Vollmer CM., Jr Readmission after major pancreatic resection: a necessary evil? J Am Coll Surg. 2011;213(4):515–523. doi: 10.1016/j.jamcollsurg.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Schneider EB, Hyder O, Wolfgang CL, et al. Patient readmission and mortality after surgery for hepato-pancreato-biliary malignancies. J Am Coll Surg. 2012;215(5):607–615. doi: 10.1016/j.jamcollsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad SA, Edwards MJ, Sutton JM, et al. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256(3):529–537. doi: 10.1097/SLA.0b013e318265ef0b. [DOI] [PubMed] [Google Scholar]
- 10.Schneider EB, Hyder O, Brooke BS, et al. Patient readmission and mortality after colorectal surgery for colon cancer: impact of length of stay relative to other clinical factors. J Am Coll Surg. 2012;214(4):390–398. doi: 10.1016/j.jamcollsurg.2011.12.025. discussion 398–399. [DOI] [PubMed] [Google Scholar]
- 11.Donabedian A. The quality of care: how can it be assessed? JAMA. 1988;260(12):1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Dimick JB. Understanding and reducing variation in surgical mortality. Annu Rev Med. 2009;60:405–415. doi: 10.1146/annurev.med.60.062107.101214. [DOI] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198(4):626–632. doi: 10.1016/j.jamcollsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Nathan H, Shore AD, Anders RA, Wick EC, Gearhart SL, Pawlik TM. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist, or hospital? J Gastrointest Surg. 2011;15(3):471–479. doi: 10.1007/s11605-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8)(suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Simons JP, Ng SC, McDade TP, Zhou Z, Earle CC, Tseng JF. Progress for resectable pancreatic [corrected] cancer? a population-based assessment of US practices. Cancer. 2010;116(7):1681–1690. doi: 10.1002/cncr.24918. [DOI] [PubMed] [Google Scholar]
- 17.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236(5):583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 21.Emick DM, Riall TS, Cameron JL, et al. Hospital readmission after pancreaticoduodenectomy. J Gastrointest Surg. 2006;10(9):1243–1252. doi: 10.1016/j.gassur.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy TJ, Cassera MA, Wolf R, Swanstrom LL, Hansen PD. Surgeon volume versus morbidity and cost in patients undergoing pancreaticoduodenectomy in an academic community medical center. J Gastrointest Surg. 2010;14(12):1990–1996. doi: 10.1007/s11605-010-1280-1. [DOI] [PubMed] [Google Scholar]
- 23.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 25.Dudley RA, Johansen KL, Brand R, Rennie DJ, Milstein A. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA. 2000;283(9):1159–1166. doi: 10.1001/jama.283.9.1159. [DOI] [PubMed] [Google Scholar]
- 26.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? a systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]